Abstract
A new series of N,N′-diarylureas (1–9) was synthesized. These compounds were investigated as inhibitors of polyphenol oxidase (PPO) which had been purified from banana by an affinity gel comprised of Sepharose 4B-l-tyrosine-p-amino benzoic acid. Ki values for (1), (2), (3), (5), (6), (7) and (8) were determined as 0.285, 17.97, 0.187, 0.108, 0.063, 0.044 and 0.047 mM, respectively. Thus (2) was by far the most effective inhibitor. Interestingly, (4) and (9) behaved as an activator of PPO in this study.
Introduction
Polyphenol oxidase (PPO) (EC 1.14.18.1) is a copper-containing enzyme, widely distributed in nature, responsible for melanization in animals and browning in plantsCitation1,Citation2. PPO also catalyzes the o-hydroxylation of monophenols and the oxidation of o-diphenols to o-quinonesCitation1. Enzymatic browning of fruits is related to oxidation of phenolic endogenous compounds into highly unstable quinones, which are later polymerized to brown, red and black pigmentsCitation3. The degree of browning depends on the nature and amount of endogenous phenolic compounds, presence of oxygen, reducing substances and metallic ions, pH and temperature and the activity of PPO, the main enzyme involved in the reactionCitation4. Enzymatic browning is also an economic problem for processors and consumersCitation1,Citation5. At least five causes of browning in processed and/or stored fruits and plants are known: enzymatic browning of the phenols, Maillard reaction, ascorbic acid oxidation, caramelization and formation of browned polymers by oxidized lipidsCitation6.
Browning reactions are major causes of quality loss during harvesting, post-harvest handling/storage and processing of fruits, plants and vegetables in food industryCitation7. The enzymatic browning cause deterioration of sensory and nutritional quality and affects appearance and organoleptic properties, inactivation of PPO is desirable for preservation of foodsCitation8. Several methods such as the addition of antioxidants and the exclusion of oxygen as well as thermal processing have been used to inhibit enzymatic browning. For inactivation of PPO, thermal processing has limits like loss of sensory and nutritional quality of food products. Therefore, high pressure treatment has been considered as an alternativeCitation9,Citation10.
Ureas are very important class of carbonyl compounds. They have extensive applications such as agrochemicals, dyes for cellulose fibers, antioxidants in gasoline, resin precursors and synthetic intermediates for fine chemicals, pharmaceuticals, cosmetics and pesticidesCitation11–16. N,N′-diarylureas are valuable starting material used in organic synthesis. They have numerous applications such as drugs, pesticides, herbicides, antioxidants and anion-binding receptorsCitation17. Many urea derivatives are very important compounds because of their biological activities. In particular, several substituted ureas have recently shown an inhibiting effect on HIV protease enzymeCitation11–13.
In the present study, we have synthesized derivatives of N,N′-diarylureas for evaluation as potential inhibitors of PPO that could be beneficial in the prevention of enzymatic browning.
Materials and methods
General procedure for the synthesis of N,N′-diarylureas
Chemicals and solvents used in the study were obtained from Sigma-Aldrich and Merck and were used without further purification. Melting points were measured on Barnstead/Electrothermal 9200 melting point apparatus. 1H and 13C NMR spectra were recorded on a Varian Mercury NMR at 300 and 75 MHz instrument in CDCl3, respectively. IR spectra were obtained with Shimadzu IR Prestige 21.
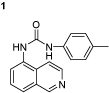
1-(isoquinolin-5-yl)-3-p-tolylurea (1): 1-isocyanato-4-methylbenzene (6.94 mmol, 0.924 g) was dissolved in toluene (10 ml) and added dropwise into the stirred solution of 5-aminoisoquinoline (6.94 mmol, 1 g) in tetrahydrofuran (15 ml). The reaction mixture was stirred at 60°C overnight. The precipitate was collected by filtration and washed with acetone (5 ml) and dried under vacuum at 40°C. Yield 95%; mp: 251–252°C; 1H NMR (DMSO-d6): δ 2.2 (s, 3H), 7.0–7.1 (d, 2H), 7.36–7.39 (d, 2H), 7.56–7.59 (dd, 1H), 7.69–7.7 (d, 1H), 7.7–7.71 (d, 1H), 8.0–8.06 (d, 1H), 8.49–8.52 (d, 1H), 8.89 (d, 1H), 8.9 (s, 1H), 8.9 (s, 1H); 13C NMR (DMSO-d6): δ 21.05, 117.99, 118.97 (2C), 121.40, 121.74, 124.40, 129.96 (2C), 130.12, 130.85, 131.51, 135.54, 137.70, 148.86, 150.98, 153.55; FT-IR ν (cm−1): 3140, 2966, 1595, 1541, 1498, 1444, 1332, 1263; (MH+): 276.1.
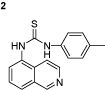
1-(isoquinolin-5-yl)-3-p-tolylthiourea (2): The experimental procedure is the same as described above. Yield 90%; mp: 184–185°C; 1H NMR (DMSO-d6): δ 3.26 (s, 3H), 7.11–7.14 (d, 2H), 7.33–7.36 (d, 2H), 7.54–7.56 (d, 1H), 7.55–7.58 (d, 1H), 7.71–7.76 (t, 1H), 7.91–7.94 (d, 1H), 8.31–8.34 (d, 1H), 8.8 (s, 1H), 9.7 (s, 1H), 9.8 (s, 1H); 13C NMR (DMSO-d6): δ 21.23, 121.99, 125.05 (2C), 126.05, 126.16, 128.29, 129.62 (2C), 129.72, 132.67, 134.68, 136.37, 137.45, 148.99, 151.17, 182.14; FT-IR ν (cm−1): 3134, 2972, 1595, 1541, 1498, 1444, 1332, 1263; MS(MH+): 293.1.
General reaction
Purification of PPO
All purification steps were carried out at 25°C. The extraction procedure was adopted from Wesche-Ebeling and MontgomeryCitation18. The bananas were washed with distilled water three times to prepare the crude extract, 50 g of bananas were cut quickly into thin slices and homogenized in a Waring blender for 2 min using 100 ml of 0.1 M phosphate buffer, pH 7.3 containing 5% poly(ethylene glycol) and 10 mM ascorbic acid. After filtration of the homogenate through muslin, the filtrate was centrifuged at 15,000g for 30 min, and the supernatant was collected. A crude protein precipitate was made by adding (NH4)2SO4 to 80% saturation. The resulting precipitate was suspended in a minimum volume of 5 mM phosphate buffer and then dialyzed against 5 the same buffer overnight. The enzyme solution was then applied to the Sepharose 4B-tyrosine-p-amino benzoic acid affinity columnCitation7, pre-equilibrated with 5 mM phosphate buffer, pH 5.0. The affinity gel was extensively washed with the same buffer before the banana PPO (BPPO) was eluted with 1 M NaCl, 5 mM phosphate, pH 7.0.
BPPO activity
Enzyme activity was determined using catechol by measuring the increase in absorbance at 420 nmCitation19 in a Biotek automated recording spectrophotometer. Enzyme activity was calculated from the linear portion of the curve. One unit of PPO activity was defined as the amount of enzyme that causes an increase in absorbance of 0.001 unit/min for 1 ml of enzyme at 25°CCitation7.
Inhibition of BPPO activity
An aliquot of each inhibitor at various final concentrations was added to the standard reaction solution immediately before the addition of enzyme extract. The concentration of inhibitor (diarylureas) giving 50% inhibition was determined from a plot of residual activity against inhibitor concentration, with 10 mM catechol as substrate. The control was activity without inhibitor.
Results and discussion
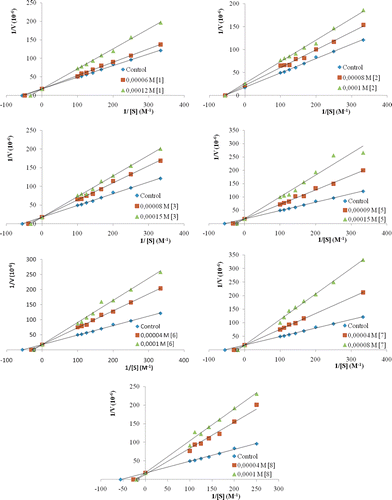
The inhibition type of diarylureas was determined by Lineweaver–Burk plots of 1/V versus 1/S at two inhibitor concentrations (). The inhibition constant, Ki, was deduced from the points of interception of the plots. Depending on kinetic analysis, competitive inhibition (1, 3, 5, 6, 7, 8) and uncompetitive inhibition (2) were all seen in this study (). Surprisingly, neither (4) nor (9) had much of an inhibiting effect, in any of the conditions used. Ki values of 0.285, 17.97, 0.187, 0.108, 0.063, 0.044 and 0.047 mM were obtained for (1), (2), (3), (5), (6), (7) and (8), respectively. Chilaka et al. reported that thiourea was a good inhibitor of PPO, with low Ki value of 0.15 mM and inhibition of PPO was uncompetitiveCitation20. We determined that (6), (7) and (8) were a better inhibitor of PPO according to thiourea. Gulcin et al. reported that sodium diethyl dithiocarbamate was the most effective inhibitor (Ki: 1.79 × 10−6 mM) on nettle PPOCitation21.
Table 1. The Ki values of diarylureas on BPPO.
Several compounds reported as PPO inhibitors were also shown to have inhibitors effect on the BPPO. The results from inhibitor studies in other plant tissues showed thiol reagents as the most effective inhibitors for those enzymesCitation22,Citation23. Reducing agents, antioxidants and enzymatic inhibitors prevent browning chemically by reducing the o-quinones. The effect of these reducing agents can be considered as temporary because these compounds are oxidized irreversibly by reaction with pigment intermediates, endogenous enzymes and metals such as copper. Among sulphur-containing agents, l-cysteine is an effective compound to prevent enzymic browning. Direct inhibition of PPO by cysteine through the formation of stable complexes with copper has also been proposedCitation24. Halim and Montgomery showed in a series of publications that Cys can inhibit enzymic browning of pear juice concentrate more effectively than sulphiteCitation25. Kahn used Cys to inhibit browning of cut or pureed avocados and bananasCitation26. Among the tested anti-browning reagent, the most effective ones were dithiothreitol and sodium metabisulphiteCitation23. The action of sulphite in the prevention of enzymatic browning can usually be explained by several processes. One is the action on o-quinones. The formation of quinone–sulphite complexes prevents the quinone polymerizationCitation27. A further action of metabisulphite on PPO is directly on the enzyme structure leading to the inactivation of PPO. Golan-Goldhirsh and Whitaker and Embs and Markakis found that during pre-incubation of PPO with sulphite (dithiothreitol, glutathione), there was a gradual loss in the ability of the enzyme to cause browningCitation27,Citation28. It has been suggested that sulphite reacts with disulphide bonds with PPO. This leads to the change in tertiary structure of enzyme and inactivation. The third process leading to PPO inhibition by bisulphate is via reduction of the intermediate quinones as described for ascorbic acidCitation27. The enzyme also seemed to be sensitive to thiourea because PPO contains copper as a co-factor, the irreversible inactivation of this enzyme can be effected by substances (such as thiol compounds thiourea, -hydroxyquinoline, etc.), which remove copper from the active site of the enzymeCitation29. Because sulphur is much more polarizable than oxygen, in this case, as already mentioned, a covalent bond is formed by donation of one of the S lone pairs into the empty 4s orbital of Cu. As sulphur is much “softer” than oxygen it acts as a buffer of the polarization effects caused by the metal cation, and these effects are not transmitted to the rest of the molecule in a significant amount. Hence, in this case, the conjugation of the near aminogroup is much smaller than in urea and both C–N bonds are almost equalCitation30.
Declaration of interest
The work was financially supported by TUBITAK (TBAG-110T133).
References
- Gowda LR, Paul B. Diphenol activation of the monophenolase and diphenolase activities of field bean (Dolichos lablab) polyphenol oxidase. J Agric Food Chem 2002;50:1608–1614.
- Shellby KS, Popham HJR. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J Insect Sci 2006;13:2442–2448.
- Blumenthal M, Goldberg A, Brinckman J. (2000). Herbal medicine: Expanded commission E monographs. Austin, TX: American Botanical Council.
- Nunez-Delicado E, Sanchez-Ferrer A, Garcia-Carmona FF, Lopez-Nicolas JM. Effect of organic farming practices on the level of latent polyphenol oxidase in grapes. J. Food Sci 2005;70:74–85.
- Marshall MR, Kim J, Wei C. Enzymatic browning in fruits, vegetables and seafoods, 2000. FAO. 1–56.
- Pizzocaro F, Torreggiani D, Gilardi G. Inhibition of apple polyphenol oxidase by ascorbic acid, citric acid and sodium chloride. J Food Process Preserv 1993;17:21–30.
- Arslan O, Erzengin M, Sinan S, Ozensoy O. Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem 2004;88:479–484.
- Hendrickx M, Ludikhuyze Van den Broeck I, Weemaes C. Effects of high pressure on enzymes related to food quality. Trends Food Sci Technol 1998;9:197–203.
- Asaka M, Hayashi R. Activation of polyphenol oxidase in pear fruit by high pressure treatment. Agric Biol Chem 1991;5:2439–2440.
- Knorr D. Effects of high hydrostatic pressure processes on food safety and quality. Food Technol 1993;47:156–161.
- Gabriele B, Salerno G, Mancuso R, Costa M. Efficient synthesis of ureas by direct palladium-catalyzed oxidative carbonylation of amines. J Org Chem 2004;69:4741–4750.
- Zheng S, Li F. A novel and efficient (NHC)CuI (NHC=N-heterocyclic carbene) catalyst for the oxidative carbonylation of amino compounds. Tetrahedron Lett 2007;48:5883–5886.
- Chiarotto I, Feroci M. Selective and environmentally friendly methodologies based on the use of electrochemistry for fine chemical preparation: an efficient synthesis of N,N’-disubstituted ureas. J Org Chem 2003;68:7137–7139.
- Atanassova IA, Petrov JS. The application of N-substituted trichloroacetamides as in situ isocyanate generating reagents for the synthesis of acylureas and sulfonylureas. Synthesis 1987;1987:734–736.
- Nomura R, Hasegawa Y. Carbonylation of amines by carbon dioxide in the presence of an organoantimony catalyst. J Org Chem 1992;57:7339–7342.
- Shi F, Deng Y. Alternatives to phosgene and carbon monoxide:synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angew Chem Int Ed 2003;42:3257–3260.
- Artamkinaa GA, Sergeeva AG. Palladium-catalyzed reaction of aryl halides with ureas. Tetrahedron Lett 2001;42:4381–4384.
- Wesche-Ebeling P, Montgomery MW. Strawberry polyphenol oxidase: extraction and partial characterization. J Food Sci 1990;55:1320–1325.
- Chilaka FC, Eze S, Anyadiegwu C, Uvere PO. Browning in processed yams: peroxidase or polyphenol oxidase? J Sci Food Agric 2002;82:899–903.
- Gulcin I, Kufrevioglu OI, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302.
- Espin JC, Morales M, Varon R, Tudela J, Garcia-Canovas F. A continuous spectrophotometric method for determining the monophenolase and diphenolase activities of apple polyphenol oxidase. Anal Biochem 1995;43:2807–2812.
- Raymond J, Rakariyatham N, Azanza JL. Purification and some properties of polyphenol oxidase from sunflower seeds. Phytochemistry 1993;34:927–931.
- Sayaverde-Soto LA, Montgomery MW. Inhibition of polyphenol oxidase by sulfite. J Food Sci 1986;51:1531–1535.
- Nicolas JJ, Richard-Forget FC, Goupy PM, Amiot MJ, Aubert SY. Enzymatic browning reactions in apple and apple products. Crit Rev Food Sci Nutr 1994;34:109–157.
- Halim DH, Montgomery MW. Polyphenol oxidase of d’Anjou pears. J Food Sci 1978;43:603–610.
- Kahn V. Effect of proteins, protein hydrolyzates and amino acids on o-dihydroxy phenolase activity of polyphenol oxidase of mushroom, avocado and banana. J Food Sci 1985;50:111–115.
- Embs RJ, Markakis P. The mechanism of sulphite inhibition of browning caused by polyphenol oxidase. J Food Sci 1965;30:753–758.
- Golan-Goldhirsh A, Whitaker JR. Effect of ascorbic acid, sodium bisulfite and thiol compounds on mushroom polyphenol oxidase. J Agric Food Chem 1984;32:1003–1008.
- Schwimmer S, eds. (1981) Source book of food enzymology. Westport, CT: AVI Publishing, 267–274.
- Trujillo C, Lamsabhi AM, Mó O, Yáñez M. The importance of the oxidative character of doubly charged metal cations in binding neutral bases. [Urea-M]2+ and [thiourea-M]2+ (M = Mg, Ca, Cu) complexes. Phys Chem Chem Phys 2008;10:3229–3235.
Appendix
1H, 13C NMR and IR spectral of diarylureas
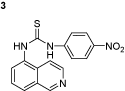
1-(isoquinolin-5-yl)-3-(4-nitrophenyl)thiourea (3): The experimental procedure is the same as described above. Yield 92%; mp: 264–265°C; 1H NMR (DMSO-d6): δ 3.3 (s, 3H), 7.1–7.13 (d, 1H), 7.13–7.16 (d, 1H), 7.48–7.51 (d, 1H), 7.49–7.52 (d, 1H), 7.56–7.6 (d, 1H), 7.7–7.72 (d, 1H), 7.2–7.5 (d, 1H), 8–8.02 (d, 1H), 8.48–8.51 (d, 1H), 8.8 (s, 1H), 8.9 (s, 1H), 9 (s, 1H); 13C NMR (DMSO-d6): δ 115.93, 116.23, 118.41, 120.59 (2C), 121.46, 121.96, 124.66, 130.10 (2C), 130.93, 135.40, 136.65, 148.85, 151.01, 153.67; FT-IR ν (cm−1): 3140, 2972, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 324.07.

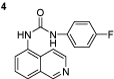
1-(4-fluorophenyl)-3-(isoquinolin-5-yl)urea (4): The experimental procedure is the same as described above. Yield 85%; mp: 182–183°C; 1H NMR (DMSO-d6): δ 7.55–7.57 (d, 1H), 7.58–7.61 (d, 1H), 7.75–7.78 (t, 1H), 7.81–7.89 (d, 2H), 7.92–7.99 (d, 1H), 8.19–8.22 (d, 2H), 8.34–8.37 (d, 1H), 8.92 (s, 1H), 10.3 (s, 1H), 10.5 (s, 1H); 13C NMR (DMSO-d6): δ 122.17, 122.69, 124.99, 125.80, 126.17, 128.74, 129.79, 132.56, 135.83, 143.17, 146.94, 148.97, 151.35, 182.09; FT-IR ν (cm−1): 3140, 2972, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 281.1.

1-(3-iodophenyl)-3-(isoquinolin-5-yl)thiourea (5): The experimental procedure is the same as described above. Yield 89%; mp: 185–186°C; 1H NMR (DMSO-d6): δ 7.0–7.13 (t, 1H), 7.45–7.48 (d, 1H), 7.49–7.51 (d, 1H), 7.52–7.56 (d, 1H), 7.52–7.59 (d, 1H), 7.7–7.75 (t, 1H), 7.92–7.94 (d, 1H), 7.97 (s, 1H), 8.30–8.34 (d, 1H), 8.9 (s, 1H), 9.9 (s, 1H), 10.0 (s, 1H); 13C NMR (DMSO-d6): δ 94.55, 122.14, 124.13, 125.95, 126.18, 128.55, 129.81, 131.00, 132.61, 132.87, 133.75, 135.99, 141.66, 149.00, 151.28, 182.12; FT-IR ν (cm−1): 3145, 2966, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 405.26.

1-(3-fluorophenyl)-3-(isoquinolin-5-yl)thiourea (6): The experimental procedure is the same as described above. Yield 86%; mp: 186–187°C; 1H NMR (DMSO-d6): δ 6.8–7.0 (t, 1H), 7.2–7.3 (d, 1H), 7.34–7.4 (t, 1H), 7.5 (s, 1H), 7.51–7.53 (d, 1H), 7.51–7.54 (d, 1H), 7.78–7.81 (t, 1H), 7.95–8.0 (d, 1H), 8.34–8.39 (d, 1H), 8.9 (s, 1H), 10.0 (s, 1H), 10.01 (s, 1H); 13C NMR (DMSO-d6): δ 120.12, 122.11, 125.95, 126.20, 128.51, 129.77, 130.58, 130.71, 132.65, 136.08, 141.90, 142.04, 148.97, 151.27, 160.83, 182.10; FT-IR ν (cm−1): 3140, 2966, 1595, 1541, 1498, 1444, 1332, 1263; MS(MH+): 297.07.
1-(2-fluorophenyl)-3-(isoquinolin-5-yl)thiourea (7): The experimental procedure is the same as described above. Yield 92%; mp: 184–185°C; 1H NMR (DMSO-d6): δ 7.1–7.2 (m, 2H), 7.2–7.26 (d, 1H), 7.5–7.6 (m, 2H), 7.56–7.59 (d, 1H), 7.73–7.78 (t, 1H), 7.95–7.98 (d, 1H), 8.25–8.37 (d, 1H), 8.9 (s, 1H), 9.5 (s, 1H), 10.09 (d, 1H); 13C NMR (DMSO-d6): δ 116.212, 116.479, 121.912, 124.615, 125.990, 126.173, 127.910, 128.025, 128.494, 129.292, 129.602, 132.526, 135.955, 148.940, 151.048, 182.962; FT-IR ν (cm−1): 3140, 2972, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 297.07.
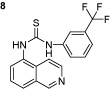
1-(isoquinolin-5-yl)-3-(3-(trifluoromethyl)phenyl)thiourea (8): The experimental procedure is the same as described above. Yield 96%; mp:186–187°C; 1H NMR (DMSO-d6): δ 7.4 (s, 1H), 7.5–7.56 (t, 1H), 7.58–7.59 (d, 1H), 7.61–7.62 (d, 1H), 7.9–8.0 (t, 1H), 8.36–8.39 (d, 1H), 8.9 (s, 1H), 10.0 (s, 1H), 10.2 (s, 1H); 13C NMR (DMSO-d6): δ 120.91, 121.55, 122.18, 122.951, 125.92, 126.20, 128.47, 128.59, 129.38, 129.80, 129.86, 130.13, 132.61, 135.83, 141.14, 148.96, 151.29; FT-IR ν (cm−1): 3140, 2972, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 347.36.

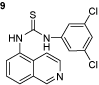
1-(3,5-dichlorophenyl)-3-(isoquinolin-5-yl)thiourea (9): The experimental procedure is the same as described above. Yield 96%; mp:170–171°C; 1H NMR (DMSO-d6): δ 339 (s, 3H), 7.3 (s, 1H), 7.5–7.58 (d, 1H), 7.58–7.60 (d, 1H), 7.6 (s, 2H), 7.78–7.81 (t, 1H), 7.98–8.0 (d, 1H), 8.3–8.34 (d, 1H), 8.9 (s, 1H), 10.0 (s, 1H), 10.2 (s, 1H); 13C NMR (DMSO-d6): δ 122.206, 122.634, 124.230, 125.883, 126.219, 128.746, 129.816, 132.584, 134.088, 135.775, 142.782, 149.001, 151.334, 182.180; FT-IR ν (cm−1): 3140, 2972, 1597, 1541, 1498, 1444, 1332, 1263; MS(MH+): 347.01.