Abstract
This study presents the synthesis and in vitro pharmacological evaluations of novel 2-(4-cyanophenyl amino)-4-(6-bromo-4-quinolinyloxy)-6-piperazinyl (piperidinyl)-1,3,5-triazines. The title compounds were assayed for their in vitro antimicrobial activity against eight bacteria (Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhi, Proteus vulgaris, Shigella flexneria) and four fungi (Aspergillus niger, Aspergillus fumigatus, Aspergillus clavatus, Candida albicans) using paper disc diffusion and agar streak dilution method as well as against Mycobacterium tuberculosis H37Rv strain using BACTEC MGIT and Lowenstein-Jensen MIC method. The bioassay results indicate that nine compounds namely 5d, 5h, 5n, 5p, 5q, 5r, 5s, 5t and 5u could be considered as possible potential agents with dual antimicrobial and antimycobacterial activities. The structures of the compounds were elucidated with the aid of IR, 1H NMR, 13C NMR, 19F NMR spectroscopy and CHN analysis.
Introduction
Bacterial infections have been most deleterious to the human health due to pathogenic microbes which continuously evolve resistance to currently used antibacterial agents. In the developing countries, this problem is especially alarming. Furthermore, increased numbers of patients with impaired immunity may further increase the burden of antimicrobial resistanceCitation1. Thus development of novel antimicrobial drugs is crucial need to combat with the multidrug-resistant infections. However, another most worrisome trend in recent years is the increase in multidrug-resistant tuberculosis strains due to the quiescent form of mycobacterium tuberculosis strains with an estimated 2 million deaths each yearCitation2–4. The WHO has estimated that, According to the stop TB partnership’s global plan to stop TB, 2006–2015, 1.3 million MDR-TB cases will need to be treated in the 27 high MDR-TB burden countries between 2010 and 2015. In 2008, there were an estimated 8.9–9.9 million incident cases of TB, 9.6–13.3 million prevalent cases of TB, 1.1–1.7 million deaths from TB among HIV-negative people and an additional 0.45–0.62 million TB deaths among HIV-positive people (classified as HIV deaths in the International Statistical Classification of Diseases), with best estimates of 9.4 million, 11.1 million, 1.3 million and 0.52 million, respectivelyCitation5. Hence, there is an urgent need to identify new regimens involving novel mechanism of action and synthetically feasible to address resistance crisis as well as the global health emergency.
As part of our ongoing studies in developing new active s-triazinyl analoguesCitation6–8, here in we report the synthesis and pharmacological activities of novel 2-(4-cyanophenyl amino)-4-(6-bromo-4-quinolinyloxy)-6-piperazinyl (piperidinyl)-1,3,5-triazines. 1,3,5-Triazine analogues constitute an important class of the realm of heterocycles, which has attracted much synthetic interest due to their wide range of biological activities such as antimicrobialCitation9–11, anticancerCitation12,Citation13, antimalarialCitation14, and antiviralCitation15 activity. A literature survey revealed that piperazines and substituted piperazines are an important family of heterocyclic compounds attracting significant interest in medicinal chemistryCitation16–20. Recently several s-triazine derivatives bearing morpholine, piperidine and some piperazine moieties are reported to possess potent antimycobacterial activityCitation21, while quinoline-based anti-TB compound TMC207 is currently in Phase II clinical trials with very promising activity against MDR-TBCitation22,Citation23. Recently, several scaffolds-based on bromo-substituted quinoline moiety and some piperazine derivatives are reported to possess potent pharmacological activitiesCitation24–26. This prior literature survey encouraged us to envisage the combination of these separate pharmacophoric groups of similar activity in a compact system to identify new candidates that may be of value in designing new, potent, selective and less toxic biologically active agents.
Materials and methods
Melting points were determined in open capillaries on a Veego electronic apparatus VMP-D (Veego Instrument Corporation, Mumbai, India) and are uncorrected. IR spectra (4000–400 cm−1) of synthesized compounds were recorded on a Shimadzu 8400-S FT-IR spectrophotometer (Shimadzu India Pvt. Ltd., Mumbai, India) using KBr pellets. Thin layer chromatography was performed on object glass slides (2 × 7.5 cm) coated with silica gel-G and spots were visualized under UV irradiation. 1H NMR and 13C NMR spectra were recorded on a Varian 400 MHz model spectrometer (Varian India Pvt. Ltd., Mumbai, India) using DMSO as a solvent and TMS as internal standard with 1H resonant frequency of 400 MHz and 13C resonant frequency of 100 MHz. 19F NMR spectra were obtained on the same spectrometer using CDCl3 as a solvent and CFCl3 as an external standard, positive for downfield shift with 19F resonant frequency of 400 MHz. The 1H NMR, 13C NMR and 19F NMR chemical shifts were reported as parts per million (ppm) downfield from TMS (Me4Si) and CFCl3 and were performed at centre for excellence, Vapi, India. The splitting patterns are designated as follows; s, singlet; br s, broad singlet; d, doublet; m, multiplet. Elemental analyses (C, H, N) were performed using a Heraeus Carlo Erba 1180 CHN analyzer (Hanau, Germany).
4-[4,6-Dichloro-1,3,5-triazin-2-ylamino]-benzonitrile (1) was synthesized according to reported literatureCitation27.
4-(4-(6-bromoquinolin-4-yloxy)-6-chloro-1,3,5-triazin-2-ylamino)-benzonitrile (3).
To a stirred solution of 6-bromo-4-hydroxyquinoline (8 g, 0.035 mol) in anhydrous THF (150 mL) 60% NaH (0.84 g, 0.035 mol) was added at room temperature during 1 h and 1 (9.31 g, 0.035 mol) was then added to the mixture. Stirring was continued for another 20 h at 45°C. Progress of the reaction was monitored by TLC using toluene: acetone (7:3) as eluent. The mixture was treated with crushed ice, filtered and dried to afford 3Citation28. M.P. 282–283°C, yield 80%, IR (KBr) cm−1, 2221 (C≡N), 1261 (C-O-C). (see and ).
Figure 1. Schematic diagram for the synthesis of 2-(4-cyanophenyl amino)-4-(6-bromo-4-quinolinyloxy)-6-piperazinyl (piperidinyl)-1,3,5-triazines.
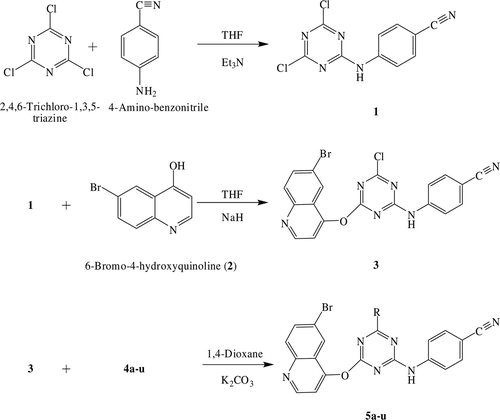
General procedure for preparation of compounds 5a-u
To a solution of 3 (0.01 mol) in 1,4-dioxane (30 mL), the respective substituted piperazine derivative was added and the reaction mixture was refluxed for 13–25 h. Potassium carbonate (0.01 mol) was used for neutralization of the reaction mixture. Progress of the reaction was monitored by TLC using toluene: acetone (8:2) as eluent. The mixture was then treated with crushed ice and neutralized by dilute HCl. The precipitate thus obtained was filtered off, dried and recrystallized from THF to afford the desired compounds 5a-u.
5a M.P. 242–244°C, yield 88% (found: C 55.59, H 3.96, N 21.61, C24H21BrN8O, calc: C 55.71, H 4.09, N 21.66%). IR (KBr) cm−1, 3280 (-NH), 2224 (C≡N), 1247 (C-O-C), 839 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.13 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.78 (d, J = 1.8 Hz, 1H, C5 proton of quinoline), 8.58 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.46 (s, 1H, C3 proton of quinoline), 8.26 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.02 (dd, J = 7.7, 1.8 Hz, 1H), 7.54–7.35 (m, 6H, Ar-H), 3.79 (br s, 4H, piperazine), 3.56 (br s, 4H, piperazine), 1.98 (s, 1H, N-CH3); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.4 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 154.2, 151.1 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 146.1–120.7 (12C, aromatic carbon atoms), 105.1 (1C, C≡N), 99.1 (1C, -C-C≡N), 48.7, 44.1 (4C, piperazine), 21.3 (1C, -CH3).
5bM.P. 235–236°C, yield 87% (found: C 56.41, H 4.21, N 20.97, C25H23BrN8O, calc: C 56.50, H 4.36, N 21.09%). IR (KBr) cm−1, 3301 (-NH), 2222 (C≡N), 1256 (C-O-C), 831 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.21 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.74 (d, J = 1.6 Hz, 1H, C5 proton of quinoline), 8.66 (d, J = 8.9 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.53 (s, 1H, C3 proton of quinoline), 8.31 (d, J = 7.7 Hz, 1H, C8 proton of quinoline), 8.11 (dd, J = 7.8, 1.2 Hz, 1H), 7.49–7.36 (m, 4H, Ar-H), 3.77 (br s, 4H, piperazine), 3.51 (br s, 4H, piperazine), 2.35 (q, J = 7.3 Hz, 2H, N-CH2), 1.88 (t, J = 6.7 Hz, 3H, CH2-CH3); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.6 (1C, C-6, s-triazine, C-N at piperazine linkage), 167.1 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.1 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 153.2, 148.0 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 144.8–121.9 (12C, aromatic carbon atoms), 105.8 (1C, C≡N), 95.7 (1C, -C-C≡N), 49.8, 42.3, 38.9 (5C, 4C of piperazine and 1C of N-CH2-CH3), 19.9 (1C, CH2-CH3).
5c M.P. 265–267°C, yield 82% (found: C 56.87, H 3.49, N 18.16, C29H22BrClN8O, calc: C 56.74, H 3.61, N 18.25%). IR (KBr) cm−1, 3280 (-NH), 2221 (C≡N), 1258 (C-O-C), 832 (s-triazine C-N str.), 756 (C-Cl); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.11 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.81 (d, J = 2.2, 1H, C5 proton of quinoline), 8.68 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.49 (s, 1H, C3 proton of quinoline), 8.26 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.16 (dd, J = 7.7, 1.4 Hz, 1H), 7.58–7.35 (m, 8H, Ar-H), 3.83 (br s, 4H, piperazine), 3.46 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.7 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.5 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 152.9, 148.2 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 146.3–119.9 (18C, aromatic carbon atoms), 106.1 (1C, C≡N), 97.7 (1C, -C-C≡N), 49.2, 47.5 (4C, piperazine).
5d M.P. 271–273°C, yield 81% (found: C 53.63, H 3.18, N 17.34, C29H21BrCl2N8O, calc: C 53.72, H 3.26, N 17.28%). IR (KBr) cm−1, 3291 (-NH), 2224 (C≡N), 1253 (C-O-C), 830 (s-triazine C-N str.), 749 (C-Cl); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.18 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.86 (d, J = 1.8, 1H, C5 proton of quinoline), 8.62 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.47 (s, 1H, C3 proton of quinoline), 8.29 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.09 (dd, J = 7.6, 1.4 Hz, 1H), 7.61–7.37 (m, 7H, Ar-H), 3.85 (br s, 4H, piperazine), 3.51 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 175.9 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.6 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 153.1, 148.7 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 145.3–117.3 (18C, aromatic carbon atoms), 105.5 (1C, C≡N), 98.1 (1C, -C-C≡N), 50.2, 42.8 (4C, piperazine).
5e M.P. 206–208°C, yield 77% (found: C 57.45, H 4.09, N 19.37, C24H20BrN7O, calc: C 57.38, H 4.01, N 19.52%). IR (KBr) cm−1, 3297 (-NH), 2222 (C≡N), 1256 (C-O-C), 835 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.20 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.80 (d, J = 1.7, 1H, C5 proton of quinoline), 8.66 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.50 (s, 1H, C3 proton of quinoline), 8.33 (d, J = 7.3 Hz, 1H, C8 proton of quinoline), 8.15 (dd, J = 7.6, 1.4 Hz, 1H), 7.52–7.40 (m, 4H, Ar-H), 3.81 (t, J = 4.9 Hz, 4H, piperidine), 3.63 (t, J = 5.7 Hz, 4H, piperidine), 1.55–1.51 (m, 2H, piperidine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.4 (1C, C-6, s-triazine, C-N at piperidine linkage), 166.7 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.6 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 152.5, 151.1 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 148.2–125.2 (12C, aromatic carbon atoms), 104.9 (1C, C≡N), 96.4 (1C, -C-C≡N), 54.9, 47.7, 38.6 (5C, piperidine).
5f M.P. 223–224°C, yield 80% (found: C 54.63, H 3.62, N 19.31, C23H18BrN7O2, calc: C 54.77, H 3.60, N 19.44%). IR (KBr) cm−1, 3288 (-NH), 2221 (C≡N), 1423 (Morpholine C-O-C str.), 1261 (C-O-C), 832 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.17 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.82 (d, J = 1.8, 1H, C5 proton of quinoline), 8.59 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.53 (s, 1H, C3 proton of quinoline), 8.30 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.13 (dd, J = 7.8, 1.3 Hz, 1H), 7.55–7.41 (m, 4H, Ar-H), 2.42–2.35 (m, 4H, -CH2, morpholine), 1.72–1.67 (m, 2H, -CH2, morpholine), 1.24–1.20 (m, 2H, -CH2, morpholine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at morpholine linkage), 165.7 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.4 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 154.3, 150.9 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 149.2–123.1 (12C, aromatic carbon atoms), 105.2 (1C, C≡N), 98.8 (1C, -C-C≡N), 57.1, 53.3 (4C, morpholine ring carbon atoms).
5g M.P. 282–283°C, yield 82% (found: C 60.24, H 3.92, N 19.46, C29H23BrN8O, calc: C 60.11, H 4.00, N 19.34%). IR (KBr) cm−1, 3285 (-NH), 2221 (C≡N), 1251 (C-O-C), 831 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.15 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.77 (d, J = 1.8, 1H, C5 proton of quinoline), 8.64 (d, J = 8.4 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.47 (s, 1H, C3 proton of quinoline), 8.28 (d, J = 7.6 Hz, 1H, C8 proton of quinoline), 8.16 (dd, J = 7.2, 1.4 Hz, 1H), 7.54–7.30 (m, 9H, Ar-H), 3.80 (br s, 4H, piperazine), 3.53 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.7 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.0 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 153.3, 149.8 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 145.9–119.3 (18C, aromatic carbon atoms), 104.9 (1C, C≡N), 97.2 (1C, -C-C≡N), 52.0, 48.2 (4C, piperazine).
5h M.P. 234–235°C, yield 77% (found: C 55.15, H 3.81, N 20.43, C25H21BrN8O2, calc: C 55.06, H 3.88, N 20.55%). IR (KBr) cm−1, 3281 (-NH), 2223 (C≡N), 1709 (-C=O), 1489 (-CH3), 1258 (C-O-C), 828 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.22 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.79 (d, J = 1.6, 1H, C5 proton of quinoline), 8.65 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.49 (s, 1H, C3 proton of quinoline), 8.32 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.08 (dd, J = 7.6, 1.4 Hz, 1H), 7.47–7.34 (m, 4H, Ar-H), 3.84 (br s, 4H, piperazine), 3.48 (br s, 4H, piperazine), 2.39 (s, 3H, COCH3); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.9 (1C, C-6, s-triazine, C-N at piperazine linkage), 169.1, 167.2 (2C, 1C at C-4, s-triazine, C-O-C at quinoline linkage and 1C at N-COCH3), 163.3 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 155.2, 151.2 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 147.2–128.1 (12C, aromatic carbon atoms), 104.9 (1C, C≡N), 99.4 (1C, -C-C≡N), 48.6, 44.6 (4C, piperazine), 23.1 (1C, COCH3).
5i M.P. 256–257°C, yield 75% (found: C 57.11, H 4.56, N 20.61, C26H25BrN8O, calc: C 57.25, H 4.62, N 20.54%). IR (KBr) cm−1, 3282 (-NH), 2221 (C≡N), 1399 (isopropyl), 1255 (C-O-C), 836 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.23 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.84 (d, J = 1.8, 1H, C5 proton of quinoline), 8.61 (d, J = 8.6 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.51 (s, 1H, C3 proton of quinoline), 8.34 (d, J = 7.9 Hz, 1H, C8 proton of quinoline), 8.04 (dd, J = 7.6, 1.4 Hz, 1H), 7.51–7.36 (m, 4H, Ar-H), 3.88 (br s, 4H, piperazine), 3.44 (br s, 4H, piperazine), 2.81–2.83 (m, 1H, N-CH), 1.96 (d, J = 6.9 Hz, 6H, -2CH3); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.2 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.4 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.3 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 152.7, 148.9 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 146.4–119.9 (12C, aromatic carbon atoms), 106.2 (1C, C≡N), 96.7 (1C, -C-C≡N), 53.2, 50.3, 39.2 (5C, 4C of piperazine and 1C of N-CH), 23.1 (2C, 2CH3).
5j M.P. 254–256°C, yield 72% (found: C 57.85, H 3.69, N 21.83, C28H22BrN9O, calc: C 57.94, H 3.82, N 21.72%). IR (KBr) cm−1, 3309 (-NH), 2218 (C≡N), 1252 (C-O-C), 831 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.19 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.82 (d, J = 1.9, 1H, C5 proton of quinoline), 8.69 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.47 (s, 1H, C3 proton of quinoline), 8.25 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.14 (dd, J = 7.6, 1.4 Hz, 1H), 8.02 (dd, J = 7.3, 1.8 Hz, 1H, pyridyl), 7.53–7.34 (m, 7H, Ar-H), 3.83 (br s, 4H, piperazine), 3.46 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 175.4 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.8 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.2 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 158.8, 158.1, 154.7, 151.1 (4C, 2C of -C2H=N-C9 or 1C of -C2H=N-C9 of Quinoline and 2C of Npip-C-Npy-CH or 1C of Npip-C-Npy-CH), 147.2–117.7 (15C, aromatic carbon atoms), 104.9 (1C, C≡N), 97.2 (1C, -C-C≡N), 52.2, 48.6 (4C, piperazine).
5k M.P. 267–268°C, yield 76% (found: C 55.62, H 3.56, N 23.95, C27H21BrN10O, calc: C 55.77, H 3.64, N 24.09%). IR (KBr) cm−1, 3294 (-NH), 2220 (C≡N), 1257 (C-O-C), 840 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.22 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.84 (d, J = 2.0, 1H, C5 proton of quinoline), 8.62 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.51 (s, 1H, C3 proton of quinoline), 8.47–8.43 (m, 2H, pyrimidyl), 8.22 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.19 (dd, J = 7.7, 1.5 Hz, 1H), 7.47–7.35 (m, 4H, Ar-H), 6.79 (t, J = 6.8 Hz, 1H, pyrimidyl), 3.79 (br s, 4H, piperazine), 3.42 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.9 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.8 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 161.2, 159.5, 157.6, 152.4 (5C, 2C of -C2H=N-C9 or 1C of -C2H=N-C9 of Quinoline and 3C of pyrimidyl ring), 148.4–120.1 (13C, aromatic carbon atoms), 105.4 (1C, C≡N), 98.7 (1C, -C-C≡N), 48.7, 42.1 (4C, piperazine).
5l M.P. 255–257°C, yield 74% (found: C 60.58, H 4.28, N 18.74, C30H25BrN8O, calc: C 60.71, H 4.25, N 18.88%). IR (KBr) cm−1, 3290 (-NH), 2221 (C≡N), 1257 (C-O-C), 833 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.17 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.82 (d, J = 1.7, 1H, C5 proton of quinoline), 8.69 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.48 (s, 1H, C3 proton of quinoline), 8.29 (d, J = 7.6 Hz, 1H, C8 proton of quinoline), 8.13 (dd, J = 7.8, 1.5 Hz, 1H), 7.51–7.29 (m, 9H, Ar-H), 3.84 (br s, 4H, piperazine), 3.51 (br s, 4H, piperazine), 2.72 (s, 2H, Npip-CH2); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.1 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 153.1, 148.6 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 145.1–119.7 (18C, aromatic carbon atoms), 105.7 (1C, C≡N), 97.9 (1C, -C-C≡N), 65.3 (1C, N-CH2), 49.1, 46.2 (4C, piperazine).
5m M.P. 239–241°C, yield 75% (found: C 62.80, H 4.53, N 16.67, C31H26BrN7O, calc: C 62.84, H 4.42, N 16.55%). IR (KBr) cm−1, 3296 (-NH), 2221 (C≡N), 1247 (C-O-C), 832 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.26 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.79 (d, J = 1.8, 1H, C5 proton of quinoline), 8.61 (d, J = 8.4 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.53 (s, 1H, C3 proton of quinoline), 8.26 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.05 (dd, J = 7.6, 1.4 Hz, 1H), 7.63–7.36 (m, 9H, Ar-H), 3.88 (4H, t, J = 6.8 Hz, piperidine), 3.67 (4H, t, J = 8.8 Hz, piperidine), 2.43 (2H, s, -CH2), 1.83 (1H, t, J = 7.4 Hz, -CH, piperidine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.5 (1C, C-6, s-triazine, C-N at piperidine linkage), 166.4 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.9 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 151.7, 150.3 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 145.9–117.8 (18C, aromatic carbon atoms), 104.9 (1C, C≡N), 98.4 (1C, -C-C≡N), 46.3, 40.2, 36.1, 29.9 (6C, 5C of piperidine and 1C of -CH2).
5n M.P. 284–285°C, yield 74% (found: C 58.91, H 4.43, N 18.36, C26H24BrN7O, calc: C 58.87, H 4.56, N 18.48%). IR (KBr) cm−1, 3299 (-NH), 2221 (C≡N), 1258 (C-O-C), 828 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.19 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.83 (d, J = 1.7, 1H, C5 proton of quinoline), 8.63 (d, J = 8.0 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.50 (s, 1H, C3 proton of quinoline), 8.25 (d, J = 7.7 Hz, 1H, C8 proton of quinoline), 8.10 (dd, J = 7.6, 1.4 Hz, 1H), 7.47–7.37 (m, 4H, Ar-H), 3.69 (dd, J = 11.9, 7.2 Hz, 2H, piperidine), 2.91 (dd, J = 12.0, 7.7 Hz, 2H, piperidine), 1.79–1.71 (m, 4H, piperidne), 1.41 (d, J = 6.5 Hz, 6H, 2CH3); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 175.8 (1C, C-6, s-triazine, C-N at piperidine linkage), 167.1 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.4 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 153.8, 149.5 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 147.0–121.4 (12C, aromatic carbon atoms), 105.9 (1C, C≡N), 96.5 (1C, -C-C≡N), 52.1, 36.6, 31.9 (5C, piperidine), 22.7 (2C, 2CH3).
5o M.P. 286–288°C, yield 72% (found: C 64.67, H 4.25, N 16.68, C36H29BrN8O, calc: C 64.58, H 4.37, N 16.74%). IR (KBr) cm−1, 3307 (-NH), 2218 (C≡N), 1255 (C-O-C), 836 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.24 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.85 (d, J = 1.8, 1H, C5 proton of quinoline), 8.62 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.51 (s, 1H, C3 proton of quinoline), 8.33 (d, J = 7.6 Hz, 1H, C8 proton of quinoline), 8.15 (dd, J = 7.7, 1.4 Hz, 1H), 7.62–7.28 (m, 14H, Ar-H), 4.41 (s, 1H, N-CH), 3.83 (br s, 4H, piperazine), 3.49 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.3 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.6 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.4 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 152.6, 150.2 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 146.5–116.9 (24C, aromatic carbon atoms), 105.6 (1C, C≡N), 97.1 (1C, -C-C≡N), 76.3 (1C, -Npip-CH), 49.5, 46.1 (4C, piperazine).
5p M.P. 274–275°C, yield 74% (found: C 61.34, H 4.14, N 15.83, C36H28BrClN8O, calc: C 61.42, H 4.01, N 15.92%). IR (KBr) cm−1, 3294 (-NH), 2224 (C≡N), 1254 (C-O-C), 839 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.12 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.84 (d, J = 1.9, 1H, C5 proton of quinoline), 8.59 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.47 (s, 1H, C3 proton of quinoline), 8.24 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.04 (dd, J = 7.5, 1.5 Hz, 1H), 7.59–7.25 (m, 13H, Ar-H), 4.02 (s, 1H, N-CH), 3.81 (br s, 4H, piperazine), 3.52 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.4 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.1 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 155.1, 150.1 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 148.2–118.4 (24C, aromatic carbon atoms), 106.2 (1C, C≡N), 98.3 (1C, -C-C≡N), 75.6 (1C, -Npip-CH), 47.8, 44.2 (4C, piperazine).
5q M.P. 235–237°C, yield 81% (found: C 58.18, H 3.63, N 18.64, C29H22BrFN8O, calc: C 58.30, H 3.71, N 18.76%). IR (KBr) cm−1, 3288 (-NH), 2221 (C≡N), 1253 (C-O-C), 834 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.26 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.74 (d, J = 1.7, 1H, C5 proton of quinoline), 8.61 (d, J = 8.5 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.45 (s, 1H, C3 proton of quinoline), 8.23 (d, J = 7.6 Hz, 1H, C8 proton of quinoline), 8.06 (dd, J = 7.7, 1.3 Hz, 1H), 7.51–7.32 (m, 11H, Ar-H), 6.95 (dd, J = 12.5, 7.2 Hz, 2H), 6.77–6.73 (m, 1H), 6.52 (dd, J = 12.7, 6.9 Hz, 1H), 3.85 (br s, 4H, piperazine), 3.40 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.8 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.3 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 151.6, 150.4, 148.4 (3C, 2C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline and 1C of C-F), 146.2–119.5 (17C, aromatic carbon atoms), 104.9 (1C, C≡N), 97.2 (1C, -C-C≡N), 51.1, 42.5 (4C, piperazine); 19F NMR (400 MHz, CDCl3, δ) ppm, -122.1 (1F, s, C-F).
5r M.P. 272–273°C, yield 79% (Found: C 58.22, H 3.61, N 18.70, C29H22BrFN8O, calc: C 58.30, H 3.71, N 18.76%). IR (KBr) cm−1, 3296 (-NH), 2223 (C≡N), 1258 (C-O-C), 832 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.20 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.77 (d, J = 2.0, 1H, C5 proton of quinoline), 8.63 (d, J = 8.5 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.53 (s, 1H, C3 proton of quinoline), 8.26 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.13 (dd, J = 7.6, 1.3 Hz, 1H), 7.57–7.31 (m, 11H, Ar-H), 7.19 (dd, J = 12.8, 7.7 Hz, 2H), 6.68–6.66 (m, 1H), 6.59 (dd, J = 12.7, 6.1 Hz, 1H), 3.89 (br s, 4H, piperazine), 3.50 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.6 (1C, C-6, s-triazine, C-N at piperazine linkage), 165.9 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.3 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 152.2, 150.4, 147.1 (3C, 2C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline and 1C of C-F), 144.7–120.1 (17C, aromatic carbon atoms), 104.9 (1C, C≡N), 96.8 (1C, -C-C≡N), 47.7, 43.9 (4C, piperazine); 19F NMR (400 MHz, CDCl3, δ) ppm, -117.9 (1F, s, C-F).
5s M.P. 294–295°C, yield 76% (found: C 55.72, H 3.31, N 17.19, C30H22BrF3N8O, calc: C 55.65, H 3.42, N 17.31%). IR (KBr) cm−1, 3307 (-NH), 2223 (C≡N), 1256 (C-O-C), 837 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.21 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.81 (d, J = 1.8, 1H, C5 proton of quinoline), 8.63 (d, J = 8.2 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.46 (s, 1H, C3 proton of quinoline), 8.27 (d, J = 7.5 Hz, 1H, C8 proton of quinoline), 8.13 (dd, J = 7.6, 1.4 Hz, 1H), 7.83 (t, J = 7.9 Hz, 1H), 7.56–7.31 (m, 7H, Ar-H), 3.82 (br s, 4H, piperazine), 3.42 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 175.5 (1C, C-6, s-triazine, C-N at piperazine linkage), 167.1 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.2 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 151.8, 150.3 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 147.2–117.7 (19C, aromatic carbon atoms C-CF3 at 130.3 & CF3 at 125.6), 105.8 (1C, C≡N), 95.2 (1C, -C-C≡N), 49.1, 46.4 (4C, piperazine); 19F NMR (400 MHz, CDCl3, δ) ppm, -63.7 (3F, s, -CF3).
5t M.P. 294–295°C, yield 72% (found: C 58.06, H 4.69, N 16.32, C33H31BrN8O4, calc: C 57.98, H 4.57, N 16.39%). IR (KBr) cm−1, 3291 (-NH), 2222 (C≡N), 1490 (-CH2), 1248 (C-O-C), 839 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.16 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.77 (d, J = 1.8, 1H, C5 proton of quinoline), 8.68 (d, J = 8.3 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.46 (s, 1H, C3 proton of quinoline), 8.33 (d, J = 7.6 Hz, 1H, C8 proton of quinoline), 8.14 (dd, J = 7.6, 1.4 Hz, 1H), 7.53–7.33 (m, 6H, Ar-H), 6.86 (d, J = 7.5 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 3.89 (br s, 4H, piperazine), 3.71 (s, 9H, 3OCH3), 3.50 (br s, 4H, piperazine); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 177.1 (1C, C-6, s-triazine, C-N at piperazine linkage), 166.1 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 164.6 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 154.2, 149.5 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 147.2–116.3 (18C, aromatic carbon atoms), 106.0 (1C, C≡N), 98.4 (1C, -C-C≡N), 66.8, 63.1, 54.4 (4C, 3C of 3OCH3 and 1C of Npip-CH2), 47.9, 41.8 (4C, piperazine).
5u M.P. 258–260°C, yield 78% (found: C 58.98, H 4.17, N 18.46, C30H25BrN8O2, calc: C 59.12, H 4.13, N 18.39%). IR (KBr) cm−1, 3293 (-NH), 2224 (C≡N), 1254 (C-O-C), 827 (s-triazine C-N str.); 1H NMR (400 MHz, DMSO-d6, δ) ppm, 9.22 (s, 1H, -NH, s-triazine to amino-benzonitrile linkage), 8.80 (d, J = 1.7, 1H, C5 proton of quinoline), 8.67 (d, J = 8.1 Hz, 1H, -N=CH-, C2 proton of quinoline), 8.49 (s, 1H, C3 proton of quinoline), 8.21 (d, J = 7.3 Hz, 1H, C8 proton of quinoline), 8.15 (dd, J = 7.6, 1.4 Hz, 1H), 7.51–7.35 (m, 6H, Ar-H), 7.23 (d, J = 8.3 Hz, 1H), 6.65 (d, J = 7.2 Hz, 1H), 4.21 (s, 3H, OCH3), 3.79 (br s, 4H, piperazine ring), 3.49 (br s, 4H, piperazine ring); 13C-NMR (100 MHz, DMSO-d6, δ) ppm, 176.6 (1C, C-6, s-triazine, C-N at piperazine linkage), 167.2 (1C, C-4, s-triazine, C-O-C at quinoline linkage), 163.3 (1C, C-2, s-triazine, C-NH at benzonitrile moiety), 155.0, 152.1 (2C, 1C of -C2H=N-C9 and 1C of -C2H=N-C9, quinoline), 149.0–119.8 (18C, aromatic carbon atoms), 105.5 (1C, C≡N), 97.9 (1C, -C-C≡N), 57.2 (1C, -OCH3), 46.2, 44.8 (4C, piperazine).
In vitro evaluation of antimicrobial activity
The synthesized s-triazinyl derivatives 5a-u were examined for antimicrobial activity against several bacteria (Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 619, Escherichia coli MTCC 739, Pseudomonas aeruginosa MTCC 741, Klebsiella pneumoniae MTCC 109, Salmonella typhi MTCC 733, Proteus vulgaris MTCC 1771, Shigella flexneria MTCC 1457) and fungi (Aspergillus niger MTCC 282, Aspergillus fumigatus MTCC 343, Aspergillus clavatus MTCC 1323, Candida albicans MTCC 183) species using paper disc diffusion techniqueCitation29. The Mueller-Hinton agar media were sterilized (autoclaved at 120°C for 30 min), poured at uniform depth of 5 mm and allowed to solidify. The microbial suspension (105 CFU/mL; 0.5 McFarland Nephelometery Standards) was streaked over the surface of media using a sterile cotton swab to ensure even growth of the organisms. The tested compounds were dissolved in dimethyl sulfoxide to give solutions of 3.12–100 µg/mL. Sterile filter paper discs measuring 6.25 mm in diameter (Whatman no. 1 filter paper), previously soaked in a known concentration of the respective test compound in dimethyl sulfoxide were placed on the solidified nutrient agar medium that had been inoculated with the respective microorganism and the plates were incubated for 24 h at (37 ± 1)°C. A control disc impregnated with an equivalent amount of dimethyl sulfoxide without any sample was also used and did not produce any inhibition. Ciprofloxacin and ketoconazole (100 µg/disc) were used as control drugs for antibacterial and antifungal activity, respectively.
MIC of the compound was determined by agar streak dilution methodCitation30. A stock solution of the synthesized compound (100 µg/mL) in dimethyl sulfoxide was prepared and graded quantities of the test compounds were incorporated in a specified quantity of molten sterile agar, that is, nutrient agar for evaluation of antibacterial and sabouraud dextrose agar for antifungal activity, respectively. The medium containing the test compound was poured into a Petri dish at a depth of 4–5 mm and allowed to solidify under aseptic conditions. A suspension of the respective microorganism of approximately 105 CFU/mL was prepared and applied to plates with serially diluted compounds with concentrations in the range of 3.12–100 µg/mL in dimethyl sulfoxide and incubated at (37 ± 1)°C for 24 h (bacteria) or 48 h (fungi). The lowest concentration of the substance that prevents the development of visible growth is considered to be the MIC value.
In vitro evaluation of antimycobacterial activity
The preliminary antimycobacterial assessment for the final synthesized compounds was carried out using BACTEC MGIT method. The Mycobacterial Growth Indicator Tubes (MGIT) containing 4 ml of modified Middle brook 7H9 Broth Base were numbered as per the title compounds to be tasted for antimycobacterial efficacy by means of various concentrations prepared. The suspension was allowed to sit for 20 min and the tubes were centrifuged at 3000 rpm for 15 min. After that prepared suspension of 104 to 107 CFU/mL of H37 RV M. tuberculosis strain was added in the medium to be incubated and 0.1 ml of egg-based medium (L. J) was also added. The MGIT tubes were then tightly recapped, mixed well and incubated into BACTEC MGIT instrument at (37 ± 1)°C until positivity is observed. The readings were measured daily starting from the second day of incubation. Positive cultures were usually detected within 10 days. For reading the actual results, the MGIT tubes were removed from incubator and placed on the UV light next to a positive control tube and an uninoculated tube (negative control). Bright fluorescence detected by the corresponding MGIT tube was noticed in the form of bright orange colour in the bottom of the tube and also an orange reflection on the meniscusCitation31. The primary screening was conducted at concentration of 6.25 µg/ml against M. tuberculosis H37 Rv in BACTEC MGIT system. Compounds demonstrating 99% inhibition in the primary screen were described as most potent compounds. All the other compounds to be tasted were re-examined for their actual MIC by using Lowenstein-Jensen MIC method. The MIC was defined as the lowest concentration inhibiting 99% of the inoculum.
The secondary antimycobacterial screening for test compounds was obtained for M. tuberculosis H37 Rv, by using L. J. (Lowenstein and Jensen) MIC methodCitation32,Citation33. Stock solutions of primary 1000, 500, 250 and secondary 200, 100, 62.5, 50, 25, 12.5, 6.25, 3.25 µg/mL dilutions of each test compound in DMSO (dimethyl sulfoxide) were added in the liquid L. J. Medium and then media were sterilized by inspissation method. A culture of M. tuberculosis H37 Rv growing on L. J. medium was harvested in 0.85% saline in bijou bottles. These tubes were then incubated at 37°C for 24 h followed by streaking of M. tuberculosis H37 Rv (5 × 104 bacilli per tube). These tubes were then incubated at (37 ± 1)°C. Growth of bacilli was seen after 12 days, 22 days and finally 28 days of incubation. Tubes having the compounds were compared with control tubes where medium alone was incubated with M. tuberculosis H37 Rv. The concentration at which no development of colonies occurred or <20 colonies was taken as MIC concentration of test compound. The standard strain M. tuberculosis H37 Rv was tested with known drugs rifampicin, isoniazid, ethambutol and pyrazinamide.
Results and discussion
Investigation on antibacterial screening data () showed some of the compounds were active against all the mentioned bacteria. Final s-triazinyl compounds 5h, 5n, 5p, 5s, 5t and 5u have the highest ability to inhibit S. aureus. Final analogues 5d, 5h, 5n, 5p, 5r, 5s, 5t showed higher effectiveness against B. cereus. The best activity was observed with compounds 5h, 5n, 5s, 5t and 5u against E. coli and P. aeruginosa. Inhibition of K. pneumoniae was also noted for s-triazine derivatives 5d, 5p, 5q, 5s and 5t. Compounds 5q, 5s and 5t had an effective action against S. typhi along with similar MIC profile of compounds 5h, 5n and 5p. Compounds 5d, 5h, 5q, 5s, 5t and 5u were superior in inhibiting the growth of P. vulgaris. Strong inhibitory effect was shown by final derivatives 5d, 5h, 5n, 5s and 5t against S. flexneria. All the remaining final s-triazine derivatives exerted good to moderate activity.
Table 1. In vitro antimicrobial activity of newly synthesized compounds.
The antifungal results data () revealed that, the synthesized compounds showed variable degree of inhibition against the tested fungi. Final s-triazine derivatives 5q, 5r, 5s and 5t displayed good antigrowth activity against A. niger. A significant inhibition was also observed for compounds 5d, 5p, 5s, 5t and 5u towards A. fumigatus. Compounds 5h, 5n, 5s, 5t and 5u act as the most potent inhibitors of the growth of A. clavatus fungi. Compounds 5q, 5r, 5s, 5t and 5u possessed the highest antifungal activity against C. albicans. All the remaining final s-triazine derivatives exerted good to moderate activity.
Table 2. In vitro antifungal activity of newly synthesized compounds.
In vitro antimycobacterial activities of compounds 5a-u were assessed against M. tuberculosis H37Rv strain. The preliminary results observed from BACTEC MGIT method furnished in indicated that final s-triazines 5n, 5t and 5u exhibited highest inhibition (99%) at a constant concentration level (6.25 μg/ml) against M. tuberculosis H37Rv. The secondary biological screening was performed using Lowenstein-Jensen MIC method and it is worthwhile to note that 5t was the only compound displaying inhibition of M. tuberculosis H37Rv completely (99%) at the MIC of 3.12 μg/mL. Compounds 5p and 5s appeared with good inhibition effect in the term of MIC at 12.5 µg/mL, while all the remaining derivatives were found to exert MIC values ranging from 25–1000 µg/mL.
Table 3. In vitro antimycobacterial activity of newly synthesized compounds.
In conclusion, it is clear that the introduction of appropriate substituent on the s-triazine ring would lead to the more bioactive compounds. Bioassay results revealed that nine compounds (5d, 5h, 5n, 5p, 5q, 5r, 5s, 5t and 5u) out of the 21 studied displayed variable in vitro antibacterial, antifungal and antimycobacterial inhibitory effects. The presence of 6-bromo-4-hydroxyquinoline heteroatom ring in the title compounds significantly increase the biological potency of the resultant compounds compared to the presence of 8-hydroxyquinoline in our previous study. Higher inhibitory effects observed in this study appear to be dependent on the mono-chloro, di-chloro, acetyl linkage, dimethyl, mono-fluoro, trifluoromethyl, trimethoxy and mono-methoxy functionality to the nitrogen atom of piperazine bases condensed to the nucleus. Therefore, it is concluded that there exists ample scope for further study in this class of compounds in order to discover varied biological profiles such as anticancer activity or anti-HIV activity. The study is currently under investigation and the results will be published in due course.
Supplementary Material
Download PDF (319.3 KB)Acknowledgments
The authors are thankful to Applied Chemistry Department of S. V. National Institute of Technology, Surat for the scholarship, encouragement and facilities. The authors wish to offer their deep gratitude to Microcare Laboratory, Surat, India for carrying out the biological screenings and Centre of Excellence, Vapi, India for carrying out 1H NMR, 13C NMR and 19F NMR analysis.
Declaration of interest
The authors report no conflict of interest.
Supplementary material
Detailed discussion on the analytical and biological characterization is included within the supplementary information. Tables containing zone of inhibition and MICs for all the newly synthesized compounds are included within the supplementary information.
References
- Nathan C. Antibiotics at the crossroads. Nature 2004;431:899–902.
- Raviglione MC. The TB epidemic from 1992 to 2002. Tuberculosis (Edinb) 2003;83:4–14.
- NIAID. http://www3.niaid.nih.gov/topics/tuberculosis/.
- TAACF. http://www.taacf.org/about-TB-background.htm.
- World Health Organization, Global tuberculosis control: A short update to the 2009 report. http://www.who.int/tb/publications/global_report/2009/update/en/index.html.
- Mahajan DH, Pannecouque C, De Clercq E, Chikhalia KH. Synthesis and studies of new 2-(coumarin-4-yloxy)-4,6-(substituted)-S-triazine derivatives as potential anti-HIV agents. Arch Pharm (Weinheim) 2009;342:281–290.
- Chikhalia KH, Patel MJ. Design, synthesis and evaluation of some 1,3,5-triazinyl urea and thiourea derivatives as antimicrobial agents. J Enzyme Inhib Med Chem 2009;24:960–966.
- Patel DH, Chikhalia KH, Shah NK, Patel DP, Kaswala PB, Buha VM. Synthesis and antimicrobial studies of s-triazine based heterocycles. J Enzyme Inhib Med Chem 2010;25:121–125.
- Zhou C, Min J, Liu Z, Young A, Deshazer H, Gao T et al. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorg Med Chem Lett 2008;18:1308–1311.
- Srinivas K, Srinivas U, Bhanuprakash K, Harakishore K, Murthy US, Rao VJ. Synthesis and antibacterial activity of various substituted s-triazines. Eur J Med Chem 2006;41:1240–1246.
- Baliani A, Bueno GJ, Stewart ML, Yardley V, Brun R, Barrett MP, Gilbert IH. Synthesis and antibacterial activity of various substituted s-triazines. Eur J Med Chem 2006;41:1240–1246.
- Menicagli R, Samaritani S, Signore G, Vaglini F, Dalla Via L. In vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: new molecules in anticancer research. J Med Chem 2004;47:4649–4652.
- Garaj V, Puccetti L, Fasolis G, Winum JY, Montero JL, Scozzafava A et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–3108.
- Melato S, Prosperi D, Coghi P, Basilico N, Monti D. A combinatorial approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: combining conventional synthesis and microwave-assistance. Chemmedchem 2008;3:873–876.
- Xiong YZ, Chen FE, Balzarini J, De Clercq E, Pannecouque C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: structural modulations of diaryltriazines with potent anti-HIV activity. Eur J Med Chem 2008;43:1230–1236.
- Kerns RJ, Rybak MJ, Kaatz GW, Vaka F, Cha R, Grucz RG et al. Structural features of piperazinyl-linked ciprofloxacin dimers required for activity against drug-resistant strains of Staphylococcus aureus. Bioorg Med Chem Lett 2003;13:2109–2112.
- Singh KK, Joshi SC, Mathela CS. Synthesis and in-vitro antibacterial activity of N-alkyl and N-aryl piperazine derivatives. Ind J Chem 2011;50(B):196–200.
- Upadhayaya RS, Sinha N, Jain S, Kishore N, Chandra R, Arora SK. Optically active antifungal azoles: synthesis and antifungal activity of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-[2-[4-aryl-piperazin-1-yl]-ethyl]-tetrazol-2-yl/1-yl)-1-[1,2,4]-triazol-1-yl-butan-2-ol. Bioorg Med Chem 2004;12:2225–2238.
- Upadhayaya RS, Vandavasi JK, Kardile RA, Lahore SV, Dixit SS, Deokar HS et al. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur J Med Chem 2010;45:1854–1867.
- Kumar CS, Vinaya K, Chandra JN, Thimmegowda NR, Prasad SB, Sadashiva CT et al. Synthesis and antimicrobial studies of novel 1-benzhydryl-piperazine sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 2008;23:462–469.
- Sunduru N, Gupta L, Chaturvedi V, Dwivedi R, Sinha S, Chauhan PM. Discovery of new 1,3,5-triazine scaffolds with potent activity against Mycobacterium tuberculosis H37Rv. Eur J Med Chem 2010;45:3335–3345.
- Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005;307:223–227.
- Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother 2008;52:2831–2835.
- Upadhayaya RS, Lahore SV, Sayyed AY, Dixit SS, Shinde PD, Chattopadhyaya J. Conformationally-constrained indeno[2,1-c]quinolines—a new class of anti-mycobacterial agents. Org Biomol Chem 2010;8:2180–2197.
- Upadhayaya RS, Shinde PD, Sayyed AY, Kadam SA, Bawane AN, Poddar A et al. Synthesis and structure of azole-fused indeno[2,1-c]quinolines and their anti-mycobacterial properties. Org Biomol Chem 2010;8:5661–5673.
- Upadhayaya RS, Shinde PD, Kadam SA, Bawane AN, Sayyed AY, Kardile RA et al. Synthesis and antimycobacterial activity of prodrugs of indeno[2,1-c]quinoline derivatives. Eur J Med Chem 2011;46:1306–1324.
- Xiong YZ, Chen FE, Balzarini J, De Clercq E, Pannecouque C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 13: synthesis of fluorine-containing diaryltriazine derivatives for in vitro anti-HIV evaluation against wild-type strain. Chem Biodivers 2009;6:561–568.
- Patel RV, Kumari P, Chikhalia KH. Novel s-triazinyl piperazines: Design, synthesis, characterization and anti-microbial activity. Arch Appl Sci Res 2010;2:232–240.
- Gillespie SH. Medical microbiology-Illustrated, Butterworth Heinemann Ltd., United Kingdom: 1994:234–247.
- Hawkey PM, Lewis DA. Medical bacteriology—a practical approach. Oxford University Press, United Kingdom: 1994:181–194.
- Anargyros P, Astill DS, Lim IS. Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J Clin Microbiol 1990;28:1288–1291.
- Isenberg HD. Clinical microbiology procedures handbook, vol. 1, American Society for Microbiology, Washington, D.C. 1992.
- Patel NB, Khan IH, Rajani SD. Antimycobacterial and antimicrobial study of new 1,2,4-triazoles with benzothiazoles. Arch Pharm (Weinheim) 2010;343:692–699.
- Dandia A, Arya K, Sati M, Sarawgi P. Green chemical synthesis of fluorinated 1,3,5-triaryl-s-triazines in aqueous medium under microwaves as potential antifungal agents. J Fluorine Chem 2004;125:1273–1277.
![Figure 2. [4(a-u), R] = Piperazine and piperidine bases coupled to compound 3.](/cms/asset/13e06b5c-147e-4e7e-a2f7-96c91f014e27/ienz_a_592491_f0002_b.gif)