Abstract
The n-hexane and CHCl3 soluble fractions of the MeOH extract of the aerial parts of Piper kadsura were found to potently inhibit nitric oxide (NO) production in LPS-activated BV-2 cells, a microglial cell line. From the active fractions, a new stereoisomer of guaiane sesquiterpene, 1α,5β-guai-4(15)-ene-6β,10β-diol, kadsuguain A (1) and a new cyclohexadienone, kadsuketanone A (2), together with twelve known compounds (3–14) were isolated. The structures of these compounds were elucidated by extensive NMR spectral studies. The absolute configuration of 2 was determined by circular dichroism (CD) spectra. Compounds 2, 6, and 11–14 significantly inhibited both nitric oxide (NO) and prostaglandin E2 (PGE2) production in the LPS-activated microglia cells. In addition, compounds 4, 6, and 11–14 exhibited cytotoxicity against the A549, SK-OV-3, SK-MEL-2, and HCT15 human tumour cells.
Introduction
Piper kadsura Ohwi (Piperaceae), is a vine-like plant with medicinal uses that naturally inhabits the forests throughout Korea, Japan, and Taiwan at low to medium altitudes and gives off a strong spicy incense [Citation1]. Its stem, known as a haifengteng, has been used as an indigenous medicine for the treatment of asthma and arthritic conditions [Citation2,Citation3]. Moreover, its fruit has been used for cooking in Japan, as it is similar to pepper and is used for improving digestive function [Citation4]. Several lignans and neolignans have been isolated from the genus Piper, and various constituents including amides, lignans, terpenes, and cyclohexanes have been isolated from P. kadsura [Citation2,Citation5–8]. The extract and components of this plant have anti-human hepatitis B virus [Citation9], anti-platelet activating factor (PAF) [Citation5,Citation10], anti-insect feeding [Citation11] and anti-inflammatory activities [Citation2]. We have found that several spicy plants can prevent or delay the onset and progression of neurodegenerative disorders [Citation12]. The n-hexane and CHCl3 soluble fractions of the MeOH extract of this spicy plant showed a potent inhibitory effect on nitric oxide (NO) production in LPS-activated BV-2 cells, a microglia cell line. Thus, in the course of our continuing search for biologically active compounds from natural Korean medicinal sources, we have reported the isolation of neolignans as active principles of P. kadsura for anti-neuroinflammatory activity [Citation12]. In our continuing study on this source, we further isolated a new stereoisomer of guaiane sesquiterpene, 1α,5β-guai-4(15)-ene-6β,10β-diol, kadsuguain A (1) and a new cyclohexadienone, kadsuketanone A (2), together with twelve known compounds (3–14), from its n-hexane and CHCl3 soluble fractions. The structures of these compounds were elucidated by extensive NMR spectral studies, including 2D-NMR experiments. The ability of the isolated compounds (1–14) to inhibit NO production was evaluated in LPS-activated BV-2 cells, a microglial cell line. Furthermore, compounds (1–14) were evaluated for their cytotoxicities against the A549, SK-OV-3, SK-MEL-2, and HCT15 cell lines.
Materials and methods
General experimental procedures
All melting points were determined on a Gallenkamp melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. Optical rotations were measured on a Jasco P-1020 polarimeter (Jasco, Easton, MD, USA). IR spectra were recorded on a Bruker IFS-66/S FT-IR spectrometer (Bruker, Karlsruhe, Germany). CD spectra were measured on a Jasco J-715 spectropolarimeter. UV spectra were recorded with a Shimadzu UV-1601 UV-Visible spectrophotometer (Shimadzu, Tokyo, Japan). FAB and HR-FAB mass spectra were obtained on a JEOL JMS700 mass spectrometer (JEOL, Peabody, MA, USA). NMR spectra, including 1H-1H COSY, HMQC, HMBC, and NOESY experiments, were recorded on a Varian UNITY INOVA 500 NMR spectrometer (Varian, Palo Alto, CA, USA) operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ). Preparative HPLC used a Gilson 306 pump (Gilson, Middleton, WI, USA) with a Shodex refractive index detector (Shodex, NY, USA). Silica gel 60 (Merck, Darmstadt, Germany, 230–400 mesh) and RP-C18 silica gel (Merck, 230–400 mesh) were used for column chromatography. Merck precoated Silica gel F254 plates and RP-18 F254s plates were used for TLC. Spots were detected on TLC under UV light or by heating after spraying with 10% H2SO4 in C2H5OH (v/v). The packing material for the molecular sieve column chromatography was Sephadex LH-20 (Pharmacia, Uppsala, Sweden).
Plant material
The aerial parts of P. kadsura were collected in Jeju-island, Korea, in October, 2006, and were identified by one of the authors (K.R.L.). A voucher specimen (SKKU 2006-10) was deposited in the herbarium of the School of Pharmacy, Sungkyunkwan University, Suwon, Korea.
Extraction and isolation
The dried aerial parts of P. kadsura (3 kg) were extracted with 80% MeOH two times at room temperature and filtered. The filtrate was evaporated in vacuum to obtain a MeOH extract (300 g), which was suspended in distilled H2O (7.2 L) and then successively partitioned with n-hexane, CHCl3, and n-BuOH, yielding 49, 12, and 26 g, respectively. The n-hexane soluble fraction (49 g) was separated on a silica gel (230–400 mesh, 550 g) column eluted with n-hexane-EtOAc (10:1 → 4:1) to yield five fractions (A - E). Fraction A (4.3 g) was separated further on a silica gel (230–400 mesh, 150 g) column eluted with n-hexane-EtOAc (10:1) and purified by preparative normal-phase HPLC, using a solvent system of n-hexane-EtOAc (30:1) over 30 min at a flow rate of 2 mL/min (Apollo Silica 5μ column; 250 × 10 mm; Shodex refractive index detector) to obtain compounds 5 (15 mg, tR 15.5 min) and 10 (5 mg, tR 17.5 min). Fraction C (6.1 g) was separated on a silica gel (230–400 mesh, 150 g) column eluted with n-hexane-EtOAc (5:1) to afford 4 fractions (Fr. C1 to Fr. C4). Fr. C2 (1.2 g) was separated further on a silica gel (230–400 mesh, 100 g) column eluted with n-hexane-EtOAc (4:1) and purified by preparative normal-phase HPLC, using a solvent system of n-hexane-EtOAc (10:1) to yield compounds 3 (32 mg, tR 13.5 min) and 4 (34 mg, tR 15 min). Fr. C4 (500 mg) was purified by preparative reversed-phase HPLC, using a solvent system of 80% MeCN over 30 min at a flow rate of 2 mL/min (Econosil RP-18 10 μm column; 250 × 10 mm; Shodex refractive index detector), to afford compounds 1 (6 mg, tR 12.5 min), 6 (7 mg, tR 14 min), and 11 (8 mg, tR 18.5 min). The CHCl3 soluble fraction (12 g) was separated on a silica gel (230–400 mesh, 350 g) column eluted with n-hexane-CHCl3-MeOH (3:4:0.5) to yield six fractions (F - K). Fraction I (1.5 g) was separated on a RP-C18 silica gel (230–400 mesh, 150 g) column eluted with 50% MeOH and Sephadex LH-20 column (CH2Cl2-MeOH, 1:1) and purified by preparative normal-phase HPLC, using a solvent system of CHCl3-EtOAc-MeOH (5:3:0.5) to yield compounds 2 (32 mg, tR 10.5 min), 7 (10 mg, tR 12 min), 8 (6 mg, tR 12.5 min), and 9 (8 mg, tR 14 min). Fraction J (750 mg) was separated over a RP-C18 silica gel (230–400 mesh, 70 g) column eluted with 50% MeOH and purified by preparative reversed-phase HPLC, using a solvent system of 45% MeCN to yield compounds 12 (5 mg, tR 15.5 min) and 13 (16 mg, tR 17.5 min). Fraction K (650 mg) was separated on a RP-C18 silica gel (230–400 mesh, 60 g) column eluted with 45% MeOH and Sephadex LH-20 column (100% MeOH) and purified by preparative normal-phase HPLC, using a solvent system of CHCl3-MeOH (20:1) to yield compound 14 (7 mg, tR 12.5 min).
Kadsuguain A (1)
Colourless gum; 6 mg. ![]() +9.8 (c 0.2, MeOH); IR (KBr) νmax: 3388, 2946, 1658, 1457, 1211, 1028, and 676 cm−1; FAB-MS (positive mode): m/z 239 [M + H]+; HR-FAB-MS (positive mode): m/z 239.2015 [M + H]+, (calcd. for C15H27O2: 239.2011); 1H (500 MHz) and 13C (125 MHz) NMR data, see .
+9.8 (c 0.2, MeOH); IR (KBr) νmax: 3388, 2946, 1658, 1457, 1211, 1028, and 676 cm−1; FAB-MS (positive mode): m/z 239 [M + H]+; HR-FAB-MS (positive mode): m/z 239.2015 [M + H]+, (calcd. for C15H27O2: 239.2011); 1H (500 MHz) and 13C (125 MHz) NMR data, see .
Table 1. The 1H and 13C NMR data for compound 1 (δ in ppm, 500 MHz for 1H and 125 MHz for 13C, in CDCl3)a.
Kadsuketanone A (2)
White powder; 32 mg. Mp 130–132°C; ![]() −5.6 (c 1.2, CHCl3); UV (MeOH) λmax (log ϵ): 284 (6.3) nm; CD (MeOH, c 1.6 × 10−4M) Δϵ (nm): −12.2 (270), +7.3 (315), and +6.4 (335); IR (KBr) νmax: 3390, 2947, 1662, 1606, 1455, 1215, 1181, 1023, and 673 cm−1; FAB-MS (positive mode): m/z 211 [M + H]+; HR-FAB-MS (positive mode): m/z 211.0965 [M + H]+, (calcd. for C11H15O4: 211.097); 1H NMR (500 MHz, CDCl3) δ: 5.6–5.53 (1H, m, H-8), 5.5 (1H, s, H-3), 5.48 (1H, s, H-6), 5.07 (1H, dd, J = 1.5, 5.5 Hz, H-9a), 5.05 (1H, dd, J = 1.5, 12 Hz, H-9b), 3.78 (3H, s, OCH3-2), 3.66 (3H, s, OCH3-5), 2.61 (2H, dd, J = 1.5, 6.5 Hz, H-7). 13C NMR (125 MHz, CDCl3) δ: 182.3 (C-4), 174.9 (C-2), 150.5 (C-5), 131.3 (C-8), 119.9 (C-9), 112.5 (C-6), 101.1 (C-3), 72.4 (C-1), 56.5 (OCH3-2), 55.4 (OCH3-5), 45.4 (C-7).
−5.6 (c 1.2, CHCl3); UV (MeOH) λmax (log ϵ): 284 (6.3) nm; CD (MeOH, c 1.6 × 10−4M) Δϵ (nm): −12.2 (270), +7.3 (315), and +6.4 (335); IR (KBr) νmax: 3390, 2947, 1662, 1606, 1455, 1215, 1181, 1023, and 673 cm−1; FAB-MS (positive mode): m/z 211 [M + H]+; HR-FAB-MS (positive mode): m/z 211.0965 [M + H]+, (calcd. for C11H15O4: 211.097); 1H NMR (500 MHz, CDCl3) δ: 5.6–5.53 (1H, m, H-8), 5.5 (1H, s, H-3), 5.48 (1H, s, H-6), 5.07 (1H, dd, J = 1.5, 5.5 Hz, H-9a), 5.05 (1H, dd, J = 1.5, 12 Hz, H-9b), 3.78 (3H, s, OCH3-2), 3.66 (3H, s, OCH3-5), 2.61 (2H, dd, J = 1.5, 6.5 Hz, H-7). 13C NMR (125 MHz, CDCl3) δ: 182.3 (C-4), 174.9 (C-2), 150.5 (C-5), 131.3 (C-8), 119.9 (C-9), 112.5 (C-6), 101.1 (C-3), 72.4 (C-1), 56.5 (OCH3-2), 55.4 (OCH3-5), 45.4 (C-7).
Piperolactam B (14)
Yellowish gum; 7 mg. UV (MeOH) λmax (log ϵ): 225 (4.5), 267 (4.6), 295 (4.5), 335 (4), and 372 (4) nm; IR (KBr) νmax: 3358, 2943, 1681, 1545, 1453, 1306, 1021, and 676 cm−1; FAB-MS (positive mode): m/z 296 [M + H]+; 1H NMR (500 MHz, C5D5N) δ: 12.00 (1H, br s, NH), 9.98 (1H, d, J = 8 Hz, H-5), 7.99 (1H, d, J = 8 Hz, H-8), 7.63 (1H, td, J = 8, 2 Hz, H-6), 7.54 (1H, dd, J = 8, 2 Hz, H-7), 7.45 (1H, s, H-9), 4.67 (3H, s, OCH3-2), 3.84 (3H, s, OCH3-3). 13C NMR (125 MHz, C5D5N) δ: 168.6 (C=O), 156.6 (C-4), 154.2 (C-2), 140.8 (C-3), 135.2 (C-10), 134.5 (C-8a), 129.2 (C-8), 128.4 (C-5), 128.1 (C-4b), 126.6 (C-7), 125.7 (C-6), 124.6 (C-10a), 113.5 (C-4a), 106.6 (C-1), 105.8 (C-9), 63.2 (OCH3-2), 61.6 (OCH3-3).
Measurement of NO production and cell viability in LPS-activated BV-2 cells
The BV-2 microglia cells were stimulated with 100 ng/ mL LPS in the presence or absence of samples for 24 h. The nitrite present in the culture media, a soluble oxidation product of NO, was measured by a Griess reaction. The supernatant (50 μl) was harvested and mixed with an equal volume of Griess reagent (1% sulphanilamide, 0.1% N-1-napthylethylenediamine dihydrochloride in 5% phosphoric acid). After 10 min, the absorbance at 540 nm was measured using a microplate reader (Emax, Molecular Device, Sunnyvale, CA, USA). Sodium nitrite was used as a standard to calculate the nitrite concentration [Citation13]. Cell viability was measured using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay [Citation14]. NG-monomethyl-L-arginine (L-NMMA, Sigma, St. Louis, MO, USA), a well-known NOS inhibitor, was tested as a positive control.
Measurement of PGE2 production in LPS-activated BV-2 cells
The BV-2 microglia cells were stimulated with 100 ng/mL LPS in the presence or absence of samples for 24 h, and the media was collected. The supernatant from the culture medium was harvested and used for measuring the level of prostaglandin E2 (PGE2). PGE2 was measured by a competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
Cytotoxicity assay
A sulphorhodamine B bioassay (SRB) was used to determine the cytotoxicity of each compound against four cultured human cancer cell lines, A549 (non small cell lung adenocarcinoma), SK-OV-3 (ovarian cancer cells), SK-MEL-2 (skin melanoma), and HCT-15 (colon cancer cells) [Citation15]. Doxorubicin (Sigma, St. Louis, MO, USA, ≥98%) was used as a positive control.
Results and discussion
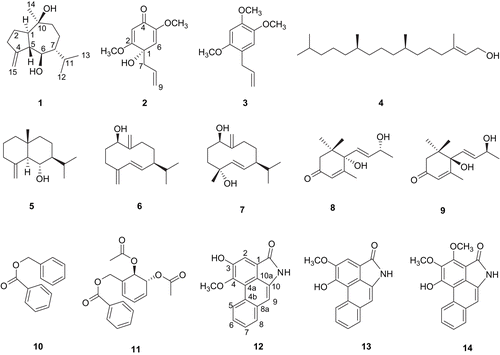
The MeOH extract of P. kadsura was fractionated by solvent (n-hexane, CHCl3, n-BuOH), and then each fraction was evaluated by assessing NO production in LPS-activated BV-2 cells, a microglia cell line. The n-hexane and CHCl3 soluble fractions showed a potent inhibitory effect on NO production. The active fractions were subjected to a series of chromatographic methods, followed by semi-preparative HPLC to afford a new stereoisomer of guaiane sesquiterpene (1) and a new cyclohexadienone (2), along with twelve known compounds (3–14) ().
Kadsuguain A (1) was obtained as a colourless gum, whose molecular formula was determined to be C15H26O2 by combined analysis of its positive-ion HR-FAB-MS showing the fragment ion [M + H]+ peak at m/z 239.2015 (calcd. for C15H27O2: 239.2011) and 13C NMR spectral data. Its IR spectrum showed a hydroxyl (3388 cm−1) and an olefinic group (1658 cm−1). The 1H NMR spectrum of 1 showed signals for two secondary methyl groups at δ 0.93 (3H, d, J = 7 Hz) and 0.86 (3H, d, J = 7 Hz), a tertiary methyl group at δ 1.18 (3H, s), and an oxygenated CH group at δ 3.5 (1H, t, J = 10.5 Hz). Two broad singlet signals at δ 5.05 (1H, s) and 5.01 (1H, s) corresponding to an exocyclic olefinic CH2 group were also observed. The 13C NMR and Distortionless Enhancement by Polarization Transfer (DEPT) spectra of 1 indicated the presence of three methyl groups at δ 29.7 (C-14), 21.5 (C-12), 16.5 (C-13), five CH2 groups including an olefinic one at δ 108.7 (C-15), five CH groups, including an oxygenated one at δ 69.3 (C-6), and two quaternary C-atoms, one of which was an oxygenated signal at δ 73.3 (C-10) and the other was an olefinic signal at δ 154.2 (C-4). The 1H and 13C NMR signals of 1 were assigned unambiguously by further detailed analysis of the 1H-1H COSY, HMQC, and HMBC spectra. The COSY spectrum showed a correlation system starting at 3-H2, continuing via 2-H2, 1-H, 5-H, 6-H, 7-H, 8-H2 and ending at 9-H2. At 7-H there was branching that ended at 12-H3 and 13-H3 via 11-H. HMBC correlations observed from H-1, H-2, and H-8 to C-10 (δ 73.3), from H-14 to C-10 (δ 73.3), and from H-1 and H-9 to C-14 (δ 29.7) indicated the connectivity of C-10 to C-1 and C-9, forming a seven-member ring with a methyl group and an OH group attached at C-10. HMBC correlations were also observed between H-15 and C-3 (δ 30.1), as well as C-5 (δ 55.6), showing the connection of C-4 to C-3 and C-5, forming a five-member ring with an exomethylene group attached at C-4. Thus, the planar structure of 1 was derived as guai-4 (15)-ene-6,10-diol. The large coupling constant (J1,5 = 10.5 Hz) indicated that the junction of the guaiane rings was trans [Citation16]. The mutual NOESY correlations H-1/H-6, H-6/Hα-8, H-1/Hα-8, H-1/H-12, H-1/H-13, H-6/H-12, H-6/H-13, and H-5/H-7 and the absence of the correlations H-1/H-5, H-5/H-12, and H-5/H-13 as well as the large coupling constant between H-5 and H-6, and between H-6 and H-7 (each 10.5 Hz) indicated that H-5 and H-7 were positioned at the same orientation (β-form) and H-1 and H-6 were then on the opposite side (α-form) [Citation17]. The configuration of C-10 is then determined by the NOESY correlations H-1/H-14 and H-6/H-14, and the lack of the correlations H-5/H-14 and H-7/H-14, suggested that methyl group (δ 29.7) at C-10 was α-oriented in equatorial position [Citation16-Citation18]. The downfield resonance (δ 2.62) of the H-5 proton, due to a cis spatial relationship with the OH-10, also supported this point [Citation16]. To establish the absolute configuration of 1, the modified Mosher’s method was performed [Citation19]. However, compound 1 failed to be esterified by (S)- or (R)-MTPA chloride, presumably due to the hindrance of the vicinal isopropyl group at C-7. Thus, the structure of 1 was determined as 1α,5β-guai-4(15)-ene-6β,10β-diol and named kadsuguain A. An 1-epimer of 1 has been reported previously [Citation18].
Kadsuketanone A (2) was obtained as an optically active white powder (mp 130–132°C; ![]() −5.6), whose molecular formula was determined to be C11H14O4 from the [M + H]+ peak at m/z 211.0965 (calcd. for C11H15O4: 211.0970) in the positive-ion HR-FAB-MS. The IR spectrum demonstrated the presence of a hydroxyl group (3390 cm−1) and α,β-unsaturated ketone function (1662 cm−1). The UV spectrum of 2 showed dienone absorption at λmax 284 nm. The 1H NMR spectral data of 2 showed two olefinic signals at δ 5.5 (1H, s, H-3), and 5.48 (1H, s, H-6). A set of ABX signals at δ 5.6–5.53 (1H, m, H-8), 5.07 (1H, dd, J = 1.5, 5.5 Hz, H-9a), and 5.05 (1H, dd, J = 1.5, 12 Hz, H-9b) and one methylene at δ 2.61 (2H, dd, J = 1.5, 6.5 Hz, H-7) were assigned to the allyl group. The 13C NMR spectra showed 11 signals, including one carbonyl carbon at δ 182.3 (C-4), six carbons for olefinic carbon at δ 174.9 (C-2), 150.5 (C-5), 131.3 (C-8), 119.9 (C-9), 112.5 (C-6), and 101.1 (C-3), one quaternary carbon at δ 72.4 (C-1), one methylene carbon at δ 45.4 (C-7), and two methoxy carbons at δ 56.5 (OCH3-2), and 55.4 (OCH3-5). The 1H and 13C NMR signals of 2 were assigned unambiguously by further detailed analysis of the HMQC and HMBC experiments. The HMBC correlations of H-6/C-2, C-4 and H-3/C-1, C-5 indicated that compound 2 was a cyclohexadienone derivative. The HMBC spectrum showed that H-7 was correlated to C-1, C-2, and C-6, suggesting that the allyl group was located at C-1. Two methoxy protons at δ 3.78 (3H, s, OCH3-2) and 3.66 (3H, s, OCH3-5) were assigned at C-2 and C-5, respectively, according to the HMBC correlations with the carbon signals at δ 174.9 and 150.5, respectively., The structure of 2 was determined based on the above considerations, this was found to be similar to the partial structure of burchellin isolated from this plant [Citation2,Citation20]. The CD spectrum of 2 exhibited a first negative Cotton effect at 270 nm, a second positive Cotton effect at 315 nm and a third positive Cotton effect at 335 nm, the Cotton effects were considered to be due to the enone chromophore, indicating the S-configuration at C-1 [Citation21]. Therefore, compound 2 is a new cyclohexadienone derivative, named kadsuketanone A, a rare analogue to occur in natural sources.
−5.6), whose molecular formula was determined to be C11H14O4 from the [M + H]+ peak at m/z 211.0965 (calcd. for C11H15O4: 211.0970) in the positive-ion HR-FAB-MS. The IR spectrum demonstrated the presence of a hydroxyl group (3390 cm−1) and α,β-unsaturated ketone function (1662 cm−1). The UV spectrum of 2 showed dienone absorption at λmax 284 nm. The 1H NMR spectral data of 2 showed two olefinic signals at δ 5.5 (1H, s, H-3), and 5.48 (1H, s, H-6). A set of ABX signals at δ 5.6–5.53 (1H, m, H-8), 5.07 (1H, dd, J = 1.5, 5.5 Hz, H-9a), and 5.05 (1H, dd, J = 1.5, 12 Hz, H-9b) and one methylene at δ 2.61 (2H, dd, J = 1.5, 6.5 Hz, H-7) were assigned to the allyl group. The 13C NMR spectra showed 11 signals, including one carbonyl carbon at δ 182.3 (C-4), six carbons for olefinic carbon at δ 174.9 (C-2), 150.5 (C-5), 131.3 (C-8), 119.9 (C-9), 112.5 (C-6), and 101.1 (C-3), one quaternary carbon at δ 72.4 (C-1), one methylene carbon at δ 45.4 (C-7), and two methoxy carbons at δ 56.5 (OCH3-2), and 55.4 (OCH3-5). The 1H and 13C NMR signals of 2 were assigned unambiguously by further detailed analysis of the HMQC and HMBC experiments. The HMBC correlations of H-6/C-2, C-4 and H-3/C-1, C-5 indicated that compound 2 was a cyclohexadienone derivative. The HMBC spectrum showed that H-7 was correlated to C-1, C-2, and C-6, suggesting that the allyl group was located at C-1. Two methoxy protons at δ 3.78 (3H, s, OCH3-2) and 3.66 (3H, s, OCH3-5) were assigned at C-2 and C-5, respectively, according to the HMBC correlations with the carbon signals at δ 174.9 and 150.5, respectively., The structure of 2 was determined based on the above considerations, this was found to be similar to the partial structure of burchellin isolated from this plant [Citation2,Citation20]. The CD spectrum of 2 exhibited a first negative Cotton effect at 270 nm, a second positive Cotton effect at 315 nm and a third positive Cotton effect at 335 nm, the Cotton effects were considered to be due to the enone chromophore, indicating the S-configuration at C-1 [Citation21]. Therefore, compound 2 is a new cyclohexadienone derivative, named kadsuketanone A, a rare analogue to occur in natural sources.
The structures of the known compounds were identified as isoasarone (3) [Citation22], trans-phytol (4) [Citation23], junenol (5) [Citation24], ent-germacra-4(15),5,10(14)-trien-1β-ol (6) [Citation25], germacra-5,10(14)-dien-1β,4β-diol (7) [Citation26], blumenol A (8) [Citation27], blumenol B (9) [Citation28], benzyl benzoate (10) [Citation29], trans-2,3-diacetoxy-1-[(benzoy1oxy)methyl]-cyclohexa-4,6-diene (11) [Citation30], aristolactam A II (12) [Citation31], and piperolactam A (13) [Citation32] by comparison of their spectroscopic data with reported values. Piperolactam B (14) was also isolated from this source, and the 1H NMR data of piperolactam B isolated from Piper longum was reported previously [Citation33]. However, the assignments of the NMR data required correction. The chemical shift (δ 4.67) for one of the methoxy groups in 14 indicated that the methoxy group was located at C-2 in 14, which was mostly shown at δ 4.4-4.6 due to the influence of the peri-carbonyl group of the lactam ring [Citation34,Citation35]. The resonances of piperolactam B (14) were reassigned unambiguously by 2D NMR (1H-1H COSY, HMQC, HMBC and NOESY).
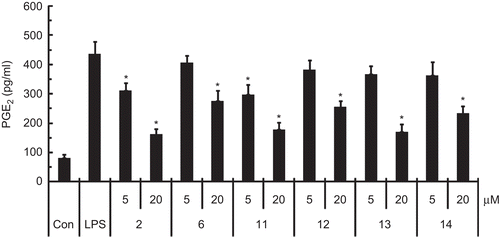
Neuroinflammation can cause neuronal damage in neurodegenerative diseases [Citation36]. Brain inflammation results from activation of microglia, the resident immune cells. Activated microglia cells produce excessive pro-inflammatory substances such as NO, cytokines, and prostaglandins [Citation37]. The NO and PGE2 produced by activated microglia is a major factor involved in neuroinflammation [Citation38]. Here, the anti-neuroinflammatory effects of 1–14 were evaluated by using LPS-activated BV-2 microglia cells. Compounds 2, 6, and 11–14 significantly inhibited the NO production in LPS-stimulated BV-2 cells. They were more potent than L-NMMA, an inducible NO synthase (iNOS) inhibitor, in inhibiting NO production. Compound 2 was the strongest inhibitor (), but the other compounds were not active (up to 20 μM). Moreover, compounds 2, 6, and 11–14 significantly reduced PGE2 production in the LPS-stimulated microglia ().
Table 2. The effects of compounds 1–14 and L-NMMA on LPS-induced NO production in BV-2 microglia cells.
Figure 2. The effects of compounds 2, 6, and 11–14 on PGE2 production in LPS-stimulated BV-2 microglia cells. PGE2 was assessed by using a competitive enzyme immunoassay kit after treatment with LPS (100 ng/mL) for 24 h in the presence or absence of compounds 2, 6, and 11–14 (5 and 20 μM). All data are presented as the mean ± SEM of three independent experiments. *p<0.05 indicates statistically significant differences compared to treatment with LPS alone.
The isolated compounds 1–14 were also evaluated for their cytotoxic activities against A549, SK-OV-3, SK-MEL-2, and HCT15 human tumour cell lines using the SRB assay. Compounds 4, 6, and 11–14 exhibited cytotoxicity against A549, SK-OV-3, SK-MEL-2, and HCT15 cells, but the other compounds were found to be inactive ().
Table 3. Cytotoxicity of compounds 1–14 against four cultured human tumour cell lines using the SRB assay in vitro.
In summary, compounds 2, 6, and 11–14 isolated from P. kadsura exhibited anti-neuroinflammatory activity by suppressing the release of NO and PGE2 in LPS-stimulated microglia cells. These results suggest that the active compounds might be good lead compounds to modulate neurological diseases associated with inflammatory processes.
Acknowledgements
The authors would like to thank Do Kyun Kim, Eun Jung Bang, and Jung Ju Seo at the Korea Basic Science Institute for the NMR and MS spectral measurements.
Declaration of interest
This study was supported by a grant from the Seoul R&BD Program (10524) funded by the Seoul Metropolitan Government, Republic of Korea.
References
- Lin TT, Lu SY. Piperaceae. In Flora of Taiwan. Taipei: Editorial Committee of the Flora of Taiwan, 1996;Vol.II:624–631.
- Lin LC, Shen CC, Shen YC, Tsai TH. Anti-inflammatory neolignans from Piper kadsura. J Nat Prod 2006;69:842–844.
- Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jain A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM. Phytochemistry of the genus Piper. Phytochemistry 1997;46:597–673.
- Shogakukan eds. Dictionary of Chinese Materia Medica. Tokyo: Shanghai Scientific Technological Publishers Shougakukan, 1985:230.
- Han GQ, Dai P, Xu L, Ma J, Li CL, Zheng QT. PAF inhibitors: Neolignans from Piper kadsura. Planta Med 1990;56:583–584.
- Chang MN, Han GQ, Arison BH, Springer JP, Hwang SB, Shen TY. Neolignans from Piper futokadsura. Phytochemistry 1985;24:2079–2082.
- Konishi T, Konoshima T, Daikonya A, Kitanaka S. Neolignans from Piper futokadsura and their inhibition of nitric oxide production. Chem Pharm Bull 2005;53:121–124.
- Matsui K, Munakata K. Four new neolignans from Piper futokadzura. Tetrahedron Lett 1976;48:4371–4374.
- Huang RL, Chen CF, Feng HY, Lin LC, Chou CJ. Anti-hepatitis B virus of seven compounds isolated from Piper kadsura (CHOISY) OHWI. J Chin Med 2001;12:179–190.
- Shen TY, Hussaini IM. Kadsurenone and other related lignans as antagonists of platelet-activating factor receptor. Methods Enzymol 1990;187:446–454.
- Matsui K, Wada K, Munakata K. Insect antifeeding substances in Parabenzoin praecox and Piper futokadzura. Agric Biol Chem 1976;40:1045–1046.
- Kim KH, Choi JW, Ha SK, Kim SY, Lee KR. Neolignans from Piper kadsura and their anti-neuroinflammatory activity. Bioorg Med Chem Lett 2010;20:409–412.
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int 2008;52:878–886.
- Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer 1989;60:206–210.
- Skehan P, Stroreng R, Scudiero D, Monks A, Mcmahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–1112.
- Bruno M, Torre MC, Rodriguez B, Omar AA. Guaiane sesquiterpenes from Teucrium leucocladum. Phytochemistry 1993;34:245–247.
- Wei HH, Xu HH, Xie HH, Xu LX, Wei XY. Sesquiterpenes and lignans from Tephrosia vogelii. Helv Chim Acta 2009;92:370–374.
- Anderson M, Bergendorff O, Shan R, Zygmunt P, Sterner O. Minor components with smooth muscle relaxing properties from scented myrrh (Commiphora guidotti). Planta Med 1997;63:250–254.
- Su BN, Park EJ, Mbwambo ZH, Santarsiero BD, Mesecar AD, Fong HHS, Pezzuto JM, Kinghorn AD. New chemical constituents of Euphorbia quinquecostata and absolute configuration assignment by a convenient mosher ester procedure carried out in NMR tubes. J Nat Prod 2002;65:1278–1282.
- Horne DA, Yakushijin K, Buchi G. Biomimetic synthesis of the neolignans kadsurenone, denudatin B, O-methyl-liliflodione, and liliflol B. Tetrahedron Lett 1999;40:5443–5447.
- Sato S, Obara H, Kumazawa T, Onodera J, Furuhata K. Synthesis of (+),(-)-model compounds and absolute configuration of carthamin; A red pigment in the flower petals of safflower. Chem Lett 1996;25:833–834.
- De Santos BV, Da-Cunha EVL, De Chaves MC, Gray AI. Phenylalkanoids from Piper marginatum. Phytochemistry 1998;49:1381–1384.
- Kim KH, Lee KH, Choi SU, Kim YH, Lee KR. Terpene and phenolic constituents of Lactuca indica L. Arch Pharm Res 2008;31:983–988.
- Cardona L, Garcia B, Gimenez JE, Pedro JR. A shorter route to the synthesis of (+)-junenol, isojunenol, and their coumarate esters from (-)-santonin. Tetrahedron 1992;48:851–860.
- Choi SZ, Choi SU, Lee KR. Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantean. Arch Pharm Res 2004;27:164–168.
- San Feliciano A, Medarde M, Gordaliza M, Lucas MJ. Structure elucidation of germacrane alcohols from Juniperus communis subsp. Hemisphaerica. J Nat Prod 1995;58:1059–1064.
- Cutillo F, Dellagreca M, Previtera L, Zarrelli A. C13 norisoprenoids from Brassica fruticulosa. Nat Prod Res 2005;19:99–103.
- Abe F, Yamauchi T. Megastigmanes and flavonoids from the leaves of Scorodocarpus borneensis. Phytochemistry 1993;33:1499–1501.
- Faler CA, Joullie MM. Aminolysis of allyl esters with bislithium aryl amides. Tetrahedron Lett 2006;47:7229–7231.
- Kodpinid M, Sadavongvivad C, Thebtaranonth C, Thebtaranonth Y. Structures of β-senepoxide, tingtanoxide, and their diene precursors. Constituents of Uvaria ferruginea. Tetrahedron Lett 1983;24:2019–2022.
- Priestap HA. 13C NMR spectroscopy of aristolochic acids and aristololactams. Magn Reson Chem 1989;27:460–469.
- Kim JK, Kim YH, Nam HT, Kim BT, Heo JN. Total synthesis of aristolactams via a one-pot Suzuki-Miyaura coupling/aldol condensation cascade reaction. Org Lett 2008;10:3543–3546.
- Desai SJ, Prabhu BR, Mulchandani NB. Aristolactams and 4,5-dioxoaporphines from Piper longum. Phytochemistry 1988;27:1511–1515.
- Olsen CE, Tyagi OD, Boll PM, Hussaini FA, Parmar VS, Sharma NK, Taneja P, Jain SC. An aristolactam from Piper acutisleginum and revision of the structures of piperolactam B and D. Phytochemistry 1993;33:518–520.
- Chen YC, Chen JJ, Chang YL, Teng CM, Lin WY, Wu CC, Chen IS. A new aristolactam alkaloid and anti-platelet aggregation constituents from Piper taiwanense. Planta Med 2004;70:174–177.
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 2003;304:1–7.
- McGeer PL, McGeer EG. The inflammatory response system of brain: Implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev 1995;21:195–218.
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci 1997;20:132–139.