Abstract
Cobalt(II), copper(II), nickel(II) and zinc(II) metal complexes with 5-chlorosalicyladehyde derived Schiff base sulfonamides have been synthesized and characterized. Structure and bonding nature of all the synthesized compounds have been deduced from physical, analytical, and spectral (IR, 1H NMR, 13C NMR, Mass, electronic) data. An octahedral geometry has been proposed for all the metal complexes. The ligands and their metal complexes have been screened for their in vitro antibacterial, antifungal, and cytotoxic properties and results are reported.
Introduction
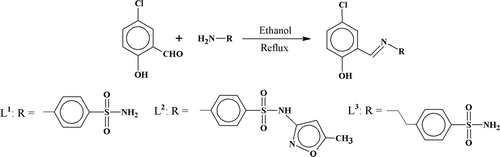
Schiff bases are known for their varied chemical and structural characteristics and wider applications as biologically active compoundsCitation1. Metal complexes derived from such molecules act in drug design and models for metalloproteinsCitation2. With the increasing incidences of mycosisCitation3, much attention has been focused on the novel methods for synthesis of more effective antimicrobial compounds with low toxicity. A particular interest in Schiff bases containing specifically oxygen and nitrogen donor atoms and their metal complexes speedily arose due to their inhibiting properties towards the growth of tumoursCitation4–7. The imine bond in Schiff bases plays role in coordination with the metals as well as acting as highly bioactive siteCitation8–14. Keeping in view all such aspects, we report in this paper the synthesis of three chloro-substituted salicylaldehyde derived Schiff base sulfonamides, 4-[(E)-(5-chloro-2-hydroxybenzylidene)amino]-benzenesulfonamide (L1), 4-{[(E)-(5-chlor-2-hydroxyphenyl) methylidene]amino}-N-(5-methyl-isoxazol-3-yl)benzenesulfonamide (L2) and 4-{2-[(5-chloro-2-hydroxybenzylidene)amino]ethyl}-benzenesulfonamide (L3) and their transition metal [cobalt (II), copper (II), nickel (II), and zinc (II)] complexes. These compounds have been characterized by their physical, analytical, and spectral data. The structure of ligands, (L1) and (L2) have been determined by X-ray diffraction method and reported by us elsewhereCitation15,Citation16. An octahedral geometry has been suggested for all the metal (II) complexes. The ligands and metal complexes have been screened for their in vitro antibacterial, antifungal, and cytotoxic activity and the data reported in this paper.
Experimental
Material and methods
Reagents and solvents used were analytical grades. All metal (II) compounds were used as their chloride salts. Elemental analyses were carried out with a CHNS/O Analyzer (Perkin Elmer USA) model. 1H and 13C-NMR spectra of compounds were recorded with a Bruker Spectrospin Avance DPX-400 using TMS as internal standard and d6-DMSO as solvent. IR of the compounds were recorded on a SHIMADZU FTIR spectrophotometer. The melting points were determined with a Gallenkamp melting point apparatus. In vitro antibacterial, antifungal, and cytotoxic properties were studied at HEJ research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
General procedure for the synthesis of compounds (L1)–(L3)
4-[(E)-(5-Chloro-2-hydroxybenzylidene)amino]benzenesulfonamide (L1)
To a stirred solution of the respective sulfanilamide (0.689 g, 0.004 moles) in ethanol (30 mL) was added a solution of 5-chlorosalisylaldehyde (0.63 g, 0.004 moles) in ethanol (15 mL). The resultant mixture was refluxed for 3 h by monitoring the formation of product through TLC. After completion of reaction, it was cooled to room temperature, filtered, and volume reduced to about one-third using rotary evaporator. The solid product thus obtained was recrystallized in hot ethanol (86% yield, 1.069 g). The same method was applied to prepare ligands (L2) and (L3).
4-[(E)-(5-Chloro-2-hydroxybenzylidene)amino]benzenesulfonamide (L1)
Bright orange crystals; Yield 86 % (1.069 g); m.p. 196–198°C; IR (KBr, cm−1): 3345 (NH2), 3318 (OH), 1602 (HC=N), 1345, 1110 (S=O), 955 (S-N), 844 (C-S), 610 (C-Cl); 1H NMR (DMSO-d6, δ, ppm): 7.2-7.5 (m, 3H, Cl-Ph), 7.7-8.2 (m, 4H, N-Ph), 8.91 (s, 1H, azomethine), 9.2 (s, 2H, -SO2NH2), 12.42 (s, 1H, OH); 13C NMR (δ, ppm): 117.4 (C3 Cl-Ph), 119.9 (C1 Cl-Ph), 122.6 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 128.6 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 138.2 (C4 N-Ph), 156.4 (C1 N-Ph), 159.2 (C2 Cl-Ph), 160.1 (C=N, azomethine); Anal. Calcd. for C13H11ClN2O3S (310.75): C, 50.24; H, 3.57; N, 9.01; Found: C, 50.35; H, 3.52; N, 9.11; Mass spectrum (ESI) [M]+ = 310.
4-{[(E)-(5-chloro-2-hydroxyphenyl)methylidene]amino}-N-(5-methylisoxazol-3-yl)benzene sulfonamide (L2)
Bright orange powder; Yield 85 % (1.33 g); m.p. 218–220°C; IR (KBr, cm−1): 3365 (NH), 3315 (OH), 1597 (HC=N), 1345, 1110 (S=O), 955 (S-N), 844 (C-S), 615 (C-Cl); 1H NMR (DMSO-d6, δ, ppm): 2.31 (s, 3H, methylisoxazole), 6.8 (s, 1H, isoxazole), 7.2-7.5 (m, 3H, Cl-Ph), 7.7-8.2 (m, 4H, N-Ph), 8.9 (s, 1H, azomethine), 8.9 (s, 1H, SO2NH-), 12.42 (s, 1H, OH); 13C NMR (δ, ppm): 12.9 (C methylisoxazole), 95.1 (C4 isoxazole), 117.4 (C3 Cl-Ph), 119.9 (C1 Cl-Ph), 122.6 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 128.6 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 138.2 (C4 N-Ph), 150.0 (C3 isoxazole), 156.4 (C1 N-Ph), 159.2 (C2 Cl-Ph), 160.9 (C=N, azomethine) 169.6 (C5 isoxazole); Anal. Calcd. for C17H14ClN3O4S (391.82): C, 52.11; H, 3.60; N, 10.72; Found: C, 52.23; H, 3.59; N, 10.81. Mass spectrum (ESI) [M]+ = 391.04.
4-{2-[(5-Chloro-2-hydroxybenzylidene)-amino]ethyl}benzenesulfonamide (L3)
Yellowish green powder; Yield 76 % (1.03 g); m.p. 166°C; IR (KBr, cm−1): 3345 (NH2), 3318 (OH), 1605 (HC=N), 1345, 1110 (S=O), 955 (S-N), 844 (C-S), 615 (C-Cl); 1H NMR (DMSO-d6, δ, ppm): 3.13 (t, 2H, CH2-aromatic), 3.34 (t, 2H, CH2-N), 7.2-7.5 (m, 3H, Cl-Ph), 7.7-8.2 (m, 4H, N-Ph), 8.91 (s, 1H, azomethine), 9.2 (s, 2H, -SO2NH2), 12.42 (s, 1H, OH); 13C NMR (δ, ppm): 37.5 (CH2-aromatic), 61.3 (CH2-N), 117.4 (C3 Cl-Ph), 126.01 (C1 Cl-Ph), 128.1 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 127.2 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 136.8 (C4 N-Ph), 142.7 (C1 N-Ph), 159.2 (C2 Cl-Ph), 160.9 (C=N, azomethine); Anal. Calcd. for C15H15ClN2O3S (338.82): C, 53.17; H, 4.46; N, 8.27; Found: C, 53.34; H, 4.52; N, 8.11; Mass spectrum (ESI) [M]+ = 338.08.
General procedure for the synthesis of complexes (1)–(12)
[Co(L1-H)2(H2O)2] (1)
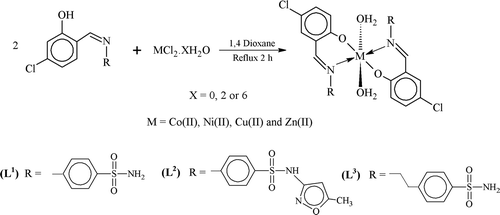
To a hot magnetically stirred dioxane (15 mL) solution of 4-[(E)-(5-chloro-2-hydroxy benzylidene)amino]benzenesulfonamide (L1; 0.622 g, 0.002 moles), an aqueous solution (15 mL) of Co(II) Cl2.6H2O (0.238 g, 0.001 moles) was added and refluxed for 2 h. TLC was used to monitor the reaction. After the completion of reaction, the solution was filtered and reduced to half of its volume by evaporating in rotary evaporator. The concentrated solution was left overnight at room temperature, which led to the formation of a solid product. It was filtered, washed with small amount of cold solution of dioxane, then with ether, dried and recrystallized in boiling dioxane. All other complexes (2)–(12) were prepared following the same method using the respective metal salts as chloride respectively with sulfonamides.
[Zn (L1-H)2(H2O)2] (4)
1H NMR (DMSO-d6, δ, ppm): 7.4-7.8 (m, 6H, Cl-Ph), 8.1-8.5 (m, 8H, N-Ph), 9.2 (s, 2H, azomethine), 9.2 (s, 4H, -SO2NH2), 10.5 (s, 4H H2O); 13C NMR (δ, ppm): 117.4 (C3 Cl-Ph), 119.9 (C1 Cl-Ph), 122.6 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 128.6 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 138.2 (C4 N-Ph), 158.5 (C1 N-Ph),160.5 (C2 Cl-phenyl), 161.7 (C=N, azomethine).
[Zn (L2-H)2(H2O)2] (8)
1H NMR (DMSO-d6, δ, ppm): 2.31 (s, 6H, methylisoxazole), 6.8 (s, 2H, isoxazole), 7.5-7.9 (m, 6H, chloro-phenyl), 8.1-8.5 (m, 8H, N-Ph), 9.1 (s, 2H, azomethine), 9.2 (s, 2H, SO2NH-), 10.5 (s, 4H, H2O); 13C NMR (δ, ppm): 12.9 (C methylisoxazole), 95.1 (C4 isoxazole), 117.4 (C3 Cl-Ph), 119.9 (C1 Cl-Ph), 122.6 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 128.6 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 138.2 (C4 N-Ph), 150.0 (C3 isoxazole), 158.5 (C1 N-Ph), 160.3 (C2 Cl-Ph), 161.5 (C=N, azomethine) 169.6 (C5 isoxazole).
[Zn (L3-H)2(H2O)2] (12)
1H NMR (DMSO-d6, δ, ppm): 3.13 (t, 4H, CH2-aromatic), 3.65 (t, 4H, CH2-N), 7.5-7.9 (m, 6H, Cl-Ph), 7.8-8.3 (m, 8H, N-Ph), 9.1 (s, 2H, azomethine), 9.3 (s, 4H, -SO2NH2), 10.5 (s, 4H, H2O); 13C NMR (δ, ppm): 37.5 (CH2-aromatic), 62.1 (CH2-N), 117.4 (C3 Cl-Ph), 126.01 (C1 Cl-Ph), 128.1 (C2, C6 N-Ph), 127.0 (C5 Cl-Ph), 127.2 (C3, C5 N-Ph), 130.7 (C6 Cl-Ph), 132.6 (C4 Cl-Ph), 136.7 (C4 N-Ph), 143.8 (C1 N-Ph), 160.3 (C2 Cl-Ph), 161.5 (C=N, azomethine).
Biological properties
Antibacterial activity (in vitro)
The agar-well diffusion methodCitation17,Citation18 was adopted to determine the antibacterial activity of newly synthesized sulfonamides (L1)–(L3) and their metal (II) complexes (1)–(12). The wells, about 6 mm in diameter, were dug in the prepared media at least 24 mm apart from each other with the help of a sterile metallic borer. 2–8 h old bacterial inocula, containing approximately 104–106 colony-forming units (CFU/mL), were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration (50 mg/mL) of the test sample was prepared in DMSO and introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug (imipenum) served as negative and positive controls, respectively. The plates were incubated at 37°C for 24 h. Antibacterial activity was determined by measuring the diameter (mm) of zones showing complete inhibition. The studies of alone DMSO showed no activity against any bacterial strains.
Antifungal activity (in vitro)
Six fungal strains, T. longifusus, C. albicans, A. flavus, M. canis, F. solani, and C. glaberata were used to study antifungal activity of all the synthesized compounds according to the literature protocolCitation18. Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 (cfu) mL−1 fungal spore suspensions and transferred to petri plates. Discs soaked in 20 ml (200 µg/mL in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for 7 days. The results were recorded as % of inhibition and compared with standard drugs miconazole and amphotericin B.
Minimum inhibitory concentration (MIC)
Compounds containing significant antibacterial activity (over 80%) were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50, and 100 μg/mL of the compounds and applying the protocolCitation19.
Cytotoxicity (in vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the matter compartment was opened to ordinary light. After 2 days, nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMF. From this stock solutions, 500, 50, and 5 µg/mL were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After 2 days, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with sea water to 5 mL per vial. After 24 h, the number of survivors was counted. Data were analyzed by Finney computer program to determine the LD50 valuesCitation20,Citation21.
Results and discussion
Chemistry
Schiff bases (L1)–(L3) were prepared by refluxing an equimolar amount of 5-chloro-2-hydroxybenzaldehyde with the respective sulfonamides, 4-aminobenzenesulfonamide, 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide and 4-(2-aminoethyl)-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide in ethanol to successfully obtain the desired new series of Schiff base sulfonamides in good yield (78–85%; . The products were obtained as solids and their purities were checked by thin layer chromatography. The ligands (L1)–(L3) were only soluble in Dioxane, DMF and DMSO. The composition of the ligands was consistent with their microanalytical data as reported in experimental.
The metal (II) complexes were prepared in a stoichiometric (metal: ligand [1:2]) molar ratio (). Physical measurements and analytical data of the metal(II) complexes (1)–(12) also agree well with the general formula, [M(L-H)2(OH2)2] ( and ).
Table 1. Physical measurements and analytical data of the metal (II) complexes, (1)–(12).
Table 2. Conductivity, magnetic and spectral data of the metal (II) complexes (1)–(12).
IR spectra
The important IR spectral bands of sulfonamide derivatives and their metal complexes are given in experimental and in . Sulfonamides (L1)–(L3) contain various potential donor sites; a broad band at 3315–3318 cm−1 and a sharp band at 1597–1605 cm−1 are assignedCitation22 to the ν(OH) and ν(C=N) modes, respectively. Two bands appearing at 1345 and 1110 cm−1due to νasymm(SO2) and νsymm(SO2), respectivelyCitation23 are present in all sulfonamides. Evidences of the nitrogen bonding of the azomethine (C=N) linkage to the metal atom stems from the shift of the ν(C=N) frequency to lower frequency by around 32–34 cm−1 (1565–1571 cm−1) in the spectra of all of its metal complexes. The following evidences further support the mode of chelation:
| i. | The disappearance of ν(OH) bands at 3315–3318 cm−1 and appearance of a new band at 1395 cm−1 due to the ν(C-O) stretching mode in the complexes reveals the deprotonation of the hydroxyl OH groups of the sulfonamides. It in turn, indicates that the proton of the OH group is replaced by the metal ions on the formation of complexes. | ||||
| ii. | Appearance of the new band at 526–538 and 438–442 cm−1 due to ν(M-O) and ν(M-N) stretching modes present in the spectra of its metal complexes and absent in the spectra of simple sulfonamidesCitation25. | ||||
| iii. | The bands in sulfonamides due to νasymm(SO2) and νsymm(SO2) appearing at 1345 and 1110 cm−1 remain almost unchanged upon coordination indicating that this group is not participating in coordination/chelation. | ||||
| iv. | The bands due to ν(S-N) and ν(C-S) stretching modes present at 955 and 844 cm−1, respectively remained unchanged, indicating no new bond formation with the metal ionsCitation24,Citation25. | ||||
1H and 13C NMR spectra
1H NMR spectra of the sulfonamides and their diamagnetic zinc (II) complexes were recorded in DMSO-d6. The 1H NMR spectral data along with the possible assignments is recorded in the experimental part. It is observed that all the protons due to heteroaromatic/aromatic groups were found as to be in their expected regionCitation26. The conclusions drawn from these studies provide further support to the binding mode discussed in their IR spectra. The coordination of the azomethine nitrogen is inferred by the downfield shifting of the −CH=N- proton signal from 8.9-8.91 ppm in the ligand to 9.1-9.2 ppm in the complexes. Hydroxyl proton at 12.42 ppm in the spectra of Zn (II) complexes of ligands (L1)–(L3) disappeared indicating deprotonation and coordination of the oxygen with the metal ion. All other protons underwent downfield shifting by 0.25–0.35 ppm due to the increased conjugationCitation27 and coordination with the metal atoms. Furthermore, the number of protons calculated from the integration curves, and those obtained from the values of the expected CHN analyses agree well with each other.
13C NMR spectra of the sulfonamides and their diamagnetic zinc (II) complexes were recorded in DMSO-d6. The 13C NMR spectral data of sulfonamides along with the possible assignments is recorded in the experimental part. The carbon atoms due to heteroaromatic/aromatic groups were found as to be in their expected regionCitation26. The conclusions drawn from these studies provide further support to the binding mode discussed in their IR and 1H NMR spectra. Downfield shifting of the−CH=N- signal from 160.1–160.9 ppm in the ligands to 161.5–161.7 ppm in its metal (II) complexes revealed coordination of the azomethine nitrogen to the metal atom. Similarly, carbons of N-phenyl and Cl-phenyl rings being near to the coordination sites also showed downfield shifting by 0.9–1.1 ppm. All other carbons underwent downfield shifting by 0.35–11.0 ppm due to the increased conjugation and coordination with the metal atomsCitation27. Furthermore, the presences of the number of carbons agree well with the expected values.
Electronic spectra
The Co (II) complexes exhibited well-resolved, low-energy bands at 7,296–7,409 cm−1, 17,451–17,519 cm−1 and a strong high-energy band at 20,508–20,646 cm−1 () which are assignedCitation28 to the transitions 4T1g(F)→4T2g(F), 4T1g(F)→4A2g(F) and 4T1g(F)→4T2g(P) in an octahedral geometryCitation29. A high-intensity band at 29,321–29,373 cm−1 was assigned to the metal to ligand charge transfer. The magnetic susceptibility measurements for the solid Co(II) complexes are also indicative of three unpaired electrons per Co(II) ion suggestingCitation30 consistency with their octahedral environment.
The electronic spectra of the Cu (II) complexes () showed two low-energy weak bands at 14,984–15,158 cm−1 and 19,166–19,209 cm−1 and a strong high-energy band at 30,357–30,387 cm−1 and may be assigned to 2B1g→2A1g and 2B1g→2Eg transitions, respectivelyCitation31. The strong high-energy band, in turn, is assigned to metal→ligand charge transfer. Also, the magnetic moment values for the Cu (II) are indicative of anti-ferromagnetic spin-spin interaction through molecular association indicative of their octahedral geometryCitation32.
The electronic spectra of the Ni(II) complexes showed d-d bands in the region 10,398–10,448, 15,695–15,789, and 26,457–26,538 cm−1. These are assignedCitation33 to the transitions 3A2g(F)→3T2g(F), 3A2g(F)→3T1g(F) and 3A2g(F)→3T2g(P), respectively, consistent with their well-defined octahedral configuration. The band at 29,878–30,956 cm−1 was assigned to metal→ligand charge transfer. The magnetic measurements showed two unpaired electrons per Ni(II) ion suggestingCitation34 also an octahedral geometry for the Ni(II) complexes. The electronic spectra of the Zn(II) complexes exhibited only a high-intensity band at 28,939–29,128 cm−1 and are assignedCitation35 to a ligand-metal charge transfer.
Conductance and magnetic susceptibility measurements
The complexes (1)–(12), are showing their nonelectrolyticCitation36 nature as their molar conductance values (in DMF) fall within the range 43.2–51.6 Ω−1 cm2 mol−1. The magnetic moment values, at room temperature, of the complexes are given in . The measured magnetic moment values for cobalt (II) lie in the range of 4.87 to 4.98 B.M. indicating half spin octahedral complex. For Cu (II) complex, the observed magnetic moment values (1.84–1.91 B.M) lie in the range expected for a d9- system, which contain one unpaired electron with octahedral geometryCitation37. The measured values (3.35–3.42 B.M.) for the nickel (II) complexes suggestCitation38 octahedral geometry for these complexes. The zinc (II) complexes were found to be diamagnetic as expected.
Pharmacology
Antibacterial activity (in vitro)
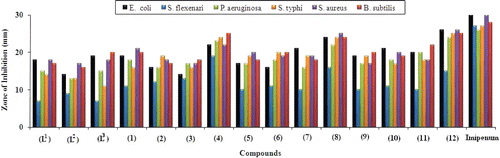
All the synthesized sulfonamides were tested against four gram-negative (E. coli, S. flexenari, P. aeruginosa, S. typhi) and two gram-positive (S. aureus, B. subtilis) bacterial strains () according to literature protocolCitation17,Citation18. The results were compared with those of the standard drug imipenum (). Moderate to significant activity against all gram positive and gram negative bacterial strains was observed for all synthesized ligands except the bacterial strain (b) which showed only small biological activity. The ligands (L1) and (L3) exhibited significant activity (>16 mm) against bacterial strains (a), (e), and (f) whereas, the ligand (L2) displayed the same significant activity against (e) and (f). On the other hand, all the three ligands showed moderate activity (>10 mm) against bacterial strains (c) and (d). The compounds (1)–(12) exhibited overall a significant activity (>16 mm) against bacterial strains (a), (c), (d), (e), and (f). However, a moderate activity (>10 mm) was observed by compound (3) against (a) and (b). Similarly, all the compounds (1)–(12) exhibited moderate activity (>10 mm) against the bacterial strain (b) except the compounds (4) and (8) which showed significant activity. Antibacterial activity is overall enhanced after complexation of the ligands. However, the zinc(II) complexes of all the ligands were observed to be the most active against all species.
Table 3. Antibacterial bioassay of ligands and metal (II) complexes.
The increased activity of the metal complexes can be explained on the basis of chelation theoryCitation39. Polarity of the metal ion reduces significantly by chelation, mainly because of partial sharing of positive charge of metal ions with the donor groups. Further, it increases the delocalization of π-electrons over the entire chelate ring and enhances the lipophilicity of the complexesCitation40–42 which favours its permeation through lipoid layers of fungus and bacterial membranes and retards their activity.
Antifungal activity (in vitro)
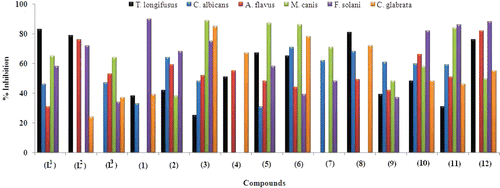
The antifungal screening of all the compounds was carried out against fungal strains T. longifusus, C. albican, A. flavus, M. canis, F. solani, and C. glaberate according to the literature protocolCitation18. The results of inhibition were compared with the standard drugs miconazole and amphotericin B ().
From the data (), it was observed that ligand (L1) showed significant activity (>75%) against (a) and (L2) against (a) and (c) fungal strains. The ligand (L1) showed moderate activity (51–75%) against (d) and (e), (L2) against (e) and (L3) against (c) and (d) fungal strains. While against all other fungal strains, the ligands were inactive or showed very weak activity (<50%). The metal(II) complexes (1)–(12) exhibited moderate (51–75%) to significant (above 75%) activity against various tested fungal strains. Complex (1) exhibited significant activity (>75%) against (e), (3) against (d), (e), and (f), (5) against (d), (6) against (d) and (f), (8) against (a), (10) against (e), (11) against (d) and (e) and the complex (12) against (a), (c), and (e) fungal strains. The complexes (4)–(6) exhibited moderate activity (51–75%) against fungal strain (a) while (2) and (6)–(11) showed moderate activity against (b). Similarly, the complexes (2)–(4), (10) and (11) displayed moderate (51–75%) activity against bacterial strain (c), (7), (10), and (12) against (d), (2) and (5) against (e) and (4), (8), and (12) against (f) fungal strain. All other complexes were inactive or showed weak activity (<50%).
Table 4. Antifungal bioassay (concentration used 200 µg/mL) of ligands and metal (II) complexes.
Minimum inhibitory concentration (MIC) for antibacterial activity
The data obtained after preliminary antibacterial screening showed that compounds (4), (8), and (12) were the most active (above 80%) and their average inhibition values were 22.5 (80.36%), 22.5 (80.36%), and 23.5 (83.93%), respectively. These compounds were therefore, selected for minimum inhibitory concentration (MIC) studies. The MIC of these compounds was in the range 1.468 × 10−8 to 1.729 × 10−7 M (). The data showed that compound (12) proved to be the most active against bacterial strain S. typhy as compared to (4) and (8).
Table 5. Brine Shrimp bioassay data of the ligands (L1)–(L3) and their complexes (1)–(12).
In vitro cytotoxic bioassay
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et alCitation20. From the data recorded in , it is evident that three compounds, (3), (7), and (11) displayed potent cytotoxic activity against Artemia salina, while the other compounds were almost inactive for this assay.
Conclusions
In the present study, in vitro antibacterial, antifungal, and cytotoxic activities of the prepared new sulfonamides and their metal (II) complexes were carried out on various bacterial/fungal strains. It was revealed that the antibacterial and antifungal activity of the ligands increased upon coordination/chelation. The enhancement of the activity in ligands upon chelation is reorganized on the basis of their structures and the mode of coordination/chelation. It has been suggested that chelation reduces the polarity of the metal ionCitation43–46 on partial sharing of its positive charge with the donor groups. The process of chelation increases the lipophilic nature of the metal atom, which in turn favoursCitation40,Citation47 its permeation through the lipoid layer of cell membrane of the microorganism. It has also been suggested that some functional groups such as azomethine or heteroaromatics present in these compounds displayCitation48,Citation49 extensive biological activities that may be responsible for the increase of hydrophobic character and liposolubility of the molecules. It ultimately enhances activity of the compounds and biological utilization ratio.
Acknowledgments
We are indebted to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for providing help in taking NMR, mass spectra, antibacterial and antifungal assays. One of us (HAZ) is grateful to Higher Education Commission (HEC), Government of Pakistan for the award to carry out this research.
Declaration of interest
The authors report no declarations of interest.
References
- Yamada S. Recent aspects of the stereochemistry of schiff-base-metal complexes. Coord Chem. Rev 1966;1(4):415–437.
- Jeewoth T, Bhowon MG and Wah HLK. Synthesis, characterization and antibacterial properties of Schiff bases and Schiff base metal complexes derived from 2,3-diamino- pyridine. Trans Met Chem 1999;24:445–448.
- Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 2003;47:3149–3154.
- Lv J, Liu T, Cai S, Wang X, Liu L, Wang Y. Synthesis, structure and biological activity of cobalt(II) and copper(II) complexes of valine-derived schiff bases. J Inorg Biochem 2006;100:1888–1896.
- del Campo R, Criado JJ, García E, Hermosa MR, Jiménez-Sánchez A, Manzano JL et al. Thiourea derivatives and their nickel(II) and platinum(II) complexes: antifungal activity. J Inorg Biochem 2002;89:74–82.
- Belaid S, Landreau A, Djebbar S, Benali-Baitich O, Bouet G, Bouchara JP. Synthesis, characterization and antifungal activity of a series of manganese(II) and copper(II) complexes with ligands derived from reduced N,N’-O-phenylenebis(salicylideneimine). J Inorg Biochem 2008;102:63–69.
- Gust R, Ott I, Posselt D, Sommer K. Development of cobalt(3,4-diarylsalen) complexes as tumor therapeutics. J Med Chem 2004;47:5837–5846.
- Hassan MU, Chohan ZH, Andrea S, Supuran CT. Carbonic anhydrase inhibitors: Schiff’s bases of aromatic and heterocyclic sulfonamides and their metal complexes. J Enz Inhib Med Chem 2004;19:263–279.
- Chohan ZH, Shaikh AU, Naseer MM. Metal-based isatin-bearing sulfonamides: Their synthesis, characterization and biological properties. Appl Organomet Chem 2006;20:729–739.
- Chohan ZH, Antibacterial copper(II) complexes of 1,1′-symmetric ferrocene-derived Schiff-base ligands: Studies of the effect of anions on their antibacterial properties. Appl Organomet Chem 2002;16:17–32.
- Hassan MU, Chohan ZH, Supuran CT. Antibacterial Zn(II) compounds of Schiff bases derived from some benzothiazoles. Main Group Metal Chem 2002;25:291–305.
- Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of benzothiazole-derived Schiff bases with antibacterial activity. J Enzyme Inhib Med Chem 2003;18:259–263.
- Chohan ZH, Scozzafava A, Supuran CT. Unsymmetrical 1,1′-disubstituted ferrocenes: Synthesis of Co(ii), Cu(ii), Ni(ii) and Zn(ii) chelates of ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with antimicrobial properties. J Enzyme Inhib Med Chem 2002;17:261–266.
- Puccetti L, Fasolis G, Vullo D, Chohan ZH, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff’s bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorg Med Chem Lett 2005;15:3096–3101.
- Chohan ZH, Shad HA, Tahir MN. 4-[(E)-(5-Chloro-2-hydroxy-benzyl-idene)amino]benzene-sulfonamide. Acta Crystallogr Sect e Struct Rep Online 2008;65:o57.
- Chohan ZH, Shad HA, Tahir MN, Khan IU. 4-{2-[(5-Chloro-2-hydroxy-benzyl-idene)amino]eth-yl}benzene-sulfonamide. Acta Crystallogr Sect e Struct Rep Online 2008;64:o725.
- Chohan ZH. Synthesis of cobalt(II) and nickel(II) complexes of Ceclor (cefaclor) and preliminary experiments on their antibacterial character. Chem Pharm Bull 1991;39:1578–1580.
- Atta-ur-Rahman Choudhary, MI, Thomsen WJ. (2001). Bioassay techniques for drug development. The Netherlands: Harwood Academic Publishers.
- McLaughlin JL, Chang CJ, Smith DL. (1991). In: Atta-ur-Rahman, ed. Studies in natural products chemistry, “Bentch-Top” bioassays for the discovery of bioactive natural products: An update, structure and chemistry (Part-B). Vol. 9. The Netherlands: Elsevier Science Publishers B.V., 383.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 1982;45:31–34.
- Finney DJ. (1971). Probit analysis. 3rd ed. Cambridge: Cambridge University Press.
- Maurya RC, Patel P. Synthesis, magnetic and special studies of some novel metal complexes of Cu(II), Ni(II), Co(II), Zn[II), Nd(III), Th(IV), and UO2(VI) with schiff bases derived from sulfa drugs, viz., Sulfanilamide/Sulfamerazine and o-vanillin. Spectr Lett 1999;32(2):213–236.
- Bellamy LJ. (1971). The infrared spectra of complex molecules. New York: John Wiley.
- Ferrero, JR. Low-frequency vibrations of inorganic and coordination compounds. New York: John Wiley.
- Nakamoto K. (1970). Infrared spectra of inorganic and coordination compounds. 2nd ed. New York: Wiley Interscience.
- Simmons WW. (1978). The Sadtler handbook of proton NMR spectra. Philadelphia: Sadtler Research Laboratories, Inc.
- Pasto DJ. (1969). Organic structure determination. London: Prentice Hall International.
- Lever ABP. (1984). Inorganic electronic spectroscopy. Amsterdam: Elsevier.
- Estes WE, Gavel DP, Hatfield WB, Hodgson DJ. Magnetic and structural characterization of dibromo- and dichlorobis(thiazole)copper(II). Inorg Chem 1978;17:1415–1421.
- Carlin RL. (1965). Transition metal chemistry. 2nd ed. New York: Marcel Decker.
- Burns GR. Metal complexes of thiocarbohydrazide. Inorg Chem 1968;7:277–283.
- Chohan ZH, Kausar S. Synthesis, structural and biological studies of nickel(II), copper(II) and zinc(II) chelates with tridentate Schiff bases having NNO and NNS donor systems. Chem Pharm Bull 1993;41:951–953.
- Balhausen CJ. (1962). An introduction to ligand field. New York: McGraw Hill.
- Chohan ZH, Rauf A, Naseer MM, Somra MA, Supuran CT. Antibacterial, antifungal and cytotoxic properties of some sulfonamide-derived chromones. J Enzyme Inhib Med Chem 2006;21:173–177.
- Chohan ZH, Kausar S. Biologically active complexes of nickel(II), copper(II) and zinc(II) with Schiff-base ligand derived from the reaction of 2-aminopyridine and pyrrol-2-carboxaldehyde–their synthesis and characterisation. Chem Pharm Bull 1992;40:2555–2556.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 1971;7:81–112.
- Lever ABP, Lewis J and Nyholm RS. Square-planar bisethylenediamine-metal complexes. J Chem Soc 1963;59:2552.
- Chohan ZH, Praveen M, Ghaffar A. Synthesis, characterisation and biological role of anions (nitrate, sulphate, oxalate and acetate) in Co(II), Cu(II) and Ni(II) metal chelates of some Schiff-base derived amino acids. Synth React Inorg Met-Org Chem 1998;28:1673.
- Jain M, Singh RV. Synthesis, characterization, and biotoxicity of N N donor sulphonamide imine silicon(IV) complexes. Bioinorg Chem Appl 2006;13743.
- Chohan ZH, Shaikh AU, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes with furanylmethyl- and thienylmethyl-dithiolenes: [1, 3-dithiole- 2-one and 1,3-dithiole-2-thione]. J Enzyme Inhib Med Chem 2006;21:733–740.
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enzyme Inhib Med Chem 2005;20:303–307.
- Chohan ZH, Hazoor A, Shad,and Nasim FH. Synthesis, characterization and biological properties of sulfonamide-derived compounds and their transitionmetal complexes. Appl Organometal Chem 2009;23:319–328.
- Patel MN, Patel NH, Patel KN, Dholakiya PP, Patel DH. Synthesis, characterization, and biocidal studies of some transition metal complexes containing bidentate monobasic hydroxyaldehydes and a neutral bidentate Schiff base. Synth React Inorg Metal-Org Chem 2003;33:51–62.
- Chohan ZH, Sherazi SKA. Synthesis and spectroscopic studies of biologically active Co(II), Cu(II) and Ni(II) complexes of hydrazine derived Schiff-base ligands. J Chem Soc Pak 1997;19:196.
- Chohan ZH, Shad HA, Youssoufi MH, Ben Hadda T. Some new biologically active metal-based sulfonamide. Eur J Med Chem 2010;45:2893–2901.
- Chohan ZH, Supuran CT. Organometallic compounds with biologically active molecules: In vitro antibacterial and antifungal activity of some 1,1′-(dicarbohydrazono)ferrocenes and their Co (II), Cu (II), Ni (II) and Zn (II) complexes. Appl Organomet Chem 2005;19:1207.
- Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (ii), copper (ii), nickel (ii) and zinc (ii) complexes of the antibiotic drug cephalothin (Keflin). J Enzyme Inhib Med Chem 2005;20:463–468.
- Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT. In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J Enzyme Inhib Med Chem 2006;21:95–103.
- Chohan ZH. Antibacterial and antifungal ferrocene incorporated dithiothione and dithioketone compounds. Appl. Organomet. Chem. 2006;20:112–116.
- Chohan ZH, Shad HA. Structural elucidation and biological significance of 2-hydroxy-1-naphthaldehyde derived sulfonamides and their first row d-transition metal chelates. J Enzyme Inhib Med Chem 2008;23:369–379.