Abstract
(2-Bromo-3,4-dimethoxyphenyl) (3,4-dimethoxyphenyl)methanone (10) and its derivatives with Br, one dibromide and isomeric three tribromides, were synthesized. Demethylation of these compounds afforded a series of new bromophenols. Inhibition of human cytosolic carbonic anhydrase II (hCA II) isozyme by these new bromophenols and naturally occurring 3,4,6-tribromo-5-(2,5-dibromo-3,4-dihydroxybenzyl)benzene-1,2-diol (3), and 5,5′-methylenebis(3,4,6-tribromo-benzene-1,2-diol) (4) was investigated. The synthesized compounds showed carbonic anhydrase inhibitory capacities with IC50 values in the range of 0.7–372 μM against hCA II. Some bromophenols investigated here showed effective hCA II inhibitory activity and might be used as leads for generating novel carbonic anhydrase inhibitors which are valuable drug candidates for the treatment of glaucoma, epilepsy, gastric and duodenal ulcers, neurological disorders, or osteoporosis.
Introduction
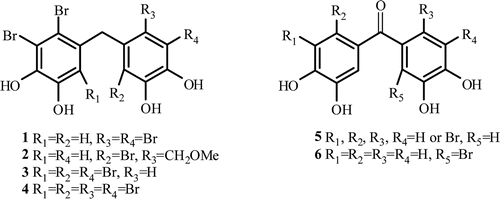
Naturally occurring bromophenols, frequently isolated from red algae of the family Rhodomelaceae, have prominent biological activitiesCitation1,Citation2. Of these natural compounds, 5,5′-methylenebis(3,4-dibromobenzene-1,2-diol) (1) and 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(methoxymethyl)benzyl)benzene-1,2-diol (2) exhibit enzyme inhibition, e.g. isocitrate lyaseCitation3 cytotoxicityCitation4, feeding deterrentCitation5, and microbialCitation6,7 activities, while 3,4,6-tribromo-5-(2,5-dibromo-3,4-dihydroxybenzyl)benzene-1,2-diol (3) and 5,5′-methylenebis(3,4,6-tribromo-benzene-1,2-diol) (4) exhibit significant aldose reductase inhibitory activityCitation8. Additionally, it was reported that bromophenol 1 is an inhibitor of protein tyrosine phosphataseCitation9. Antioxidant activities of 1 and 4 have also been reportedCitation10,Citation11. Recently, we have achieved an alternative synthesis of 1Citation10, first total synthesis of 2, 3, 4Citation12,Citation13, and a series of diphenylmethanone like bromophenols 5Citation10. We have reported that compound 1 and a series of 5 show high antioxidant and radical scavenging activitiesCitation10. Compound 6 and its derivatives with different number of bromines are also diphenylmethanone like compounds which are similar to 5 ().
The carbonic anhydrases (CA; Carbonate hydrolyase, EC 4.2.1.1) are a ubiquitous family of zinc-containing enzymes that classically participate in the maintenance of pH homeostasis in human body, catalyzing the reversible hydration of carbon dioxide in a two-step reaction to yield bicarbonate and protonsCitation14. Sixteen isozymes have been described so far, that differ in their subcellular localization, catalytic activity and susceptibility to different classes of inhibitors. Some of these isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane-bound (CA IV, CA IX, CA XII and CA XIV), two are mitochondrial (CA VA and CA VB), and one is secreted in saliva (CA VI). It has been reported that CA XV isoform is not expressed in humans or in other primates, but it is abundant in rodents and other higher vertebratesCitation15–17. CAs are produced in a variety of tissues where they participate in several important biological processes such as acid-base balance, respiration, carbon dioxide and ion transport, bone resorption, ureagenesis, gluconeogenesis, lipogenesis and body fluid generationCitation15,Citation18,Citation19. The two major CA isozymes (CA I and CA II) are present at high concentrations in the cytosol in erythrocytes, and CA II has the highest turnover rate among all CAs. Many of the CA isozymes involved in these processes are important therapeutic targets with the potential to be inhibited to treat a range of disorders including oedema, glaucoma, obesity, cancer, epilepsy and osteoporosisCitation18–20.
Interaction of most CA isozymes with several types of phenols, such as simple phenol and its substituted derivatives, clioquinol, salicyclates and some of their derivatives, has been recently investigatedCitation20–23. Here, we extend these earlier investigations to a novel series of bromophenols.
Chemicals are generally known to activate or inhibit several enzymes in vivo and affect metabolic pathways. Inhibitory effects of different anions, metal ions, drugs, phenols and sulfonamides, which are specific inhibitors, have been so far investigated against many CAsCitation21,Citation24–28. CA II inhibitors are used for several purposes, in particular for the treatment of glaucoma, epilepsy, and as diuretics or antitumor agents/diagnostic toolsCitation18,Citation19,Citation29.
Many chemical substances and synthesized drugs affect metabolisms by changing enzyme activitiesCitation30–33. As CA II inhibitors are valuable molecules for therapeutical and pharmacological applications, we have synthesized novel bormophenols in the current research and evaluated their potency to be novel carbonic anhydrase inhibitors.
Materials and methods
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Column chromatography (CC): silica gel (SiO2; 60 mesh, Merck, Darmstadt, Germany). Preparative thick layer chromatography: 1 mm of SiO2 60 PF (Merck) on glass plates. Mp: cap. melting-point apparatus (BUCHI 530: Flawil, Switzerland); uncorrected. IR Spectra: solns. in 0.1 mm cells with a Mattson 1000 FT-IR spectrophotometer (Cambridge, England). 1H- and 13C- NMR spectra: 200 (50) and 400 (100)-MHz Varian spectrometer (Danbury, CT); δ in ppm; Me4Si as the internal standard. Elemental analyses: Leco CHNS-932 apparatus (MI, USA). Antioxidant activities of samples were determined in a spectrophotometer (UV-1208, Shimadzu, Japan).
Synthesis of (2-bromo-3,4-dimethoxyphenyl)(3,4-dimethoxyphenyl)methanone (10)
Polyphosphoric acid (PPA), prepared from conc. H3PO4 (85%, 2.63 g) and P2O5 (4.72 g, 33.2 mmol), was heated to 80°C in a beaker (100 mL). To this mixture were added 8 (0.84 g, 4.6 mmol) and 9Citation36 (1.0 g, 4.6 mmol) quickly. The mixture was stirred with a glass stick at 80°C for 45 min and was then carefully poured onto 35 mL of ice/water. The organic phase was extracted with EtOAc (2 × 125 mL). The combined organic layers were dried over Na2SO4 and the solvent was evaporated. Monobromide 10 (85%) was the sole product and was crystallized from ethyl acetate as white crystals. Mp 166–167°C; 1H-NMR (400 MHz, CDCl3): δ 7.54 (d, J = 2.2 Hz, 1 H), 7.23 (dd, A part of AB-system, J = 8.3 Hz, 2.2 Hz, 1 H), 7.07 (d, A part of AB-system, J = 8.4 Hz, 1 H), 6.93 (d, B part of AB-system, J = 8.4 Hz, 1 H), 6.82 (d, B part of AB-system, J = 8.4 Hz, 1 H), 3.93 (s, methoixde, 6 H), 3.92 (s, methoxide, 3 H), 3.88 (s, methoixde, 3 H); 13C-NMR (100 MHz, CDCl3): δ 194.24 (CO), 154.89 (C), 154.05 (C), 149.45 (C), 146.95 (C), 134.35 (C), 129.95 (C), 126.51 (CH), 124.78 (CH), 116.18 (C), 111.46 (CH), 111.18 (CH), 110.14 (CH), 60.84 (OCH3), 56.41 (OCH3), 56.31 (OCH3), 56.25 (OCH3); IR (CH2Cl2, cm−1): 3003, 2938, 2839, 1657, 1586, 1512, 1487, 1463, 1417, 1394, 1341, 1294, 1274, 1240, 1217, 1171, 1135, 1032, 991, 904, 879, 813, 796, 759, 729, 636, 569; Anal. Calcd for C17H17BrO5: C, 53.56; H 4.49. found: C, 53.52; H 4.40.
Bromination of compound 10
To a stirring solution of monobromide 10 (2.0 g, 5.2 mmol) in CHCl3 (50 mL) was added a solution of bromine (5.0 g, 31.2 mmol, 6 eq.) in CHCl3 (30 mL) drop wise at room temperature (RT) over 10 min. After the reaction mixture was stirred at RT for 3 days, the solvent was evaporated. Chromatography of the residue (2.71 g) on silica gel (SiO2, 100 g) with ethyl acetate/hexane (5:95) gave dibromide 11 (0.68 g, 28%), 14 (0.32 g, 12%), 13 (0.71 g, 26%) and 12 (0.76 g, 27%), respectively.
(2-Bromo-3,4-dimethoxyphenyl)(2-bromo-4,5-dimethoxyphenyl)methanone (11)
Mp 122–123°C as white crystals; 1H-NMR (400 MHz, CDCl3) δ 7.21 (d, A part of AB-system, J = 8.4 Hz, 1 H), 7.06 (s, 1 H), 7.03 (s, 1 H) 6.88 (d, B part of AB-system, J = 8.4 Hz, 1 H) 3.93 (s, methoxide, 3 H), 3.92 (s, methoxide, 3 H), 3.86 (s, methoxide, 3 H), 3.85 (s, methoxide, 3 H); 13C-NMR (100 MHz, CDCl3) δ 194.19 (CO), 156.31 (C), 152.18 (C), 148.49 (C), 147.26 (C), 133.38 (C), 131.96 (C), 127.67 (CH), 117.97 (C), 116.56 (CH), 114.19 (CH), 113.61 (C), 110.83 (CH), 60.80 (OCH3), 56.56 (OCH3), 56.45 (OCH3), 56.37 (OCH3); IR (CH2Cl2, cm−1): 3005, 2964, 2842, 2591, 1668, 1584, 1505, 1486, 1463, 1445, 1399, 1375, 1336, 1271, 1211, 1171, 1159, 1059, 1030, 994, 919, 867, 820, 785, 735, 702, 647, 584; Anal. Calcd for C17H16Br2O5: C, 44.38, H 3.51 found: C, 44.38; H 3.52.
(2-Bromo-3,4-dimethoxyphenyl)(2,3-dibromo-4,5-dimethoxyphenyl)methanone (12)
Mp 100–101°C as pale yellow crystals; 1H-NMR (400 MHz, CDCl3) δ 7.24 (d, A part of AB-system, J = 8.8 Hz, 1 H), 6.98 (s, 1 H), 6.86 (d, part of AB-system, J = 8.8 Hz, 1 H), 3.93 (s, methoxide, 3 H), 3.91 (s, methoxide, 3 H), 3.87 (s, methoxide, 3 H), 3.86 (s, methoxide, 3 H); 13C-NMR (100 MHz, CDCl3) δ 193.60 (CO), 157.03 (C), 152.76 (C), 147.67 (C), 138.11 (C), 131.33 (C), 129.07 (CH), 126.18 (C), 123.27 (C), 118.75 (C), 114.70 (C), 113.20 (CH), 110.54, (CH), 60.93 (OCH3), 60.81 (OCH3), 56.62 (OCH3), 56.41 (OCH3); IR (CH2Cl2, cm−1): 3003, 2938, 1673, 1588, 1564, 1507, 1464, 1403, 1337, 280, 1262, 1217, 1166, 1141, 1070, 1032, 996, 924, 865, 837, 790, 733, 681, 609. Anal. Calcd for C17H16Br3O5: C, 37.88, H 2.80 found: C, 37.93; H 2.85.
(2-Bromo-4,5-dimethoxyphenyl)(2,6-dibromo-3,4-dimethoxyphenyl)methanone (13)
Mp 115–117°C as pale yellow crystals; 1H-NMR (400 MHz, CDCl3) δ 7.35 (s, 1 H), 7.11 (s, 1 H), 7.02 (s, 1 H) 3.94 (s, methoxide, 3 H), 3.91 (s, methoxide, 3 H), 3.87 (s, methoxide, 3 H), 3.84 (s, methoxide, 3 H); 13C-NMR (100 MHz, CDCl3) δ 192.88 (CO), 153.66 (C), 152.80 (C), 152.14 (C), 148.59 (C), 137.82 (C), 130.58 (C), 129.52 (CH), 117.00 (C), 116.81 (C), 116.74 (CH), 114.36 (CH), 114.23 (C), 61.39 (OCH3), 61.25 (OCH3), 56.62 (OCH3), 56.50 (OCH3); IR (CH2Cl2, cm−1): 2938, 2841, 1671, 1579, 1541, 1512, 1464, 1419, 1399, 1366, 1300, 1270, 1212, 1185, 1142, 1080, 1032, 1004, 918, 804, 775, 734, 665; Anal. Calcd for C17H16Br3O5: C, 37.88, H 2.80 found: C, 37.86; H 2.84.
(2-Bromo-4,5-dimethoxyphenyl)(2,5-dibromo-3,4-dimethoxyphenyl)methanone (14)
Mp 138–139°C as colourless crystals; 1H-NMR (400 MHz, CDCl3) δ 7.42 (s, 1 H), 7.10 (s, 1 H), 7.09 (s, 1 H) 3.86 (s, methoxide, 3 H), 3.85 (s, methoxide, 3 H), 3.85 (s, methoxide, 3 H), 3.84 (s, methoxide, 3 H); 13C-NMR (100 MHz, CDCl3) δ 191.38 (CO), 154.50 (C), 153.46 (C), 148.58 (C), 146.65 (C), 134.96 (C), 128.23 (C), 117.55 (CH), 116.61 (C), 116.29 (CH), 115.80 (C), 115.02 (CH), 114.60 (C), 60.97 (OCH3), 56.63 (2 OCH3), 56.43 (OCH3); IR (CH2Cl2, cm−1): 3003, 2936, 2841, 1679, 1655, 1586, 1508, 1476, 1442, 1380, 1338, 1298, 1262, 1212, 1158, 1065, 1027, 992, 931, 844, 815, 785, 756, 733, 701, 588; Anal. Calcd for C17H16Br3O5: C, 37.88, H 2.80 found: C, 37.66; H 2.82.
Standard procedure for demethylation of compounds with OMe by ether cleavage (2-bromo-3,4-dihydroxyphenyl)(3,4-dihydroxyphenyl)methanone (6)
A solution of monobromide 10 (0.43 g, 1.32 mmol) in CH2Cl2 (15 mL) was cooled to 0°C and then a solution of BBr3 (0.9 mL) in CH2Cl2 (10.0 mL) was added drop wise under N2(g) over 5 min. After the cold bath was removed, the mixture was stirred at RT and under N2 for 1 day. Methanol (35 mL) was slowly added over 15 min and then the solvent was evaporated. After water (45 mL) and EtOAc (2 × 40 mL) were added, the mixture was shaken. The organic phase was separated and the water phase was extracted with EtOAc (2 × 30 mL). The combined organic phases were dried over Na2SO4 and the solvent was evaporated. Bromophenol 6 (0.40 g, 93%) was obtained as pale yellow amorphous. Mp 77–78°C; 1H-NMR (400 MHz, CD3COCD3) δ 9.06 (m, 1 OH), 8.77 (m, 1 OH), 8.43 (m, 1 OH) 8.28 (m, 1 OH), 7.32 (d, J = 2.2 Hz, 1 H), 7.17 (dd, A part of AB-system, J = 8.1, 2.2 Hz, 1 H), 6.94 (d, A part of AB-system, J = 8.1 Hz, 1 H), 6.90 (d, B part of AB-system, J = 8.1 Hz, 1 H), 6.74 (d, B part of AB-system, J = 8.1 Hz, 1 H); 13C-NMR (100 MHz, CD3COCD3) δ 193.59 (CO), 150.79 (C), 146.86 (C), 145.12 (C), 143.33 (C), 133.88 (C), 129.86 (C), 124.26 (CH), 120.21 (CH), 116.69 (CH), 115.08 (CH), 113.89 (CH), 107.53 (C); IR (CH2Cl2, cm−1): 3434, 2967, 2075, 1638, 1595, 1524, 1442, 1388, 1300, 1201, 1120, 1032, 1015, 943, 816, 782, 763; Anal. Calcd for C13H9BrO5: C, C, 48.03; H 2.79 found: C, 48.01; H 2.80.
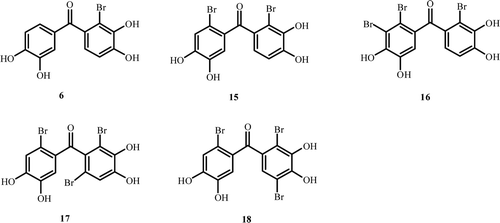
Synthesis of bromophenols 15–18 from the corresponding compounds 11–14, respectively.
The standard procedureCitation10,Citation12,Citation13,Citation35 described above for the synthesis of 6 with BBr3 was applied. From these reactions, bromophenols 15–18 were obtained.
(2-Bromo-3,4-dihydroxyphenyl)(2-bromo-4,5-dihydroxyphenyl)methanone (15)
It was crystallized from ethyl acetate/hexane as pale yellow crystals (0.382 g, 85%); mp 186–187°C; 1H-NMR (400 MHz, CD3COCD3) δ 9.29 (s, 1 OH), 8.96 (s, 1 OH), 8.53 (s, 1 OH) 8.26 (s, 1 OH), 7.11 (s, 1H), 6.96 (s, 1H), 6.91 (d, A part of AB-system, J = 8.2 Hz, 1 H), 6.86 (d, B part of AB-system, J = 8,2 Hz, 1 H); 13C-NMR (100 MHz, CD3COCD3) δ 193.29 (CO), 149.15 (C), 148.58 (C), 144.52 (C), 143.70 (C), 132.57 (C), 131.49 (C), 123.31 (CH), 120.58 (CH), 118.66 (CH), 113.64 (CH), 111.01 (C), 108.98 (C); IR (CH2Cl2, cm−1): 3368, 2947, 2834, 2526, 2041, 1655, 1594, 1452, 1419, 1295, 1115, 1032, 668; Anal. Calcd for C13H8Br2O5: C, 38.65; H 2.00. found: C, 38.64; H 2.01.
(2-Bromo-3,4-dihydroxyphenyl)(2,3-dibromo-4,5-dihydroxyphenyl)methanone (16)
Yellow amorphous (0.34 g, 95%); mp 121–123°C; 1H-NMR (400 MHz, CD3COCD3) δ 6.96 (s, 1 H), 6.91 (s, 1 H), 6.90 (s, 1 H); 13C-NMR (100 MHz, CD3COCD3), δ 193.05 (CO), 149.24 (C), 146.94 (C), 144.55 (C), 143.96 (C), 133.79 (C), 131.19 (C), 124.47 (CH), 116.19 (CH), 114.38 (C), 113.54 (CH), 113.37 (C), 109.43 (C); IR (CH2Cl2, cm−1): 3400, 2950, 2839, 2076, 1648, 1452, 1396, 1295, 1114, 1019, 667; Anal. Calcd for C13H7Br3O5: C, 32.33; H 1.46. found: C, 32.11; H 1.45.
(2-Bromo-4,5-dihydroxyphenyl)(2,6-dibromo-3,4-dimethoxyphenyl)methanone (17)
Red amorphous; (0.47 g, 87%); mp 240–242°C′(its color was changed at ≥ 180°C); 1H-NMR (400 MHz, CD3COCD3) δ 7.20 (s, 1 H), 7.15 (s, 2 H); 13C-NMR (100 MHz, CD3COCD3) δ 190.55 (CO), 150.60 (C), 147.04 (C), 144.49 (C), 143.42 (C), 133.90 (C), 122.01 (CH), 120.16 (CH), 118.75 (C), 118.29 (CH), 113.62 (C), 112.90 (C), 108.33 (C); IR (CH2Cl2, cm−1): 3681, 2973, 2863, 2071, 1654, 1587, 1495, 1476, 1454, 1286, 1213, 1144, 1054, 1033; Anal. Calcd for C13H7Br3O5: C, 32.33; H 1.46. found: C, 32.33; H 1.45.
(2-Bromo-4,5-dihydroxyphenyl)(2,5-dibromo-3,4-dihydroxyphenyl)methanone (18)
It was crystallized from ethyl acetate/hexane as pale yellow crystals (0.382 g, 85%); mp 186–188°C (its color changed at ≥ 160°C); 1H-NMR (400 MHz, CD3COCD3), 9.09 (bs, 1 OH), 9.05 (bs, 1 OH), 8.80 (bs, 1 OH) 8.58 (bs, 1 OH), δ 7.16 (s, 1 H), 7.13 (s, 1 H), 7.02 (s, 1 H); 13C-NMR (100 MHz, CD3COCD3), δ 192.02 (CO), 149.66 (C), 146.28 (C), 144.65 (C), 144.30 (C), 133.35 (C), 130.49 (C), 125.70 (CH), 120.78 (CH), 118.94 (CH), 111.33 (C), 108.45 (C), 108.15 (C); IR (CH2Cl2, cm−1): 3436, 3225, 2076, 1638, 1285, 1033, 720; Anal. Calcd for C13H7Br3O5: C, 32.33; H 1.46. found: C, 32.33; H 1.49.
CA purification assay
The purification of the CA II isozyme was performed in a simple single-step method by means of Sepharose-4B-aniline-sulfanilamide affinity column chromatoghrapyCitation36. hCA II was purified 311-fold with a specific activity of 2500 EU mg−1 and an overall yield of 16%. Erythrocytes were purified from fresh human blood obtained from the Blood Centre of the Research Hospital at Atatürk University. The blood samples were centrifuged at 1500 rpm for 15 min and the plasma and buffy coat were removed. The red cells were isolated and washed twice with 0.9% NaCl and hemolyzed with 1.5 volumes of ice-cold water. The ghost and intact cells were removed by centrifugation at 20,000 rpm for 30 min at 4°C. The pH of the hemolysate was adjusted to 8.7 with solid Tris. Firstly, Sepharose-4B was oxidized by KMnO4 and subsequently activated by SOCl2. Subsequently, aniline was attached to the activated gel as a spacer arm and finally diazotized sulfanilamide was clamped to the para position of aniline molecule as ligand. The hemolysate was applied to the prepared Sepharose 4B-aniline-sulfanylamide affinity column which had been equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human carbonic anhydrase II (hCA II) isozyme was eluted with 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6). All procedures were performed at 4°C.
Hydratase activity assay
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to our previous studiesCitation37,Citation38. CO2-hydratase activity as an enzyme unit was calculated by using the equation (t0-tc/tc) where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively.
Protein determination
Protein quantity was determined spectrophotometrically at 595 nm according to the Bradford method during the purification steps, using bovine serum albumin as the standardCitation39.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10 and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedure. A 20-μg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coomassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dyeCitation40.
Crystal structure determination
For the crystal structure determination, the single-crystal of 13 and 14 were used for data collection on a four-circle Rigaku R-AXIS RAPID-S diffractometer (equipped with a two-dimensional area IP detector). The graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å) and oscillation scans technique with Δω = 5° for one image were used for data collection. The lattice parameters were determined by the least-squares method on the basis of all reflections with F2 > 2σ (F2). Integration of the intensities, correction for Lorentz and polarization effects and cell refinement were performed using CrystalClear (Rigaku/MSC Inc., 2005) softwareCitation41. The structures were solved by direct methods using SHELXS-97Citation42 and refined by a full-matrix least-squares procedure using the program SHELXL-97. H atoms were positioned geometrically and refined using a riding model. The final difference Fourier maps showed no peaks of chemical significance. Crystal data for 13: C17H15O5Br3, crystal system, space group: triclinic, P-1; (no:2); unit cell dimensions: a = 8.2823(2), b = 10.2487(2), c = 12.7924(3) Å, α = 72.529(5), β = 68.923(5), γ = 87.664(7)°; volume: 963.71(6) Å3; Z = 2; calculated density: 1.86 mg/m3; absorption coefficient: 6.302 mm−1; F(000): 524; θ range for data collection 2.6–30.5°; refinement method: full-matrix least-square on F2; data/parameters: 3936/228; goodness-of-fit on F2: 1.267; final R indices [I > 2σ(I)]: R1 = 0.089, wR2 = 0.103; R indices (all data): R1 = 0.137, wR2 = 0.115; largest diff. peak and hole: 0.391 and −0.556 e Å−3; CCDC: 774839. Crystal data for 14: C17H15O5Br3, crystal system, space group: triclinic, P-1; (no:2); unit cell dimensions: a = 8.3610(5), b = 8.3972(5), c = 14.7542(7)Å, α = 98.097(2), β = 95.915(3), γ = 107.200(2)°; volume: 968.2(2) Å3; Z = 2; calculated density: 1.85 mg/m3; absorption coefficient: 6.273 mm−1; F(000): 524; θ range for data collection 2.6–30.5°; refinement method: full-matrix least-square on F2; data/parameters: 4293/230; goodness-of-fit on F2: 1.333; final R indices [I > 2σ(I)]: R1 = 0.086, wR2 = 0.135; R indices (all data): R1 = 0.139, wR2 = 0.146; largest diff. peak and hole: 0.367 and −0.662 e Å−3; crystallographic data were deposited in CSD under CCDC registration number 774807.
Results and discussion
We have synthesized natural product bromophenols 3 and 4 from corresponding materials by the known method as shown in Citation13. Reactions of compound 7 with 1,4-dibromo-2,3-dimethoxybenzene and 1,2,5-tribromo-3,4-dimethoxybenzene in the presence of PPA at 80°C gave methylether substituted diarylmethanes in high yields as sole product. The ether cleavage reaction of these diarylmethanes with BBr3 under mild conditions afforded naturally occurring bromophenols 3 and 4Citation13.
Scheme 1. (A) 1,4-dibromo-2,3-dimethoxybenzene, PPA/80°C, (B) BBr3/CH2Cl2, 0–25°C, (C) 1,2,5-tribromo-3,4-dimethoxybenzene, PPA/80°C.
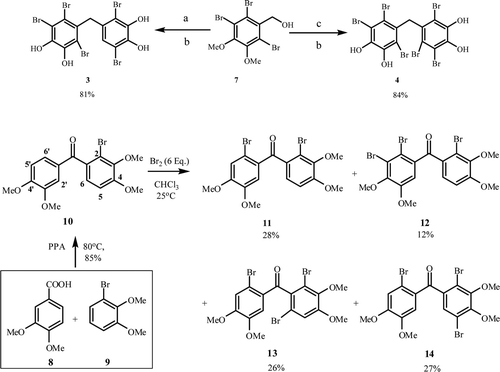
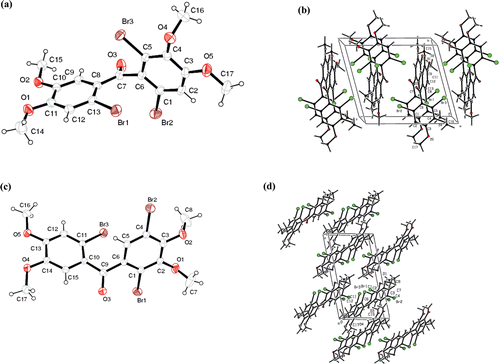
(2-Bromo-3,4-dimethoxyphenyl)(3,4-dimethoxyphenyl)methanone (10) was synthesized from the reaction of 3,4-dimethoxybenzoic acid (8) and 3-bromoveratrole (9) with PPA in 85% yield as sole product (). Bromination of monobromide 10 (in CHCl3) with Br2 (6 eq.) at RT for 3 days followed by CC allowed us to isolate four products 11–14 (). The NMR analysis of 13 and 14 did not allow determination of their structures. Therefore, the exact structures of them were determined by X-ray diffraction analysis ().
Figure 2. (A) The molecular structure of tribromide 13 showing the atom numbering scheme. (B) Packing diagram for 13. (C) The molecular structure of tribromide 14 showing the atom numbering scheme. (D) Packing diagram for 14.
Bromophenols derived from compounds 10–14 may be potential biologically active compounds, because they are similar to 5 with high antioxidant and radical scavenging activitiesCitation10. Therefore, bromophenols 6 and 15–18 were synthesized from compounds 10–14 by ether cleavage reaction with BBr3 in high yields (). Spectroscopic data of 6 and 15–18 are consistent with the proposed structures.
Inhibitory effects of the compounds on CA II catalytic activity were tested under in vitro conditions; IC50 values were calculated and are given in .
Table 1. IC50 values (concentration that causes 50% inhibition of the enzyme activity) for the molecules.
We report here the first study on the inhibitory effects of the bromophenols derivatives 3–6 and 10–18 on the hydratase activity of hCA II. The data in show the following regarding the inhibition of hCA II by bromophenol derivatives.
The strongest inhibitory activity has been observed with compounds 11, 15–18, (). Three derivatives, 3, 10, 13, showed weak hCA II inhibitory activity with IC50-s in the range of 86.4–372 μM, (), whereas the remaining four derivatives were quite effective hCA II inhibitors, with IC50-s in the range of 26.4–58 µM, (). The best hCA II inhibitor in this series of derivatives was the bulky, (2-Bromo-3,4-dihyroxyphenyl)(2,3-dibromo-4,5-dihyroxyphenyl)methanone (16), with a IC50 of 0.7 µM.
As revealed by a comparison of the inhibition ranges of molecules, 13 has a higher IC50 value than those of its isomers 12 and 14, and, likewise, 17 has a higher IC50 than those of 16 and 18.
The phenolic compounds have been investigated as CA inhibitors (CAIs) in this study. The rationale of investigating these compounds as CAIs lies in the fact that phenol has been shown to be the only competitive inhibitor with CO2 as the substrate for the main isoform of CA, i.e., human CA II (hCA II)Citation20. In a very sound study, Christianson and colleagues reported on the X-ray crystal structure for the adduct of hCA II with phenolCitation20, showing this compound to bind to CA by anchoring its OH moiety to the zinc-bound water/hydroxide ion of the enzyme active site through a hydrogen bond as well as to the NH amide of Thr199, an amino acid conserved in all α-CAs and critically important for the catalytic cycle of these enzymesCitation18,Citation19.
CAIs are a class of pharmaceuticals used as antiglaucoma agents, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, neurological disorders, or osteoporosis. Thus, discovery of novel CAIs is of great importance for pharmacological and medicinal approaches, and many inhibitors have been designed and synthesized in the literature. However, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profile when compared to sulfonamides and their bioisosteres, and to find novel applications for the inhibitors of these widespread enzymes.
Conclusions
Diphenylmethanone derivative 10 was obtained and its bromination gave dibromide 11 and tribromides 12–14. From these compounds, potential biological active bromophenols 6 and 15–18 were synthesized in high yields. The structures of the products were determined and characterized by spectroscopic methods. Bromophenol derivatives 1–13 used in this study affect the activity of CA II isozyme due to the presence of the different functional groups (OH, OCH3) in their aromatic scaffold. It has been determined in our study that compounds 15, 16 and 18 are effective inhibitors for CA II when compared to Acetazolamide, which is used as the reference inhibitor for carbonic anhydrase. Our findings here indicate thus another class of possible CAIs of interest, in addition to the well-known sulfonamides/sulfamates/sulfamides. These findings point out that substituted phenolic compounds may be used for generation of potent CAIs.
Acknowledgment
The authors are indebted to the Department of Chemistry (Atatürk University) for research conditions.
Declaration of interest
This research was financed by grants from of TÜBİTAK (The Scientific and Technological Research Council of Turkey) (Project no: TBAG-107T348).
References
- Gribble GW. Naturally Occurring Organohalogen Compounds-A Survey. J Nat Prod 1992;55:1353–1395.
- Gribble GW. The diversity of naturally occurring organobromine compounds. Chem Soc Rev 1999;28:335–346.
- Lee HS, Lee TH, Lee JH, Chae CS, Chung SC, Shin DS, Shin J, Oho KB. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J Agric Food Chem 2007;55:6923–6928.
- Xu X, Song F, Wang S, Li S, Xiao F, Zhao J, Yang Y, Shang S, Yang L, Shi J. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J Nat Prod 2004;67:1661–1666.
- Kurata K, Taniguchii K, Takashima K, Hayashi I, Suzuki M. Feeding-deterrent bromophenols from Odonthalia corymbifera. Phytochemistry 1997;45:485–487.
- Oh KB, Lee JH, Chung SC, Shin J, Shin HJ, Kim HK, Lee HS. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg Med Chem Lett 2008;18:104–108.
- Xu N, Fan X, Yan X, Li X, Niu R, Tseng CK. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003;62:1221–1224.
- Wang W, Okada Y, Shi H, Wang Y, Okuyama T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J Nat Prod 2005;68:620–622.
- Fan X, Ma C, Han L, Shi D, Liu Q. Extraction of bromophenol compounds from red algae and their uses in the treatment of diabetes and obesity. CN Patent 1,853,618 2006; Chem Abstr 2007;146:13018v.
- Balaydin HT, Gülçin Í, Menzek A, Göksu S, Sahin E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–695.
- Duan XJ, Li XM, Wang BG. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J Nat Prod 2007;70:1210–1213.
- Akbaba Y, Balaydın HT, Göksu S, Şahin E, Menzek A. Total Synthesis of the biologically active, naturally occurring 3,4-dibromo-5-[2-bromo-3,4-dihydroxy-6-(methoxymethyl)benzyl]benzene-1,2-diol and regioselective O-demethylation of aryl methyl ethers. Helv Chim Acta 2010;93:1127–1135.
- Balaydin HT, Akbaba Y, Menzek A, Sahin E, Goksu S. First and short syntheses of biologically active, naturally occurring brominated mono- and dibenzyl phenols. Arkivoc 2009;XIV:75–87.
- Ekinci D, Beydemir S, Alim Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–587.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Cankaya M, Hernandez AM, Ciftci M, Beydemir S, Ozdemir H, Budak H et al. An analysis of expression patterns of genes encoding proteins with catalytic activities. BMC Genomics 2007;8:232.
- Hilvo M, Tolvanen M, Clark A, Shen B, Shah GN, Waheed A et al. Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J 2005;392:83–92.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of mammalian isoforms I-XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–7428.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Abdülkadir Coban T, Beydemir S, Gülcin I, Gücin I, Ekinci D, Innocenti A et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg Med Chem 2009;17:5791–5795.
- Supuran CT. Diuretics: From classical carbonic anhydrase inhibitors to novel applications of the sulfonamides. Curr Pharm Des 2008;14:641–648.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–750.
- Coban TA, Beydemir S, Gülçin I, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: An in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–270.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Alici HA, Ekinci D, Beydemir S. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem 2008;41:1384–1390.
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–120.
- Ekinci D, Beydemir S. Evaluation of the impacts of antibiotic drugs on PON 1; a major bioscavenger against cardiovascular diseases. Eur J Pharmacol 2009;617:84–89.
- Ekinci D, Beydemir S. Effect of some analgesics on paraoxonase-1 purified from human serum. J Enzyme Inhib Med Chem 2009;24:1034–1039.
- Stevens RV, Bisacchi GS. An efficient and remarkably regioselective synthesis of benzocyclobutenones from benzynes and 1,1-dimethoxyethylene. J Org Chem 1982;47:2393–2396.
- Vickery EH, Pahler LF, Eisenbraun EJ. Selective O-demethylation of catechol ethers. Comparison of boron tribromide and iodotrimethylsilane. J Org Chem 1979;44:4444–4446.
- Ekinci D, Beydemir S. Risk assessment of pesticides and fungicides for acid–base regulation and salt transport in rainbow trout tissues. Pestic Biochem Phys 2010;97:66–70.
- Soyut H, Beydemir S. Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition. Protein Pept Lett 2008;15:528–535.
- Soyut H, Beydemir S, Hisar O. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 2008;123:179–190.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Cleavage of structural proteins during in assembly of the head of Bacteriophage T4. Nature 1970;227:680–685.
- Rigaku/MSC, Inc., 9009 new Trails Drive, The Woodlands, TX 77381, USA.
- Sheldrick GM. SHELXS97 and SHELXL97. Crystal structure solution and refinement programs. 1997.