Abstract
This study aimed to determine plasma and neutrophil oxidase activities that may contribute to vascular inflammation in Behçet’s disease (BD) patients. Cyclooxygenase (COX), NADPH oxidase and myeloperoxidase (MPO) activity was determined in neutrophils isolated from BD patients and healthy controls. Functional assay of NADPH oxidase was significantly increased in BD patients, both at basal conditions and in response to fMLP stimulation. There was a significant increase in plasma MPO activity in the disease group as compared to controls. Total COX activity was significantly increased in BD neutrophils. The increase in total COX activity was accompanied with enhanced activity of COX-2, differentiated by using the COX-1 isoform-specific inhibitor SC-560. Neutrophil nitrate/nitrite levels showed no significant difference in BD; however, plasma nitrate/nitrite contents in BD patients were significantly greater compared to controls. In conclusion, increased plasma MPO, neutrophil NADPH and COX activities may contribute to intravascular inflammation documented in BD patients.
Introduction
Participation of leukocytes in the pathophysiology of Behçet’s disease (BD) is supported by studies that show increased neutrophil CD64 expression during exacerbation of the diseaseCitation1 and by increased expressions of CR3 and cALLa on peripheral blood polymorphonuclear (PMN) cells, reflecting phenotypically activated neutrophilsCitation2. Notably, proinflammatory mediators, including interleukin-1 beta (IL-1β), IL-6, IL-8, soluble IL-2 receptor (sIL-2R), and tumour necrosis factor-alpha (TNF-α) are all elevated in the serum of BD patientsCitation3. Once secreted, IL-1 and TNF-α activate vascular endothelium, leading to increased expression of E-selectin and P-selectin in BDCitation4, which function as receptors for leukocyte adhesive proteins. IL-1, TNF-α, and IL-6 also activate hepatocytes to synthesize acute-phase proteins, which are also elevated in BD patientsCitation5,Citation6.
The activation state of neutrophils in BD patients is also evident by the expression of several surface antigens and levels of circulating proteins released by neutrophils. The high-affinity Fc receptor (FcgR1/CD64) is considered a marker of PMN activation and is strongly positive in BD patients, especially in patients with active BD than in patients with inactive diseaseCitation7. Another sensitive marker of neutrophil activation is decreased surface expression of L-selectin (CD62L), which is shed upon PMN stimulation via proteolytic cleavageCitation8. The expression of CD62L is reported to be significantly decreased in non-symptomatic BD patientsCitation9. Neutrophil activation also causes degranulation, leading to increased plasma levels of elastaseCitation10, which is reported to be significantly increased in BDCitation11.
One of the important features of vascular inflammation, the major underlying pathology in BD, is the migration of leukocytes from the circulation, across the endothelium, into vascular tissueCitation12. In vivo leukocyte–endothelial interactions are confined to the venular segments of the microcirculation and are rarely observed in arteriolesCitation13. The preferential adhesion of leukocytes to venular endothelium is not explained by the existence of lower shear rates in venules, compared to arterioles, but rather suggests a heterogeneous distribution of adhesion molecules between arterioles and venulesCitation14. In fact, intravital confocal microscopy and immunofluorescent labelling have shown that the spatial distribution and expression levels of adhesion molecules in the microcirculation significantly impact on leukocyte adhesion in vivoCitation15. Nitric oxide (NO) functions as a potent antagonist of inflammation, when its concentration is greater than that of superoxide, decreasing cytokine-induced adhesion molecule expression and endothelial activationCitation16. Considering that cytokine-induced leukocyte–endothelial adhesion predominantly occurs in the microcirculationCitation17, it is relevant that compensatory NO release is operative in BD to inhibit preferential adhesion of neutrophils to the endotheliumCitation18.
Physiologic actions of NO are highly dependent on steady state concentrations of reactive oxygen species and tissue-oxidant defence mechanisms. Oxidases and oxygenases are sources of oxygen radical production and can lead to an overall impairment of vascular NO signalling, via the metalloprotein and free radical–mediated consumption of this vasoactive moleculeCitation19. This study therefore aimed to determine the activity of enzymes that generate vascular oxidants, NADPH oxidase, MPO and COX, in neutrophils isolated from BD patients and healthy controls. Nitrite (NO2−) and nitrate (NO3−) levels were measured in both plasma and neutrophils to estimate NO production. Erythrocyte sedimentation rate, C-reactive protein (CRP) and plasma MPO activity, was also analyzed in BD patients as indicators of immune system activation.
Materials and methods
Patients
The study group included 20 patients (9 female, 11 male) with a mean age of 40 years (age range: 22–56) who were admitted to Akdeniz University Physical Medicine and Rheumatology Clinic with a diagnosis of BD and 20 healthy volunteers (9 female, 11 male) with a mean age of 36 years (age range: 22–56). The diagnosis of BD was made according to the criteria of the International Study Group for BDCitation20. Patients and healthy volunteers enrolled in the study were free from non-steroidal anti-inflammatory drugs for over 14 days and from azathioprine and colchicine for at least 24 h before blood collection, respectively. None of the BD patients and controls received an irregular diet. All experimental protocols were approved by the Institutional Review Board for Human Use at Akdeniz University Faculty of Medicine, conforms to Helsinki Declaration and all patients gave written informed consent. Erythrocyte sedimentation rate in participating 18 BD subjects were determined by a fully automated analyzer (Test 1, Alifax SpA. Padova, Italy), while CRP was measured on an automated analyzer (Roche/Hitachi Diagnostic Systems) via an immunoturbidimetric assay.
Neutrophil isolation
Neutrophils were isolated from whole blood via a Histopaque density gradient as described previouslyCitation21. Briefly, fresh heparinised venous blood obtained from BD patients and controls were layered over a Ficoll-Hypaque gradient (Histopaque 1077 and 1119, Sigma-Aldrich Chemie, Steinheim, Germany) having densities of 1.077 and 1.119, respectively. After 30-min centrifugation (700g) at room temperature, the mononuclear cells were removed and the granulocyte cell layer was transferred and washed twice with phosphate-buffered saline. Purified PMN were re-suspended in modified Hank’s balanced salt solution containing 137 mM NaCl, 5.4 mM KCl, 5.5 mM glucose, 0.34 mM KH2PO4, 0.5 mM Na2HPO4, 1 mM CaCl2, 0.8 mM MgSO4, 11.9 mM NaHCO3, and 10 mM HEPES at pH 7.4. Purified cells were found to be > 95% viable by trypan blue exclusion and the purity of PMN was found to be > 95% by Wright staining. The cells were counted electronically via Beckman Coulter LH 750 analyzer (Beckman Coulter, Inc. Miami, FL) and the final cell number was adjusted to 6 × 106 cells/mL in Hank’s balanced salt solution.
Measurement of neutrophil superoxide release
Neutrophils were assayed for superoxide production on the same day following isolation. The release of superoxide (O2•−) was quantified spectrophotometrically by CuZn superoxide dismutase (SOD) inhibitable reduction of cytochrome c at 550 nm (εM = 21 mM−1·cm−1), as described previouslyCitation22. Briefly, neutrophil cell suspension (6 × 106 cells/mL) was incubated with or without SOD (100 U/mL; Sigma-Aldrich Chemie) in the presence of cytochrome c (50 mM; Sigma-Aldrich Chemie) for 1 hour at 37°C. In some experiments, O2•− release was measured in cells pre-treated with 10 mM N-formyl-methionyl-leucyl-phenylalanine (fMLP; Fluka, Sigma-Aldrich Chemie), which induced neutrophil oxidative response.
Measurement of myeloperoxidase activity
Cell pellets were sonicated (Branson Sonifier 250, G. Heinemann Ultraschall- und Labortechnik, Schwabisch Gmund, Germany) in 1 mL ice-cold lysis buffer containing 0.1 M NaH2PO4 (Merck, Darmstadt, Germany) at pH 5.5, 0.1% Triton X-100 (Sigma-Aldrich Chemie) and 10 mL protease inhibitor cocktail (Sigma-Aldrich Chemie). Myeloperoxidase (MPO) activity was determined by adding an aliquot of cell lysate or plasma to 43 mM NaH2PO4 (pH 5.4), 1.2 mM tetramethylbenzidine (TMB; Merck, Darmstadt, Germany), and 100 mM H2O2 (Sigma-Aldrich Chemie). Absorbance kinetics was assessed spectrophotometrically at 655 nm by H2O2-dependent oxidation of TMB (εM = 3.9 × 104 mM−1.cm−1). One unit of enzyme activity was defined as the amount of enzyme that caused the oxidation of 1 mmol TMB per minute at 25°C.
Measurement of neutrophil cyclooxygenase activity
Cell pellets were sonicated in 400 mL 0.1 M ice-cold Tris-HCl (SERVA, Heidelberg, Germany) buffer at pH 7.8 containing 1 mM EDTA (Sigma, Sigma-Aldrich Chemie). Cell homogenates were centrifuged at 10,000g for 15 min at 4°C and supernatants were kept at −80°C until assayed. Cyclooxygenase (COX) activity was measured using a COX activity assay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The COX activity assay kit measures enzyme activity colorimetrically by monitoring the appearance of oxidized N,N,N’,N-tetramethyl-p-phenylenediamine at 590 nm. The reaction rate was determined by using the N,N,N0,N0-tetramethylp-phenylenediamine extinction coefficient (εM = 8.26 mM−1 cm−1). Activity of COX-2 was differentiated using the COX-1 isoform-specific inhibitor SC-560 according to manufacturer’s instructions.
Nitrite and nitrate assay
Neutrophil cell suspensions (6 × 106 cells/mL) were sonicated on ice and cell lysates were transferred to an ultrafiltration unit and centrifuged through a 10-kDa molecular mass cut-off filter (Amicon, Millipore Corporation, Bedford, MA) to remove protein. The filtrate was stored at −80°C until analyses via a colorimetric assay kit. Analyses were performed in duplicate via the Greiss reaction using a colorimetric assay kit (Calbiochem, Darmstadt, Germany). Nitrate reductase was provided with the assay kit that was used to measure NO2− + NO3− levels. A 0.01-unit of enzyme was added to each well and the plate was incubated for 3 h at room temperature to convert NO3− into NO2−.
Protein measurements
Protein concentrations were measured at 595 nm by a modified Bradford assay using Coomassie Plus reagent with bovine serum albumin as a standard (Pierce Chemical Company, Rockford, IL).
Results
Erythrocyte sedimentation rate and CRP
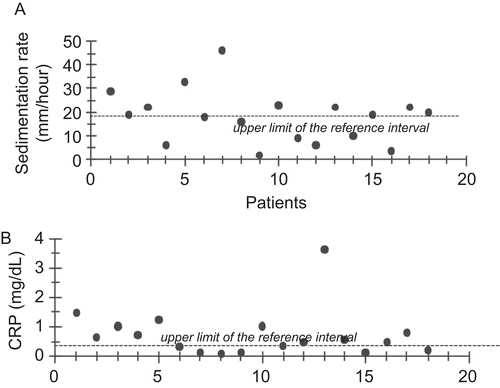
Erythrocyte sedimentation rate (mean ± SD; n = 18–20) was significantly higher (p < 0.05) in BD patients (17.90 ± 10.70 mM/h) as compared to the control group (10.25 ± 5.17 mM/h). Measured ESR values were above the upper limit of the reference interval in 61% of BD patients tested (). Likewise, CRP levels were significantly elevated (p < 0.05) in BD patients compared to controls with values of 1.01 ± 0.88 mg/dL vs. 0.33 ± 0.13 mg/dL, respectively. CRP levels were above the upper limit of the reference interval in 50% of the patients tested ().
Neutrophil superoxide release
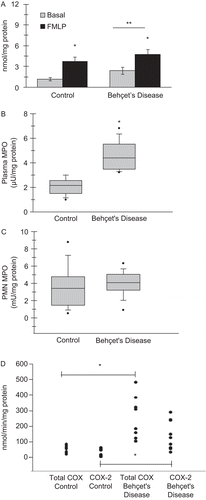
Neutrophil superoxide release (mean ± SEM; n = 19–20) was significantly increased (p < 0.05) in BD patients compared to controls, both at basal conditions (1.19 ± 0.25 vs. 2.40 ± 0.51 nmol/mg protein, respectively) and in response to 10 µM fMLP stimulation (4.77 ± 0.74 vs. 3.70 ± 0.68 nmol/mg protein, respectively) (). Statistical analysis was by two-way analysis of variance with all pair-wise multiple comparison procedures carried out via Tukey’s test.
Figure 2. (A) Neutrophil superoxide release in Behçet disease patients and controls, both at basal conditions and in response to 10 µM formyl-methionyl-leucyl-phenylalanine (fMLP) stimulation. Values are mean ± SEM (n = 19–22). *p < 0.05 compared to basal. (B) Plasma MPO activity and (C) neutrophil MPO activity in Behçet disease patients and controls. Values are mean ± SD (n = 10–11). *p < 0.001. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. (D) Neutrophil COX activity in Behçet disease patients and controls. Values are mean ± SD (n = 8–9). *p < 0.001.
Plasma and neutrophil MPO activity
Plasma MPO activity (mean ± SD; n = 10–11), as shown in , was significantly increased (p < 0.001) in BD (4.56 ± 1.2 µU/mg protein) compared to controls (2.08 ± 0.7 µU/mg protein). As shown in , neutrophil MPO activity was not significantly different in BD (3.95 ± 1.5 mU/mg protein) when compared to controls (3.61 ± 2.5 mU/mg protein). Statistical analysis was preformed via Student’s t-test.
Neutrophil COX activity
Total COX activity (mean ± SD; n = 8–9) as shown in , was significantly increased in BD neutrophils (241.6 ± 137.6 nmol/min/mg protein) compared to controls (57.2 ± 22.1 nmol/min/mg protein). COX-2 activity measured by using the COX-1 isoform-specific inhibitor SC-560 was also significantly increased in BD neutrophils (142.3 ± 94.4 nmol/min/mg protein) as compared to control (27.4 ± 23.1 nmol/min/mg protein). Statistical analysis was performed by Mann–Whitney rank sum test.
Plasma and neutrophil nitrat/nitrite levels
Nitrate and NO2− levels, as shown in , were significantly increased in the plasma of BD patients when compared to the control group. No significant difference could be observed in neutrophil NO3− and NO2− levels. Statistical analysis was by Student’s t-test.
Table 1. Plasma and neutrophil nitrite and nitrate levels in Behçet disease patients and controls.
Discussion
Although previous work have demonstrated the presence of neutrophil activation in BD, to our knowledge, this is the first study evaluating COX enzyme activity in conjunction with redox enzyme activities and NO production in neutrophils isolated from BD patients.
The significant increase in total COX activity observed in BD neutrophils was accompanied with elevated COX-2 activity. The capacity of human neutrophils to induce COX-2 expression under inflammatory conditions has been demonstrated previouslyCitation23; however, this report is the first to show increased COX-2 activity in circulating PMN isolated from BD patients. Expression of COX-2 is increased, by TNF-α, IL-1, and IL-8Citation24,Citation25, which are all elevated in the plasma of BD patientsCitation3. Prostaglandins are potent signalling molecules formed by the activity of COX and once synthesized by various inflammatory stimuli, prostaglandins lead to many diverse effects, some of which include vasoconstriction, platelet aggregation, smooth muscle cell contraction, chemotaxis, fever, and pain responseCitation26, all of which are associated with the pathogenesis of the vascular-inflammation and end-organ damage in BD.
Presented data herein also show that ESR and CRP were above the upper limit of the reference interval in 61 and 50% of the patients tested, respectively. These results are in agreement with previous work, which report similar increases in ESR and CRP levels in BD patientsCitation5,Citation6.
Superoxide release was significantly increased in BD patients compared to controls, both at basal conditions and in response to 10 µM fMLP stimulation (). These results are also in agreement with previous work, which report similar levels of spontaneous, zymosan-induced and lipopolysaccharide-stimulated superoxide release in neutrophils isolated from BD patientsCitation27,Citation28.
Plasma MPO levels of BD patients were previously evaluated and found to be increased compared to healthy controlsCitation29; however, this is the first study to measure both neutrophil and plasma MPO activity in BD patients. Although MPO activity measured in BD neutrophils was similar to healthy controls, increased levels of circulating plasma MPO is in agreement with reported literatureCitation29. The initiation of an inflammatory response is causally linked to activation and adherence of neutrophils. It is known that when neutrophils are activated by a proinflammatory signal, the granules fuse with the plasma membrane in a simultaneous manner and discharge their contents into the extracellular mediumCitation30. This process, also called degranulation, is a way by which MPO is released into sites of inflammation. Neutrophil-derived, haem-containing MPO catalytically consumes NO directly and via the generation of radical intermediates, thereby modulating NO bioactivity and adversely impacting on vascular inflammationCitation31. The NO metabolite NO2−, which is also significantly increased in the plasma of BD patients (), is a substrate for MPO and becomes oxidized to the reactive oxidizing and nitrating species nitrogen dioxide (•NO2Citation31). Importantly, the avidity of MPO for endothelial and other cells results in its transcytosis into the subcellular matrixCitation32, which can further lead to enhancement of tissue inflammation.
Serum NO levels were found to be either significantly elevatedCitation33 or decreasedCitation34 in BD patients compared with control subjects. The major metabolic pathway for endogenously formed NO in healthy human subjects is uptake into the red blood cells and conversion to NO3− and methemoglobinCitation35. In vivo activation of NO synthesis can therefore account for increased plasma levels of NO2− or NO3− in BD patients reported herein. Although earlier studies have shown constitutive expression of inducible nitric oxide synthase (NOS-2) in whole cell lysates of human neutrophilsCitation36, no significant difference was observed in neutrophil NO2−/NO3− levels in BD patients as compared to controls. This observation is in accord with as previous study which has reported insufficient NOS activity in leukocytesCitation37. Nonetheless, transient in vivo activation of NOS-2 can lead to the production of NO2− or NO3−, the stable metabolites of NO in neutrophil supernatants.
As our understanding of the pathophysiology of BD improves, more focused therapy will likely become available. Topical steroids, colchicine, and anti-inflammatory agents remain the basic therapy for mild mucocutaneous disease. Ocular disease in BD is improved by immunosuppressive therapy, and early aggressive treatment of ocular and visceral disease may alter the disease course. Thalidomide and interferon have also shown encouraging results in the therapy of BD patientsCitation38. Vasculitis is the major underlying pathology in BD and arachidonic acid metabolites are accepted to be responsible for vasculitis. Prostaglandin E2 and leukotriene C4 levels of BD patients before and after colchicine therapy were compared, and it was found that colchicine inhibits inflammation and PMN chemotaxis by inhibiting the COX and lipoxygenase pathwaysCitation39. It has been established that inhibition of COX-1 results in adverse effects such as gastrointestinal irritation and damage, platelet dysfunction and bronchospasmCitation40. The potential benefits of COX-2 specific inhibitors in the treatment of BD disease are supported by reported findings in this study, which show elevated COX-2 activity in BD PMN.
In summary, enzymatic sources of reactive inflammatory mediators and NO metabolites were examined in neutrophils and plasma isolated from BD patients and healthy controls. A major aspect of BD vasculopathy is the presence of inflammation. The observed increase in plasma MPO levels in addition to elevated neutrophil COX-2 and NADPH oxidase activity in BD patients can aggravate inflammatory conditions associated with the disease. Therapeutic strategies designed to counteract inflammatory and oxidative abnormalities present in neutrophils of BD patients may reduce tissue injury and vascular inflammation related to enhanced leukocyte activation observed in BD patients.
Declaration of interest
This work was supported by a grant (No: 2008.04.0103.002) from Akdeniz University Research Foundation.
References
- Ureten K, Ertenli I, Oztürk MA, Kiraz S, Onat AM, Tuncer M et al. Neutrophil CD64 expression in Behçet’s disease. J Rheumatol 2005;32:849–852.
- Mishima K, Ichikawa Y, Shimizu H, Yoshida M, Arimori S. Cell surface antigens expressed on polymorphonuclear cells in the peripheral blood from patients with inflammatory rheumatic diseases: Two-color flow-cytometric analysis. Tokai J Exp Clin Med 1992;17:41–51.
- Evereklioglu C, Er H, Türköz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediators Inflamm 2002;11:87–93.
- Haznedaroglu E, Karaaslan Y, Büyükasik Y, Kosar A, Ozcebe O, Haznedaroglu C et al. Selectin adhesion molecules in Behçet’s disease. Ann Rheum Dis 2000;59:61–63.
- Durmazlar SP, Ulkar GB, Eskioglu F, Tatlican S, Mert A, Akgul A. Significance of serum interleukin-8 levels in patients with Behcet’s disease: High levels may indicate vascular involvement. Int J Dermatol 2009;48:259–264.
- Bozoglu E, Dinc A, Erdem H, Pay S, Simsek I, Kocar IH. Vascular endothelial growth factor and monocyte chemoattractant protein-1 in Behçet’s patients with venous thrombosis. Clin Exp Rheumatol 2005;23:S42–S48.
- Alpsoy E, Kodelja V, Goerdt S, Orfanos CE, Zouboulis ChC. Serum of patients with Behçet’s disease induces classical (pro-inflammatory) activation of human macrophages in vitro. Dermatology (Basel) 2003;206:225–232.
- Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood 1991;77:2553–2555.
- Assaad-Khalil SH, Abou-Seif M, Youssef I, Farahat N. L-selectin expression on leukocytes of patients with Behçet’s disease. Adv Exp Med Biol 2003;528:273–278.
- de Haas M, Kerst JM, van der Schoot CE, Calafat J, Hack CE, Nuijens JH et al. Granulocyte colony-stimulating factor administration to healthy volunteers: Analysis of the immediate activating effects on circulating neutrophils. Blood 1994;84:3885–3894.
- Tsutsui K, Hasegawa M, Takata M, Takehara K. Increased plasma granulocyte elastase levels in Behçet’s disease. J Rheumatol 1998;25:326–328.
- Sakane T, Takeno M, Suzuki N, Inaba G. Behçet’s disease. N Engl J Med 1999;341:1284–1291.
- House SD, Lipowsky HH. Leukocyte-endothelium adhesion: Microhemodynamics in mesentery of the cat. Microvasc Res 1987;34:363–379.
- Perry MA, Granger DN. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest 1991;87:1798–1804.
- Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 2006;291:H2116–H2125.
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995;96:60–68.
- Kalogeris TJ, Kevil CG, Laroux FS, Coe LL, Phifer TJ, Alexander JS. Differential monocyte adhesion and adhesion molecule expression in venous and arterial endothelial cells. Am J Physiol 1999;276:L9–L19.
- Duygulu F, Evereklioglu C, Calis M, Borlu M, Cekmen M, Ascioglu O. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active Behçet’s disease: A pilot study. Clin Rheumatol 2005;24:324–330.
- Aslan M, Freeman BA. Oxidases and oxygenases in regulation of vascular nitric oxide signaling and inflammatory responses. Immunol Res 2002;26:107–118.
- International Study Group for Behçet’s Disease: Criteria for diagnosis of Behçet’s disease. Lancet 1990;335:1078–1080.
- Aslan M, Yucel G, Bozcuk H, Savas B. The effect of recombinant human granulocyte/macrophage-colony-stimulating factor (rHu GM-CSF) and rHu G-CSF administration on neutrophil chemiluminescence assay in patients following cyclic chemotherapy. Cancer Immunol Immunother 1998;47:176–181.
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 2001;98:15215–15220.
- Aslan M, Canatan D. Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp Hematol 2008;36:1535–1544.
- Maloney CG, Kutchera WA, Albertine KH, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory agonists induce cyclooxygenase type 2 expression by human neutrophils. J Immunol 1998;160:1402–1410.
- Pouliot M, Gilbert C, Borgeat P, Poubelle PE, Bourgoin S, Créminon C et al. Expression and activity of prostaglandin endoperoxide synthase-2 in agonist-activated human neutrophils. Faseb J 1998;12:1109–1123.
- Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA 1994;91:2046–2050.
- Mege JL, Dilsen N, Sanguedolce V, Gul A, Bongrand P, Roux H et al. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behçet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol 1993;20:1544–1549.
- Niwa Y, Miyake S, Sakane T, Shingu M, Yokoyama M. Auto-oxidative damage in Behçet’s disease–endothelial cell damage following the elevated oxygen radicals generated by stimulated neutrophils. Clin Exp Immunol 1982;49:247–255.
- Yazici C, Köse K, Calis M, DemIr M, Kirnap M, Ates F. Increased advanced oxidation protein products in Behçet’s disease: A new activity marker? Br J Dermatol 2004;151:105–111.
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–376.
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002;296:2391–2394.
- Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest 2001;108:1759–1770.
- Yildirim M, Baysal V, Inaloz HS, Doguc D. The significance of serum nitric oxide levels in Behçet’s disease and recurrent aphthous stomatitis. J Dermatol 2004;31:983–988.
- Orem A, Vanizor B, Cimsit G, Kiran E, Deger O, Malkoç M. Decreased nitric oxide production in patients with Behçet’s disease. Dermatology (Basel) 1999;198:33–36.
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 1996;380:221–226.
- Cedergren J, Follin P, Forslund T, Lindmark M, Sundqvist T, Skogh T. Inducible nitric oxide synthase (NOS II) is constitutive in human neutrophils. Apmis 2003;111:963–968.
- Yan L, Vandivier RW, Suffredini AF, Danner RL. Human polymorphonuclear leukocytes lack detectable nitric oxide synthase activity. J Immunol 1994;153:1825–1834.
- Goker B, Goker H. Current therapy for Behçet’s disease. Am J Ther 2002;9:465–470.
- Gürer MA, Keskin N, Gülekon A, Karel L, Aksakal B, Baysal V. Arachidonic acid metabolites and colchicine in Behçet’s disease (BD). Prostaglandins Leukot Essent Fatty Acids 1991;43:257–259.
- Brooks P, Emery P, Evans JF, Fenner H, Hawkey CJ, Patrono C et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology (Oxford) 1999;38:779–788.