Abstract
Dual cyclooxygenase/lipoxygenase (COX/LOX) inhibitors constitute a valuable alternative to classical nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors for the treatment of inflammatory diseases. A series of 3-(5-phenyl/phenylamino-[1,3,4]oxadiazol-2-yl)-chromen-2-one and N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide derivatives were synthesized and screened for anti-inflammatory, analgesic activity. All the derivatives prepared are active in inhibiting oedema induced by carrageenan. Compound 4e was found more potent with 89% of inhibition followed by compound 4b (86%). Compounds with >70% of anti-inflammatory activity were tested for analgesic, ulcerogenic, and lipid peroxidation profile. Selected compounds were also evaluated for inhibition of COXs (COX-1 and COX-2) and LOXs (LOX-5, LOX-12, and LOX-15). Compound 4e was comparatively selective for COX-2, LOX-5, and LOX-15. Study revealed that these derivatives were more effective than ibuprofen with reduced side effects. It can be suggested that these derivatives could be used to develop more potent and safer NSAIDs.
| Abbreviations | ||
| HETE | = | Hydroxy eicosatetraenoic acid |
| AA | = | arachidonic acid |
| BDH | = | British Drug House |
| bs | = | broad singlet |
| CDCl3 | = | deuterated chloroform |
| COX | = | cyclooxygenase |
| d | = | doublet |
| DMSO | = | dimethyl sulphoxide |
| DMSO-d6 | = | deuterated dimethyl sulphoxide |
| EDTA | = | ethylenediaminetetraacetic acid |
| EIA | = | enzyme immunoassay |
| GI | = | gastro intestinal |
| HCl | = | hydrochloric acid |
| Hz | = | hertz |
| IC50 | = | dose that causes half-maximal inhibition (median inhibition concentration) |
| J | = | coupling constants |
| KCl | = | potassium chloride |
| LDL | = | low-density lipoprotein |
| LOX | = | lipoxygenase |
| LTB4 | = | leukotriene B4 |
| m | = | multiplet |
| MDA | = | malondialdehyde |
| NMR | = | nuclear magnetic resonance |
| NSAIDs | = | nonsteroidal anti-inflammatory drugs |
| PBML | = | peripheral blood mononuclear cell leukocyte |
| PGs | = | prostagladins |
| ppm | = | parts per million |
| s | = | singlet |
| SDS | = | sodium dodecyl sulphate |
| SS1 | = | solvent system 1 |
| SS2 | = | solvent system 2 |
| TBA | = | thiobarbituric acid |
| TLC | = | thin-layer chromatography |
Introduction
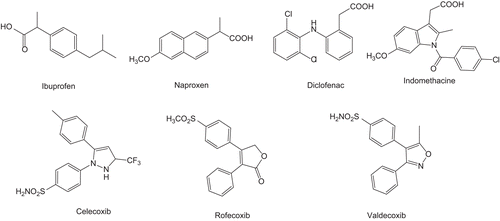
Inflammation is a normal reaction to injury or infection. It is caused by the body release of hormone-like substances called prostaglandins (PGs) and leukotrienes (LTs). These inflammatory-inducing agents are synthesized from arachidonic acid (AA) by the enzymes cyclooxygenase (COX) and lipoxygenase (LOX). COX enzymes catalyse the first committed step in the biosynthesis of PGs and thromboxanes, and are the pharmacological targets of nonsteroidal anti-inflammatory drugs (NSAIDs).Citation1,Citation2 NSAIDs have been subdivided into two classes: (1) classical NSAIDs and (2) selective COX-2 inhibitors. Ibuprofen, indomethacin, diclofenac, naproxen () comes under first category. Despite an extensive chemical diversity, they all possess a carboxylate function that like one of the AA forms an ion pair with Arg-120 at the bottom of the COX active site.Citation3 They therefore share common side effects like gastrointestinal (GI) lesions and renal toxicity, leading at high doses to erosions, ulcerations, bleedings, and even to death.Citation4 This is because of their nonspecific inhibition of both COX isoforms. Classical NSAIDs reduce the production of proinflammatory PGs at sites of injury and also the formation of physiological PGs in the stomach and the kidney. These observations provided a rationale for the development of COX-2 selective inhibitors like celecoxib, rofecoxib that should retain the potent anti-inflammatory and analgesic effects of classical NSAIDs with less GI adverse effects.Citation5 They were shown to preferentially inhibit the inducible isoform, that is, COX-2. Currently, >500 COX-2-specific inhibitors have been designed. The main structural features of these compounds are the absence of the carboxylate group, characteristic of classical NSAIDs, and generally, the presence of a sulphone (–SO2–) or sulphonamide (–SO2NH2) moiety (), which can interact with Arg-513 in the hydrophilic side pocket of the COX-2 active site.Citation6 But the development of selective COX-2 inhibitors did not solve the purpose completely due to certain associated reasons, for example, COX-2 is constitutively expressed in the kidney and the reproductive tract, also cyclic hormonal induction of COX-2 plays an important role in ovulation.Citation6 Second, the adverse cardiovascular effectsCitation7 (by decreasing vasodilatory and antiaggregatory PGI2 production) have resulted in the voluntary withdrawal of Vioxx® (rofecoxib) worldwide. In these cases, the anti-inflammatory efficacy of selective COX-2 inhibitors was only observed at doses that inhibited COX-1.Citation8 In conclusion, it appears that selective COX-2 inhibitors do not fully satisfy the search for new safer anti-inflammatory agents. LOX is a family of non-haeme iron-containing dioxygenases, and exists in three isoforms: LOX-5, LOX-12, and LOX-15. The LOX-5 pathway, which is the second major metabolic pathway of AA, plays an important role in the pathophysiology of several inflammatoryCitation9,Citation10 and allergic diseases and generates products particularly important in inflammation (LTs), and is up-regulated during COX blockade. This up-regulation of LOX has been associated with various adverse effects, such as asthma. LOX-5 has been shown to be involved in the production of LTs, which are known to contribute to the progression of osteoarthritis, asthma, and inflammation.Citation11–14 LOX-15 has been implicated in the oxidation of low-density lipoprotein (LDL), which ultimately causes atherosclerosis.Citation15,Citation16 Most recently, it has also been demonstrated that increased expression of LOX-12/LOX-15 causes heart failure, in transgenic mice.Citation17 Therefore, agents that inhibit both the enzymes viz. COX and LOX may arrive as winners in this race as exemplified by Licofelone (ML-3000), a dual COX/LOX-5 inhibitor and a potent anti-inflammatory agent without GI side effects.Citation18 Therefore, dual inhibition of COX and LOX is an interesting alternative to provide safer NSAIDs.Citation1 Compounds with dual COX/LOX inhibitory activity have recently emerged as potential anticancer agents.Citation19,Citation20
Five-membered heterocyclic compounds like oxazole, pyrazole, indole, triazole, oxadiazole have been widely explored for their biological activities especially for anti-inflammatory activity, for example, celecoxib bears pyrazole nucleus, phenyl butazones have pyrazole- 2,5-dione, rofecoxib has furanone, and valdecoxib has isoxazole nucleus. 1,3,4-Oxadiazoles forms an important class of heterocyclic compounds with broad spectrum of biological activities. Substituted 1,3,4-oxadiazoles have revealed antibacterial,Citation21 antimycobacterial,Citation22 antifungal,Citation23 anti-inflammatory and analgesic,Citation24–26 anticonvulsant,Citation27 antihyperglycemic,Citation28 anticancer,Citation29 anti-HIV-1,Citation30 and tyrosinase inhibitory activity.Citation31
1,2,4-Oxadiazole derivatives have been reported as potent anti-inflammatory agents with selectivity for COX-2.Citation32–34 3-Phenyl-1,2,4-oxadiazole derivative has been reported to exhibit analgesic activity far superior than aspirin.Citation35 Compounds containing coumarin moiety have also been reported to possess wide spectrum of biological activity like anti-inflammatory, anticancer, antirheumatic, and so on. It has been reported that styryl carbonyl derivatives possess appreciable anti-inflammatory activity.Citation36,Citation37 Therefore, coumarin nucleus that incorporates the styryl carbonyl moiety into a rigid framework was selected to be a part of newly synthesized compounds. Furthermore, coumarin/chromone and related derivatives are recognized as inhibitors of both the mediators of inflammation, that is, LOX and COX pathways of AA metabolism.Citation33,Citation38
Considering the proinflammatory properties of LTs and prostanoids, agents that are able to block equally the synthesis of both eicosanoids (dual inhibitors) should not only present a superior anti-inflammatory profile but also fewer side effects than NSAIDs and selective COX-2 inhibitors.Citation39 In continuation to our work to develop agents to treat inflammatory conditions,Citation40,Citation41 we described in this article the synthesis and biological evaluation of a class of 3-(5-phenyl/phenylamino-[1,3,4]oxadiazol-2-yl)-chromen-2-one and N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide derivatives as dual inhibitors of COXs and LOXs.
Materials and methods
Reagents and solvents were purchased from local suppliers of Sigma, British Drug House (BDH) and were used without further purification. Celecoxib and ibuprofen were obtained as gift sample from Cadila Pharma, India and Zydus Cadila, India, respectively. Proton nuclear magnetic resonance (1H-NMR) spectra were determined in deuterated chloroform (CDCl3), or deuterated dimethyl sulphoxide (DMSO-d6) solution on a Bruker Avance 300 spectrometer. Proton chemical shifts (δ) are expressed in parts per million (ppm) relative to tetramethylsilane as an internal standard. Spin multiplicities are given as s (singlet), d (doublet), bs (broad singlet), and m (multiplet). Coupling constants (J) are given in hertz (Hz). Liquid chromatography was performed with a forced flow (flash chromatography) with Merck Grade Silica gel (70–230 mesh). The solvents used for elution varied depending on the compound and included either one or a combination of the following: toluene:ethylacetate:formic acid (SS1) and chloroform:methanol (SS2). Analytical thin-layer chromatography (TLC) was performed with Sigma-Aldrich 0.25-mm silica gel plates (60 Å), visualized under 254 nm ultraviolet light or iodine spray. The compounds were purified by Combiflash Retrieve, Septech. All yields were of purified product and were not optimized. Melting points were determined in an open capillary tube using a Decibel digital melting point apparatus and are uncorrected.
Chemistry
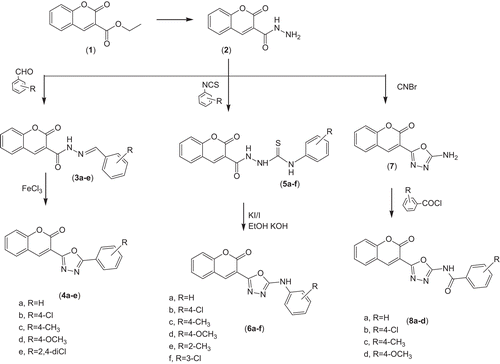
Carbethoxy coumarin (1) and aryl isothiocyanates were prepared by reported methods,Citation42,Citation43 the 2-oxo-2H-chromene-3-carboxylic acid hydrazide (2), substituted 2-oxo-2H-chromene-3-carboxylic acid benzylidene-hydrazide (3a–e), and 3-(5-amino-[1,3,4]oxadiazol-2-yl)-chromen-2-one (7) were synthesized by previously reported procedures.Citation41 Compounds (5a–f) were prepared by condensing compound 2 with aryl isothiocyanates following reported procedures.Citation44
General procedure for synthesis of oxadiazole (4a–e)
To a solution of compound 3 (0.01 mol) in acetic acid (8 mL) was added ferric chloride (0.1 g) and water (4 mL) and stirred for 1 h at room temperature followed by dilution with water to 100 mL. The reaction mixture was left overnight at room temperature when a solid mass separated was collected by filtration on suction pump and washed with water thoroughly. The solid so obtained was dried and purified to get the desired compound.
3-(5-Phenyl-[1,3,4]oxadiazol-2-yl)-chromen-2-one (4a): Yield: 48%; m.p.: 151–153°C; Rf (SS1): 0.67; IR (cm−1): 1060 (C-O-C), 1450, 1504, 1602, 1645 (ring stretching of oxadiazole ring), 1705 (C=O); 1H-NMR (ppm): 6.89–6.92 (m, 2H, H6,8), 7.48–7.54 (m, 2H, H5,7), 7.64–7.71 (m, 5H, ArH), 8.06 (s, 1H, H4); Anal. Calcd. for C17H10N2O3: C, 70.34; H, 3.47; N, 9.65. Found: C, 70.29; H, 3.49; N, 9.63.
3-[5-(4-Chloro-phenyl)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (4b): Yield: 52%; m.p.: 200–202°C; Rf (SS2): 0.61; IR (cm−1): 1710 (C=O), 1650, 1615, 1480, 1380, 1070 (C-O-C), 760 (C-Cl); 1H-NMR (ppm): 7.2–7.24 (m, 2H, H6,8), 7.5 (d, 2H, J = 8.1 Hz, ArH), 7.6–7.65 (m, 2H, H5,7), 8.05 (s, 1H, H4), 8.18 (d, 2H, J = 8 Hz, ArH); Anal. Calcd. for C17H9ClN2O3: C, 62.88; H, 2.79; N, 8.63. Found: C, 62.79; H, 2.58; N, 8.60.
3-(5-p-Tolyl-[1,3,4]oxadiazol-2-yl)-chromen-2-one (4c): Yield: 51%; m.p.: 160–162°C; Rf (SS1): 0.68; IR (cm−1): 1070 (C-O-C), 1454, 1500, 1517, 1633 (C=N), 1660 (CO), 1712 (C=O); 1H-NMR (ppm): 2.16 (s, 3H, CH3), 7.04–7.10 (m, 2H, H6,8), 7.59–7.63 (m, 2H, H5,7), 7.43 (d, 2H, J = 8 Hz, ArH), 7.89 (d, 2H, J = 8.2 Hz, ArH), 8.12 (s, 1H, H4); Anal. Calcd. for C18H12N2O3: C, 71.05; H, 3.97; N, 9.21. Found: C, 71.13; H, 3.89; N, 9.12.
3-[5-(4-Methoxyphenyl)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (4d): Yield: 49%; m.p.: 168–171°C; Rf (SS1): 0.71; IR (cm−1): 1066 (C-O-C), 1150 (OCH3), 1455, 1506, 1577, 1613 (C=N), 1696 (C=O); 1H-NMR (ppm): 3.47 (s, 3H, OCH3), 7.27–7.3 (m, 2H, H6,8), 7.53 (d, 2H, J = 8 Hz, ArH), 7.6–7.64 (m, 2H, H5,7), 7.71 (d, J = 7.8 Hz, 2H, ArH), 8.05 (s, 1H, H4); Anal. Calcd. for C18H12N2O4: C, 67.50; H, 3.78; N, 8.75. Found: C, 67.49; H, 3.85; N, 8.68.
3-[5-(2,4-Dichloro-phenyl)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (4e): Yield: 58%; m.p.: 194–196°C; Rf (SS2): 0.65; IR (cm−1): 771 (C-Cl), 1065 (C-O-C), 1455, 1506, 1577, 1610 (C=N), 1705 (C=O); 1H-NMR (ppm): 7.27–7.31 (m, 2H, H6,8), 7.42–7.49 (m, 2H, ArH) 7.56–7.59 (m, 2H, H5,7), 7.65 (d, J = 2.2, 1H, ArH), 8.13 (s, 1H, H4); Anal. Calcd. for C17H8Cl2N2O3: C, 56.85; H, 2.25; N, 7.80. Found: C, 56.94; H, 2.20; N, 7.67.
General procedure for synthesis of oxadiazole (6a–f)
Compound (5) (0.01 mol) dissolved in ethanol (20 mL) was added 15 mL of 6 N NaOH and 10% iodine solution (in potassium iodide) drop wise until the colour of iodine persisted. The reaction mixture was refluxed for 5–7 h. On completion of reaction, the contents were cooled to room temperature. A solid mass separated was collected and thoroughly washed with water and purified to obtain the product.
3-(5-Phenylamino-[1,3,4]oxadiazol-2-yl)-chromen-2-one (6a): Yield: 55%; m.p.: 128–130°C; Rf (SS1): 0.65; IR (cm−1): 1125 (C-O-C), 1451, 1505, 1588, 1615, 1720 (C=O), 3318 (NH); 1H-NMR (ppm): 6.8–6.85 (m, 2H, H6,8), 7.18–7.22 (m, 3H, ArH), 7.41–7.46 (m, 2H, H5,7), 7.53 (d, J = 7.4 Hz, 2H, ArH), 8.2 (s, 1H, H4) 9.37 (br, s, 1H, NH); Anal. Calcd. for C17H11N3O3: C, 66.88; H, 3.63; N, 13.76. Found: C, 66.72; H, 3.67; N, 13.54.
3-[5-(4-Chloro-phenylamino)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (6b): Yield: 48%; m.p.: 175–177°C; Rf (SS2): 0.67; IR (cm−1): 778 (C-Cl), 1071 (C-O-C), 1454, 1505, 1577, 1630 (C=N), 1728 (CO), 3317 (NH); 1H-NMR (ppm): 6.91–6.96 (m, 2H, H6,8) 7.22 (d, 2H, J = 8.0 Hz, ArH), 7.32 (d, 2H, J = 8.2 Hz, ArH), 7.48–7.51 (m, 2H, H5,7), 9.92 (br, s, 1H, NH), 8.46 (s, 1H, H4); Anal. Calcd. for C17H10ClN3O3: C, 60.10; H, 2.97; N, 12.37. Found: C, 60.18; H, 2.92; N, 12.45.
3-(5-p-Tolylamino-[1,3,4]oxadiazol-2-yl)-chromen-2-one (6c): Yield: 51%; m.p.: 181–183°C; Rf (SS1): 0.71; IR (cm−1): 1705 (CO), 1615 (C=N), 1075 (C-O-C), 3318 (NH), 1579, 1506, 1455; 1H-NMR (ppm): 2.51 (s, 3H, CH3), 7.72 (d, 2H, J = 8.2 Hz, ArH), 6.92–6.95 (m, 2H, H6,8) 8.26 (d, 2H, J = 8 Hz, ArH) 7.61–7.68 (m, 2H, H5,7) 8.97 (br, s, 1H, NH), 8.40 (s, 1H, H4); Anal. Calcd. for C18H13N3O3: C, 67.71; H, 4.10; N, 13.16. Found: C, 67.57; H, 4.07; N, 13.04.
3-[5-(4-Methoxy-phenylamino)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (6d): Yield: 53%; m.p.: 168–170°C; Rf (SS1): 0.69; IR (cm−1): 1713 (CO), 1597 (C=N), 1115 (C-O-C), 3317 (NH), 1577, 1500, 1454; 1H-NMR (ppm): 3.65 (s, 3H, OCH3), 6.83 (d, 2H, J = 8.0 Hz, ArH), 6.97–7.06 (m, 2H, H6,8), 7.46 (d, 2H, J = 8.1 Hz, ArH), 7.61–7.67 (m, 2H, H5,7), 10.24 (br, s, 1H, NH), 8.21 (s, 1H, H4); Anal. Calcd. for C18H13N3O4: C, 64.47; H, 3.91; N, 12.53. Found: C, 64.35; H, 3.86; N, 12.36.
3-(5-o-Tolylamino-[1,3,4]oxadiazol-2-yl)-chromen-2-one (6e): Yield: 55%; m.p.: 161–163°C; Rf (SS1): 0.58; IR (cm−1): 1065 (C-O-C), 1450 (oxa), 1504, 1589, 1613 (C=N), 1708 (CO), 3320 (NH); 1H-NMR (ppm): 2.38 (s, 3H, CH3), 7.20–7.22 (m, 2H, ArH), 6.96–7.08 (m, 2H, H6,8),7.36–7.41 (m, 2H, H5,7), 7.71–7.75 (m, 2H, Ar), 8.43 (s, 1H, H4), 10.3 (br, s, 1H, NH); Anal. Calcd. for C18H13N3O3: C, 67.71; H, 4.10; N, 13.16. Found: C, 67.83; H, 4.14; N, 13.10.
3-[5-(3-Chloro-phenylamino)-[1,3,4]oxadiazol-2-yl]-chromen-2-one (6f): Yield: 38%; m.p.: 148–150°C; Rf (SS2): 0.72; IR (cm−1) 1710 (CO), 1096 (C-O-C), 3409 (NH), 1625, 1579, 1501, 1451; 1H-NMR (ppm): 6.95–7.03 (m, 4H, ArH), 7.18–7.21 (m, 2H, H6,8), 7.57–7.61 (m, 2H, H5,7), 10.02 (br, s, 1H, NH), 8.39 (s, 1H, H4); Anal. Calcd. for C18H13N3O3: C, 67.71; H, 4.10; N, 13.16. Found: C, 67.59; H, 4.21; N, 13.09.
General procedure for the synthesis of N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide (8a–d)
To a solution of 3-(5-amino-[1,3,4]oxadiazol-2-yl)-chromen-2-one (0.01 mol) in absolute ethanol was added substituted aromatic acid chloride and heated gently under reflux for 6–8 h. After complexion of reaction, the solvent was removed till small volume was left. This was poured onto crushed ice; after cooling to room temperature, solid separated was filtered, washed with water, dried, and purified to get the title product.
N-[5-(2-Oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide (8a): Yield: 44%; m.p.: 174–176°C; Rf (SS1): 0.66; IR (cm−1): 1159 (C-O-C), 1633 (C=N), 1730 (CO), 3284 (NH); 1H-NMR (ppm): 7.20–7.23 (M, 1H, ArH), 7.28–7.32 (m, 2H, H6,8), 7.43–7.47 (m, 2H, H5,7), 7.56–7.6 (m, 3H, ArH), 8.17 (s, 1H, H4), 9.92 (br, s, 1H, NH); Anal. Calcd. for C18H11N3O4: C, 64.86; H, 3.33; N, 12.61. Found: C, 64.74; H, 3.41; N, 12.53.
4-Chloro-N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide (8b): Yield: 52%; m.p.: 203–204°C; Rf (SS2): 0.65; IR (cm−1): 772 (C-Cl), 1140 (C-O-C), 1725 (CO), 3281 (NH); 1H-NMR (ppm): 6.98 (d, 2H, J = 8.0 Hz, ArH), 7.12 (br, s, 1H, NH), 7.18–7.23 (m, 2H, H6,8), 7.56–7.61 (m, 2H, H5,7), 7.45 (d, 2H, J = 8.2 Hz, ArH), 8.21 (s, 1H, H4); Anal. Calcd. for C18H10ClN3O4: C, 58.79; H, 2.74; N, 11.43. Found: C, 58.70; H, 2.68; N, 11.35.
4-Methyl-N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide (8c): Yield: 58%; m.p.: 180–188°C; Rf (SS1): 0.69; IR (cm−1): 1162 (C-O-C), 1614 (C=N), 1721 (CO), 3210 (NH); 1H-NMR (ppm): 2.3 (s, 3H, CH3), 7.38 (d, J = 8.1 Hz, 2H, ArH), 7.28–7.35 (m, 2H, H6,8), 7.45–7.49 (m, 2H, H5,7), 7.54 (d, J = 8.0 Hz, 2H, ArH), 8.05 (s, 1H, H4), 8.55 (br, s, 1H, NH); Anal. Calcd. for C19H13N3O4: C, 65.70; H, 3.77; N, 12.10. Found: C, 65.79; H, 3.65; N, 12.16.
4-Methoxy-N-[5-(2-oxo-2H-chromen-3-yl)-[1,3,4]oxadiazol-2-yl]-benzamide (8d): Yield: 54%; m.p.: 168–170°C; Rf (SS1): 0.66; IR (cm−1): 1162 (C-O-C), 1618 (C=N), 1738 (CO), 3212 (NH); 1H-NMR (ppm): 3.71 (s, 3H, OCH3), 6.94 (d, J = 7.8 Hz, 2H, ArH), 7.16–7.20 (m, 2H, H6,8), 7.54 (d, J = 8 Hz, 2H, ArH), 7.64–7.7 (m, 2H, H5,7), 8.12 (s, 1H, H4), 10.3 (br, s, 1H, NH); Anal. Calcd. for C19H13N3O5: C, 62.81; H, 3.61; N, 11.57. Found: C, 62.76; H, 3.54; N, 11.45.
Anti-inflammatory assay
The test compounds (4a–e, 6a–f, and 8a–d) were evaluated using the in vivo rat carrageenan-induced foot paw oedema at 20 mg/kg body weight as reported in literature.Citation45
Analgesic assay
Compounds (4a, 4b, 4c, 4e, 6b, 6e, 8b) that showed 70% and above inhibition in carrageenan-induced oedema were screened for analgesic activity at 20 mg/kg body weight using the 4% sodium chloride-induced writhings (abdominal constriction) assay as reported in literature.Citation46
Acute ulcerogenesis assay
Compounds (4a, 4b, 4c, 4e, 6b, 6e, 8b) were tested for acute ulcerogenic studies by reported method of Cioli et al.Citation47 The studies were carried out on healthy Wistar rats (150–200 g) at a dose three times the anti-inflammatory dose viz. 60 mg/kg. The animals were divided into different groups of six each, group I served as control and received vehicle only and group II received pure ibuprofen 60 mg/kg. Other groups were administered test compounds in dose molecularly equivalent to 60 mg/kg of ibuprofen. The animals were fasted 8 h prior to a single dose of each of the vehicle, standard and test compounds, respectively, and sacrificed 17 h later during which period food and water were available. The animals were sacrificed and gastric mucosa of the rats was examined for lesions and ulcers by means of a 4× binocular magnifier. The scoring was done according to the reported procedure.Citation47
Lipid peroxidation assay
Lipid peroxidation studies were carried out according to the method of Ohkawa et al.Citation48 After scoring the gastric mucosa of animals for ulcerogenic effect of synthesized drugs, the gastric mucosa of animals was scraped with two glass slides, weighed (100 mg), and homogenized in 1.8 mL of 1.15% ice-cold potassium chloride (KCl) solution. The homogenate was supplemented with 0.2 mL of 8.1% sodium dodecyl sulphate (SDS), 1.5 mL of acetate buffer (pH 3.5), and 1.5 mL of 0.8% thiobarbituric acid (TBA). The mixture was heated at 95°C for 60 min. The cooled reactants were shaken vigorously for 1 min and centrifuged for 10 min at 4000 rpm after supplementing with 5 mL of a mixture of n-butanol and pyridine (15:1 v/v). The supernatant organic layer was collected and absorbance was measured at 532 nm on UV spectrophotometer. The results are expressed as nmoles of malondialdehyde (MDA)/100 mg tissue, using extinction coefficient 1.56 × 105 per cm/M.
COX inhibition studies
Selected 1,3,4-oxadiazole derivatives (4a, 4b, 4c, 4e, 6b, 6e, 8b) were tested for their ability to inhibit COX-1 and COX-2 using a COX-(ovine) inhibitor screening kit (Catalogue No. 560101; Cayman Chemical, Ann Arbor, MI). Stock solutions of test compounds were dissolved in a minimum volume of dimethyl sulphoxide (DMSO) to obtain 0.001, 0.01, 0.1, 1, 10, 100, and 500 μM in a final volume of 1 mL. In brief, haematin reconstituted purified COX-1 and COX-2 enzymes in a reaction buffer containing Tris–hydrochloric acid (HCl) (0.1 M, pH 8.0), 5 mM ethylenediaminetetraacetic acid (EDTA), and 2 mM phenol were pre-incubated at room temperature for 1 h with test compounds followed by the addition of AA (100 μM) for 2 min at 37°C. Reactions were terminated by adding 50 μL of 1 M HCl followed by the addition of 100 μL of stannous chloride. The final product PGF2α formed was measured by enzyme immunoassay (EIA) and the dose that causes half-maximal inhibition (IC50) values were determined following the instructions given in the kit manual. Percent inhibition was calculated by comparison of compound treated to various control incubations. The concentration of the test compound causing 50% inhibition (IC50, μM) was calculated from the concentration–inhibition response curve.
LOX inhibition assay
LOX-5,Citation49 LOX-12,Citation50 and LOX-15Citation51 activities were carried out on 4a, 4c, 4e following the procedures as described in literature. The enzyme sources for LOX-5, LOX-12, and LOX-15 are human peripheral blood mononuclear cell leukocyte (PBML) cells, human platelets, and rabbit reticulocytes, respectively. The substrates for LOX-5, LOX-12, and LOX-15 used in the assays were endogenous AA, 30 mM AA, and 256 mM linoleic acid, respectively. The enzyme inhibition was quantitated by measuring LTB4, 12-hydroxy eicosatetraenoic acid (12-HETE), and 15-HETE by EIA and spectrophotometrically.
Results and discussion
Chemistry
The synthetic route used to synthesize the compounds is outlined in . 2,5 Disubstituted 1,3,4-oxadiazole (4a–e) derivatives were obtained from Schiff bases (3a–e) of compound (2) by cyclization using aqueous ferric chloride solution. Aryl semicarbazides (5a–f) were obtained by treating compound (2) with different aryl isothiocyanates in alcohol followed by cyclization to corresponding 1,3,4-oxadiazole (6a–f) derivatives using alkaline iodine solution. 2-(Coumarin-3yl)-5-amino-1,3,4-oxadiazole (7) was condensed with various acid chlorides in pyridine to yield N-substituted 1,3,4-oxadiazoles (8a–d). The structures of synthesized compounds were confirmed on the basis of different spectral studies. The physical data, FTIR and 1H-NMR spectral data for all the synthesized compounds are reported in experimental protocols.
The FTIR spectra of the compounds synthesized exhibited very similar features and showed the expected bands for the characteristic groups that are present in the compounds such as C=O and the C=N stretching vibrations. In case of 1,3,4-oxadiazole derivatives, the presence of C=N stretching band at 1645–1597 cm−1 and (C-O-C) stretching band at 1170–1065 cm−1 is an evidence of ring closure. The N-H stretching bands of the compounds were observed between 3410 and 3210 cm−1. In the proton NMR spectral data, all protons were seen according to the expected chemical shift and integral values. The aromatic protons appeared in the range 6.6–8.5 ppm. For the compounds (3a–e), the singlet signals belonging to benzylidene (=CH-Ar) group were observed at aromatic region, and disappearance of the signals belonging to –NHNH2 indicated functionalization of hydrazide (2) to hydrazone (3a–e). Disappearance of resonance due to benzylidene group supported the formation of oxadiazole nucleus (4a–e), –NH– proton was observed as broad singlet between 8 and 11 ppm (probably due to their ability to get exchanged with D2O) each signal showing integration for one proton. H4 of coumarin was located as singlet between 8 and 8.5 ppm.
Biological activity
We have designed some new 1,3,4-oxadiazole derivatives containing coumarin moiety and evaluated for their anti-inflammatory activity. Anti-inflammatory activity was checked by their ability to inhibit carrageenan-induced inflammation in vivo. Compounds that showed 70% and above inhibition in oedema were further screened for analgesic activity, acute ulcerogenicity, as well as lipid peroxide profile. Selected compounds were screened for their ability to inhibit COX-1, COX-2, LOX-5, LOX-12, and LOX-15 enzymes in vitro.
The anti-inflammatory activity was carried out by Winter et al.Citation45 method, analgesic activity by Fukawa et al.Citation46 method, acute ulcerogenic activity by Cioli et al.Citation47 method, and lipid peroxidation studies by Ohkawa et al.Citation48 method. The data are presented in . IC50 values for inhibition of COX-1 and COX-2 enzymes by these compounds were determined by an EIA. Inhibition of human LOX-5 from human PBML cells, LOX-12 from human platelets, and LOX-15 from rabbit reticulocytes were determined by EIA and spectrophotometric quantitation.
Table 1. Biological evaluation of synthesized 2,5-disubstituted 1,3,4-oxadiazole derivatives.
The anti-inflammatory activity of all the oxadiazole derivatives (4a–e, 6a–f, and 8a–d) synthesized is presented in . The percent oedema inhibition relative to control was measured after 2 and 3 h of the treatment and the inhibition of swelling in carrageenan-induced oedema in rat paw brought about by oral administration of the drugs is reported as paw volume ± SEM and percentage inhibition in oedema (). The percentages of inhibition in swelling by the compounds were calculated using Equation (1).
Vt and Vo relates to the average volume in the hind paw of the rats (n = 6) before any treatment and after anti-inflammatory agent treatment, respectively.
The inhibition of oedema observed was in the range of 35% to 89%. Compound 4e was the most active compound (89% inhibition of oedema) and was found potent than ibuprofen and equivalent to celecoxib in inhibiting the oedema at 3 h. Compounds (4a, 4b, 4c, 4e, 6b, 6e, 8b) with percentage inhibition >70% were tested for their ability to inhibit COX and LOX enzymes in vitro. The in vitro COX inhibition assay showed selectivity of 4e towards COX-2 (COX-1 IC50 = 41.6 μM; COX-2 IC50 = 0.4 μM, SI for COX-2 = 104) (). Compound 4e was also good in inhibiting LOX enzyme in in vitro analysis compared with standard.
Table 2. In vitro COX inhibition data for 1,3,4-oxadiazole derivatives (4a, 4b, 4c, 4e, 6b, 6e, 8b).
Structure–activity relationship
A comparison of the structure–activity relationship (SAR) data for the 2,5 disubstituted 1,3,4-oxadiazole derivatives showed that the presence of a substitution with positive hydrophobicity and electronic effect are good for anti-inflammatory activity like chloro substitution at p-position of phenyl ring at fifth position of oxadiazole (4b). It was observed that changing hydrophobicity (σ) or electronic (π) parameter adversely affected the activity, decrease in activity was observed by replacing chloro group with methyl (4c) (+π and −σ) or methoxy group (4d) (– π and –σ). Accordingly the activity should then increase with increase in the π and σ values. Therefore, effect of addition of another chlorine group in the molecule was observed. The results showed that the activity indeed increased by adding another chloro group into the nucleus (4e) confirming the above observation.
Compounds (6a–f and 8a–d) exhibited less activity comparatively, which may be due to increase the distance between the oxadiazole nucleus and phenyl ring due to presence of –NH–, and –NHCO– group between the two rings. The presence of single bond also results in formation of large number of conformers, which may be another reason for less activity of these derivatives. Compounds (6b, 6e, and 8b) showed inhibition of oedema by 74%, 72%, and 77%, respectively, but they were nonselective in nature in inhibiting COX-1 and COX -2 (COX-1 IC50 >100, >100, >100 μM; COX-2 IC50 > 100, 36.6, >100 μM, respectively) in in vitro analysis ().
LOX inhibition studies of 4a, 4c, and 4e showed that these molecules have a moderate to good activity towards LOX-5 and LOX-15 and very low activity towards LOX-12. Compound 4e was the most active against LOX-5 and LOX-15 with 48% and 19% inhibition at 10 μM, respectively, and was found also more effective than the standard in inhibiting LOX-5 and LOX-15 ().
Table 3. In vitro LOX inhibition data at 10 µM for 1,3,4-oxadiazole derivatives (4a, 4b, 4c).
Compounds 4a, 4b, 4c, 4e, 6b, 6e, 8b were screened for analgesic activity, acute ulcerogenicity, as well as lipid peroxide profile. The analgesic activity of the compounds was done at the same dose as used for anti-inflammatory activity. The percent protection in mice brought about by administration of the drugs is shown in . The compounds tested showed analgesic activity in the range of 51–74%. The percent protection was calculated using Equation (2).
Compound 4e showed 73.5% of protection against sodium chloride-induced writhings compared with 71.9% protection with ibuprofen. Similar SAR pattern in analgesic effect of compounds was observed as was seen in anti-inflammatory activity. Compound bearing electronegative group on phenyl ring directly attached to the oxadiazole nucleus exhibited good analgesic effect compared with other derivatives.
The acute ulcerogenic effect of synthesized compounds was studied at 60 mg/kg in rats. It was observed that the ulcerogenic effect of test compounds (4a, 4b, 4c, 4e, 6b, 6e, 8b) was appreciably less than ibuprofen. Less number of ulcers was seen in animals treated with test compounds compared with the animals treated with ibuprofen. The tested compounds showed severity index ranging from 0.33 to 0.83, whereas the standard drug ibuprofen showed severity index of 2.0 (). Compounds 4a and 4e showed severity index of 0.42 and 0.33, respectively, which is less than one-fourth of the value of ibuprofen. These findings support the statement that these compounds are relatively selective for COX-2. Compounds that are less irritant to gastric mucosa are also reported to show reduced MDA content, a by-product of lipid peroxidation.Citation48 Therefore, by determining the MDA levels it can be ascertained that the compounds are actually less irritant to gastric mucosa. To correlate the ulcerogenic profile of compounds, the lipid peroxidation values were also determined. The lipid peroxidation was measured as nmoles of MDA/100 mg of tissue. Animals treated with ibuprofen exhibited 6.8, whereas control group showed 1.96 and the groups treated with synthesized compounds showed lipid peroxidation in the range of 2.5–5 (), suggesting that these derivatives are less irritant to gastric mucosa.
Conclusion
The studies showed that the dual inhibition of COX and LOX as promising strategy for treating inflammatory conditions due to their less associated side effects. Fifteen new 2,5-disubstituted 1,3,4-oxadiazoles were successfully synthesized and characterized. All the compounds showed anti-inflammatory profile with fewer side effects and also exhibited protection against sodium chloride-induced writhings. Compounds 4a, 4b, and 4e were most active compounds. Compound 4a was also found selective for COX and LOX both.
Oxadiazole derivatives therefore present an opportunity to develop new NSAIDs with reduced side effects. The capacity to block both COX and LOX appears advantageous as this will help in developing agents with less unwanted effects because of increased expression of LOX otherwise. These observations suggest that these derivatives could be used to develop leads for more potent dual inhibitors of COX and LOX as nonsteroidal anti-inflammatory agents.
Declaration of interest
The authors are thankful to UGC for financial support. The authors are also grateful to Janab Abdul Mueed Sb. for providing infrastructure and facility to carry out this work.
References
- Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol 2001;62:1433–1438.
- Smith WL, Marnett LJ. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta 1991;1083:1–17.
- Dannhardt G, Kiefer W. Cyclooxygenase inhibitors—current status and future prospects. Eur J Med Chem 2001;36:109–126.
- Fosslien E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci 1998;28:67–81.
- Goldenberg MM. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clin Ther 1999;21:1497–1513; discussion 1427.
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 1998;38:97–120.
- Mukherjee D. Selective cyclooxygenase-2 (COX-2) inhibitors and potential risk of cardiovascular events. Biochem Pharmacol 2002;63:817–821.
- Parente L, Perretti M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochem Pharmacol 2003;65:153–159.
- Samuelsson B, Borgeat P, Hammaratrom S, Murphy RC. Leukotrienes: a new group of biologically active compounds. Adv Prostaglandin Thromboxane Res 1980;6:1–18.
- Samuelsson B. Leukotrienes and other lipoxygenase products. Prog Lipid Res 1986;25:13–18.
- Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem 2003;38:645–659.
- McMillan RM, Walker ERH. Designing therapeutically effective 5-lipoxygenase inhibitors. Trends Pharmacol Sci 1992;13:323–327.
- Ford-Hutchinson AW, Gresser M, Young RN. 5-Lipoxygenase. Annu Rev Biochem 1994;63:383–386.
- Young RN. Inhibitors of 5-lipoxygenase: a therapeutic potential yet to be fully realized? Eur J Med Chem 1999;34:671–685.
- Vila L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med Res Rev 2004;24:399–424.
- Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med 2004;14:191–195.
- Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med 2009;206:1565–1574.
- Rotondo S, Dell’Elba G, Krauze-Brzósko K, Manarini S, Martelli N, Pecce R et al. Licofelone, a dual lipoxygenase–cyclooxygenase inhibitor, downregulates polymorphonuclear leukocyte and platelet function. Eur J Pharmacol 2002;453:131–139.
- Pommery N, Taverne T, Telliez A, Goossens L, Charlier C, Pommery J et al. New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy. J Med Chem 2004;47:6195–6206.
- Vijayakrishnan R, Rao GS. A computer modeling approach towards designing dual LOX/COX inhibitors as potent anti-cancer drugs. Biophys J 2009;94:1090.
- Holla BS, Gonsalves R, Shenoy S. Synthesis and antibacterial studies of a new series of 1,2-bis(1,3, 4-oxadiazol-2-yl)ethanes and 1,2-bis(4-amino-1,2, 4-triazol-3-yl)ethanes. Eur J Med Chem 2000;35:267–271.
- Macaev F, Rusu G, Pogrebnoi S, Gudima A, Stingaci E, Vlad L et al. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–anti-mycobacterial activities. Bioorg Med Chem 2005;13:4842–4850.
- Liu F, Luo XQ, Song BA, Bhadury PS, Yang S, Jin LH et al. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg Med Chem 2008;16:3632–3640.
- Burbuliene MM, Jakubkiene V, Mekuskiene G, Udrenaite E, Smicius R, Vainilavicius P. Synthesis and anti-inflammatory activity of derivatives of 5-[(2-disubstituted amino-6-methyl-pyrimidin-4-yl)-sulfanylmethyl]-3H-1,3,4-oxadiazole-2-thiones. Farmaco 2004;59:767–774.
- Palaska E, Sahin G, Kelicen P, Durlu NT, Altinok G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 2002;57:101–107.
- Amir M, Shikha K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur J Med Chem 2004;39:535–545.
- Zarghi A, Tabatabai SA, Faizi M, Ahadian A, Navabi P, Zanganeh V et al. Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-1,3,4-oxadiazoles. Bioorg Med Chem Lett 2005;15:1863–1865.
- Liu KG, Smith JS, Ayscue AH, Henke BR, Lambert MH, Leesnitzer LM et al. Identification of a series of oxadiazole-substituted alpha-isopropoxy phenylpropanoic acids with activity on PPARalpha, PPARgamma, and PPARdelta. Bioorg Med Chem Lett 2001;11:2385–2388.
- Kumar D, Sundaree S, Johnson EO, Shah K. An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anticancer agents. Bioorg Med Chem Lett 2009;19:4492–4494.
- Emam AA, Deeb OA, Al-Omar M, Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. J Bioorg Med Chem 2004;12:5107–5113.
- Khan MT, Choudhary MI, Khan KM, Rani M, Atta-ur-Rahman. Structure–activity relationships of tyrosinase inhibitory combinatorial library of 2,5-disubstituted-1,3,4-oxadiazole analogues. Bioorg Med Chem 2005;13:3385–3395.
- Farooqui M, Bora R, Patil CR. Synthesis, analgesic and anti-inflammatory activities of novel 3-(4-acetamido-benzyl)-5-substituted-1,2,4-oxadiazoles. Eur J Med Chem 2009;44:794–799.
- Srivastava RM, de Almeida Lima A, Viana OS, da Costa Silva MJ, Catanho MT, de Morais JO. Antiinflammatory property of 3-aryl-5-(n-propyl)-1,2,4-oxadiazoles and antimicrobial property of 3-aryl-5-(n-propyl)-4,5-dihydro-1,2,4-oxadiazoles: their syntheses and spectroscopic studies. Bioorg Med Chem 2003;11:1821–1827.
- Velázquez C, Rao PN, McDonald R, Knaus EE. Synthesis and biological evaluation of 3,4-diphenyl-1,2,5-oxadiazole-2-oxides and 3,4-diphenyl-1,2,5-oxadiazoles as potential hybrid COX-2 inhibitor/nitric oxide donor agents. Bioorg Med Chem 2005;13:2749–2757.
- Grimm EL, Brideau C, Chauret N, Chan CC, Delorme D, Ducharme Y et al. Substituted coumarins as potent 5-lipoxygenase inhibitors. Bioorg Med Chem Lett 2006;16:2528–2531.
- Alka K, Vasant KA, Rao MNA. Anti-inflammatory activity of cinnamic acids. Pharmazie 1989;44:870–873.
- Maddi V, Raghu KS, Rao MN. Synthesis and anti-inflammatory activity of 3-(benzylideneamino)coumarins in rodents. J Pharm Sci 1992;81:964–966.
- Kimura Y, Okuda H, Arichi S, Baba K, Kozawa M. Inhibition of the formation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid from arachidonic acid in polymorphonuclear leukocytes by various coumarins. Biochim Biophys Acta 1985;834:224–229.
- Celotti F, Laufer S. Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res 2001;43:429–436.
- Akhter M, Husain A, Azad B, Ajmal M. Aroylpropionic acid based 2,5-disubstituted-1,3,4-oxadiazoles: synthesis and their anti-inflammatory and analgesic activities. Eur J Med Chem 2009;44:2372–2378.
- Khan MSY, Akhter M. Synthesis of some new 2,5-disubstituted 1,3,4-oxadiazole derivatives and their biological activity. Indian J Chem 2003;42B:900–904.
- Horning EC, Horning MG, Dimming DA. Organic Synthesis, Collective Vol. 3. New York: John Wiley & Sons Inc.; 1995, pp. 165–167.
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Miscellaneous aromatic nitrogen compound. Vogel’s Textbook of Practical Organic Chemistry. England: Addison Wesley Longman Limited; 1998, pp. 966.
- Yar MS, Siddiqui AA, Ali MA. Synthesis and anti tuberculostatic activity of novel 1,3,4-oxadiazole derivatives. J Chin Chem Soc 2007;54:5–8.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547.
- Fukawa K, Kawano O, Hibi M, Misaki N, Ohba S, Hatanaka Y. A method for evaluating analgesic agents in rats. J Pharmacol Methods 1980;4:251–259.
- Cioli V, Putzolu S, Rossi V, Scorza Barcellona P, Corradino C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol 1979;50:283–289.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358.
- Carter GW, Young PR, Albert DH, Bouska J, Dyer R, Bell RL et al. 5-Lipoxygenase inhibitory activity of zileuton. J Pharmacol Exp Ther 1991;256:929–937.
- Romano M, Chen XS, Takahashi Y, Yamamoto S, Funk CD, Serhan CN. Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem J 1993;296 (Pt 1):127–133.
- Auerbach BJ, Kiely JS, Cornicelli JA. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem 1992;201:375–380.