Abstract
Gelatinase B/matrix metalloproteinase-9 (MMP-9) is a regulatory and effector metalloproteinase in inflammation. TNF-α is an important proinflammatory cytokine and is released by the action of a Zn2+-containing converting enzyme (TACE/ADAM-17). Both metallo-enzymes play important roles during the development of shock syndromes. Combinatorial chemical synthesis and subsequent library deconvolution were previously used to define a peptide inhibitor (Regasepin1) acting, almost to the same degree, on neutrophil collagenase/MMP-8 and MMP-9 in vitro, and protecting mice against lethal endotoxinemia in vivo. We have now extended this approach by incorporating D-form amino acids and residues preferred by TACE. A new peptide library was designed and synthesized, and by deconvolution new peptide inhibitors were defined. These included a TACE-specific inhibitor, an MMP-9- specific inhibitor, and inhibitors for both enzymes.
Keywords::
| Abbreviations | ||
| ADAM, | = | a disintegrin and metalloprotease |
| MMP, | = | matrix metalloproteinase |
| TACE, | = | TNF-α converting enzyme |
| LPS, | = | lipopolysaccharide |
| HBTU, | = | N-[(1H-benzotriazol-1-yl) (dimethylamino) methylene]-N-methylmethanaminium hexafluophosphate N-oxide |
| HOBt, | = | N-hydroxybenzotriazole |
| DIEA, | = | diisopropylethylamine |
| DMF, | = | dimethylformamide |
| DCM, | = | dichloromethane |
| TFA, | = | trifluoroacetic acid |
| EDT, | = | 1, 2-ethanedithiol |
Introduction
Matrix metalloproteinases (MMPs) form a group of more than 20 enzymes in the human speciesCitation1. All MMPs possess a zinc ion, coordinated by three histidines, in their active site. Another signature of MMPs is activation by the so-called cysteine switch mechanismCitation1–4. MMPs are divided functionally into constitutive and induced enzymes. In general, the constitutively expressed enzymes fulfil more homeostatic functions, whereas the induced members are linked to regulatory processes and pathological conditions. Gelatinase B is induced by many inflammatory mediators, suggesting that it may be an excellent pharmaceutical target in inflammatory diseasesCitation5.
Several acute inflammatory and chronic autoimmune diseases are mediated by gelatinase B. For instance, gelatinase B levels and activities are elevated in the synovial fluid of patients with rheumatoid arthritis (RA), whereas gelatinase A levels remain rather constantCitation6. By knock-out experiments in mice, it has been deduced that gelatinase B has a disease-promoting role in arthritisCitation7. In acute inflammatory processes, gelatinase B potentiates the human neutrophil activating chemokine interleukin-8 at least tenfold and thus further fuels acute inflammationCitation8. The role of gelatinase B in the control of acute and chronic inflammation is evidenced by the finding that it cleaves extracellular structural proteins, proteinase inhibitors such as α1-antitrypsinCitation9, cell surface molecules, and, last but not least, signaling molecules, including hormones, cytokines, and chemokinesCitation2,Citation5,Citation10. As a consequence, novel gelatinase inhibitors are in high demand for the treatment of inflammatory and vascular diseasesCitation11,Citation12.
Shock syndromes, including sepsis and endotoxinemia, are frequent causes of death. They cause multi-organ failure by an excessive and acute host inflammatory response toward microbial productsCitation13, e.g. endotoxins/lipopolysaccharides (LPS) and peptidoglycans (PGN). These effects are mediated by Toll-like receptor (TLR) signaling by many cell typesCitation14. Neutrophils possess TLRs and are abundant cells in the circulation. They degranulate during endotoxin shock and are involved in vascular damage. These cells not only produce neutrophil collagenase/MMP-8, gelatinase B/MMP-9 and MMP-25, but also release mediators capable of activating MMPsCitation15. An important pro-inflammatory cytokine is TNF-α. It induces the production and secretion of MMPs by many cell types. Furthermore, soluble TNF-α is released from cell membranes by TACE/ADAM-17, another metalloproteinase. TNF-α signaling is critical as a pathogenic mechanism in endotoxin shock. In the present work a newly designed peptide library for inhibition of MMP-9 and TACE was evaluated and peptide inhibitors for both enzymes were selected, and they also inhibited MMP-8 activity. Inhibitors of these target enzymes might be valuable tools in inflammatory diseases.
Methods
Enzyme activity of recombinant human MMP-3, MMP-8, MMP-9 and human TACE
Recombinant human MMP-9 was expressed in Sf9 insect cells and purified by gelatin-Sepharose affinity chromatography as described previouslyCitation16,Citation17. It was activated with 0.01 µM of the catalytic domain of MMP-3 (cd-MMP-3, Calbiochem) in assay buffer (100 mM Tris/HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl2 and 0.01% Tween-20) at 92 ng/µl (1 µM) as a stock solution as describedCitation8. Hydrolysis of the substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (substrate I, R&D Systems Europe Ltd) was monitored by measuring the increase in fluorescence (excitation wavelength = 328 nm, emission wavelength = 392 nm)Citation18. All assays were performed at 37°C in 100 µl assay buffer. The reaction was started by adding 10 µl gelatinase B stock solution (0.92 ng/µl) to a 100 µl reaction system with a substrate concentration of 10 µM, after which the rate of substrate hydrolysis was monitored.
Recombinant human MMP-8 was purchased as an active form enzyme (Sino Biological Inc.). The enzyme activity was detected with the same fluorogenic peptide substrate as that for MMP-9. All MMP-8 assays were performed at 37°C in 100 µl assay buffer with 20 µl recombinant human MMP-8 (2 ng/µl). The final substrate concentration was 10 µM.
The activity of recombinant human MMP-3 (catalytic domain, Calbiochem) was detected with the substrate Mca-Arg-Pro-Lys-Pro-Val-Glu-Nval-Trp-Arg-Lys(Dnp)-NH2 (substrate II, R&D Systems Europe Ltd) (excitation wavelength = 320 nm, emission wavelength = 405 nm). These assays were performed at 37°C in 100 µl assay buffer. The reaction was started by adding 10 µl MMP-3 stock solution (1 ng/µl) to a 100 µl reaction system with a final substrate concentration of 10 µM, after which the rate of substrate hydrolysis was monitored.
TACE was purchased as an active form enzyme (R&D Systems). Hydrolysis of the TACE-substrate Mca-Pro-Leu-Ala-Gln-Ala-Val-Dpa-Arg-Ser-Ser-Ser-Arg-NH (substrate III, R&D Systems Europe Ltd) was monitored by measuring the increase in fluorescence (excitation wavelength = 320 nm, emission wavelength = 405 nm). TACE assays were performed at 37°C in 100 µl assay buffer. The reaction was started by adding 4 µl TACE stock solution (1 ng/µl) to a 100 µl reaction system with a final substrate concentration of 10 µM, after which the rate of substrate hydrolysis was monitored.
Enzyme kinetic work
A 100 µl reaction system was set up, which included 10 µl MMP-9 (10 ng/µl), 50 µl peptide inhibitors (final concentrations were indicated in ), 30 µl assay buffer, and 10 µl fluorogenic peptide substrate (substrate I). The final fluorogenic peptide substrate concentrations of 4, 2, 1.33, 1 and 0.8 µM in the presence or absence of peptide inhibitors were used to generate the Lineweaver-Burk plots. By comparision of the Lineweaver-Burk plots genrated in the presence or absence of a peptide inhibitor in the same graph, the inhibition mode of the peptide inhibitor was derived.
Detection of enzyme inhibition
A TECAN Safire plate reader was used to detect MMP-9 activity. To screen inhibitor samples against MMP-9, a 100 µl enzyme incubation system was used. This included 10 µl recombinant human gelatinase B (0.92 ng/µl), 10 µl substrate I (4 mM, R&D Systems, diluted to 100 µM before the assays), 40 µl inhibitor peptide mixtures (20 µM total peptide concentration for samples from libraries A, and 4 µM for samples from library B), and 40 µl assay buffer (100 mM Tris/HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl2 and 0.01% Tween-20). The mixture was incubated at 37°C. A sample without any peptide inhibitors was included as positive control. The kinetics of each reaction was detected and fluorescence versus time was measured. All data were transferred to Excel files and the slope of each fluorescence-time graph was calculated. This represented the relative enzyme activity in the corresponding reaction mixture. Decreases of the slopes represented inhibition of the enzyme activity by the peptide samples. The used plate reader simultaneously detected the fluorescence of seven wells.
TACE activity was detected in a similar way. To screen inhibitor samples against TACE, a 100 µl enzyme incubation system was used, which included 4 µl recombinant human TACE (1 ng/µl), 10 µl substrate III (6 mM, R&D Systems, diluted to 100 μM before the assays), 40 µl peptide mixtures (20 µM total peptide concentration for samples from library A, and 4 µM for samples from library B), and 46 µl assay buffer (25 mM Tris/HCl, pH 9.0, 0.005% Tween-20). The mixture was incubated at 37°C. A sample without any peptide inhibitors was included as positive reaction control. Collected data were processed in a similar way as those for the analysis of MMP-9.
Chemical synthesis
All peptide synthesis was by Fmoc chemistry. Mixtures of four equivalent amounts of HBTU (N-[(1H-benzotriazol-1-yl) (dimethylamino) methylene]-N-methylmethanaminium hexafluophosphate N-oxide), HOBt (1-hydroxybenzotriazole), DIEA (diisopropylethylamine), and Fmoc-amino acids with respect to the available amines in the solid phase were used. The synthesis was carried out on Rink resin (Advanced ChemTech), with an Fmoc-derived acid labile benzhydryl amine as linker. Pre-activation was not applied, and the reaction was for 8 hours. The coupling of individual amino acids was followed by capping of the residual amines in a mixture of pyridine/acetic anhydride/N-methylimidazol (4:1:0.5) for 10 minutes. Fmoc deprotection was achieved by shaking with 20% piperidine in DMF (dimethylformamide) for 15 minutes. The peptides were recovered from the solid support with the use of TFA in a mixture of TFA/H2O/thioanisole/EDT (1, 2-ethanedithiol)/crystalline phenol (10:0.5:0.5:0.25:0.75).
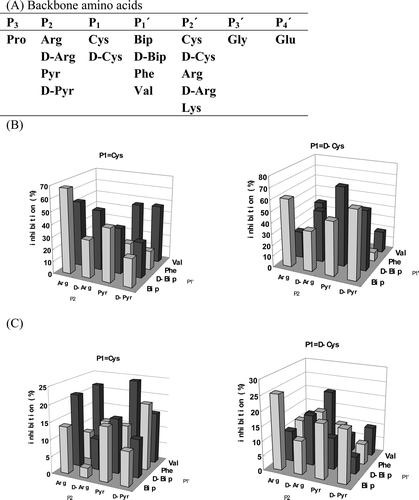
Peptide libraries were synthesized under the above mentioned chemical conditions, and all the amino acids were double-coupled. The mix-and-split method was used. Synthesis of library A was started with 0.3 g Rink resin (0.37 mmol free N-terminal/g dry resin). After coupling of amino acids for position P2′ (), the resin was mixed together and suspended in 50 ml DMF/DCM (dichloromethane) (1:1). The suspension was shaken in three dimensions for 15 minutes, and then the resin was separated into aliquots by equal division of the suspension. The number of aliquots was determined by the number of incoming amino acids in position P1′. After coupling of the amino acids for position P1′, P1 and P2, the resin was not mixed anymore so that 32 samples were generated which represented the combinatorial possibilities for the three positions (P2×P1×P1′ = 4 × 2×4). After comparison of the inhibitory effect of the peptide mixtures of the 32 samples ( and ) the preferable amino acid in these three positions was deduced. Six samples were selected for library B (). Library B was synthesized with splitting and without anymore mixing. Thirty samples were generated (6 × 5) so that all the combinatorial possibilities for the four positions (from P2 to P2′) were included. Each sample of library B contained only one peptide.
Table 1. Design of library B.
Figure 1. Design of library A and inhibitor screen of the samples of library A with MMP-9 and TACE. (A) Amino acid candidates selected for different positions (from P2 to P2′) for library A. (B) and (C) Inhibitory effects of samples from library A on MMP-9 (B) and TACE (C) activity. Each bar represents the inhibitory effect (Z-axis) of a mixture of five peptides. The X-axis indicates the amino acids selected for P2 position. The Y-axis indicates the amino acids selected for P1′ position. The histograms on the left indicate the inhibitory effects of samples with cysteine at the P1 position, whereas the right side figure indicates the inhibitory effects of samples with D-form cysteine at the P1 position. Pyr indicates pyridylalanine, Bip indicates biphenylalanine.
The study was approved by the Investigational Review Board of China Pharmaceutical University.
Results
Design and synthesis of inhibitor libraries
Previously, an ideal inhibitor length of seven amino acids (from P3 to P4′) was selected as the backbone for the inhibitory librariesCitation12. We now selected the amino acids as indicated in . The new amino acid candidates were selected only for positions from P2 to P2′ as Pro is prefered for P3 positionCitation18 and the amino acids in P3′ and P4′ positions extend out of the active site of the MMPs and therefore were kept constant (Gly-Glu)Citation18. The amino acids indicated in the top line encode Regasepin1Citation12. Pyr at P2 and Arg at P2′ came from the sequence of Regasepin2Citation19. D-form amino acids were introduced to evaluate their use. Val at P1′ and Lys at P2′ were preferred amino acids of TACE and selected to enhance TACE-specificity. Cys at P1 was supposed to play a role as a Zinc-binding group. All the library samples were screened with two target enzymes: recombinant human MMP-9 and recombinant human TACE.
The peptide library was deconvoluted by the iterative deconvolutionCitation20 method. The basic idea is to define the most suitable amino acid residue(s) for each position (from P2 to P2′) step by step and by lining up the best amino acid residues, until the peptide with the strongest inhibitory effect was found. A two-step screening strategy was used, and during the first step thirty-two samples were generated, which included all the combinatorial possibilities in positions P2, P1 and P1′ (P2×P1×P1′ = 4 × 2×4 = 32). Each of the thirty-two samples is a mixture of five peptides (five amino acid candidates in P2′). By comparison, the most effective inhibitor sequences for positions P2, P1 and P1′ were defined. As the samples of the library were screened with two target enzymes, two inhibitory profiles were generated — one for MMP-9 () and the other for TACE ().
Based on the screening results from library A, six sequences (from P2 to P1′) were selected for library B. Of these six sequences, D-Pyr-D-Cys-Bip, Arg-D-Cys-Bip, Arg-Cys-D-Bip, Pyr-Cys-Val were derived from the samples that inhibited both MMP-9 and TACE activity. Pyr-D-Cys-D-Bip specifically inhibited MMP-9 activity, whereas D-Arg-D-Cys-Val specifically inhibited TACE activity. During the second-step screening, thirty samples were generated for library B (six sequences for the P2 to P1′positions and 5 different residues of P2′) and each sample contained a definite peptide (see ).
The 30 peptides from Library B were individually synthesized, and their inhibitory effects on MMP-9 and TACE were measured. The average percentages of inhibition after three reproducible screenings are shown in and . From the screening results, it was deduced that several peptides showed inhibition of both enzymes. The sequence Arg-Cys-D-Bip-D-Arg (from P2′ to P2) showed a 62% inhibition of MMP-9 activity compared to the control sample, whereas it only inhibited 9% TACE activity at the same concentration. Another peptide with sequence D-Pyr-D-Cys-Bip-D-Cys inhibited 30% TACE activity, whereas it left MMP-9 uninhibited.
Table 2. Percentage inhibition of MMP-9 activity by peptides from library B.
Table 3. Percentage inhibition of TACE activity by peptides from library B.
We used sensitive and reliable methods to efficiently screen all library samples. The enzyme activity was represented as the slope of the straight line formed by at least 4 data points (the fluorescence of the sample was detected every minute for 4 minutes). In accordance with previous results, the peptide with sequence Arg-Cys-Bip (P1′ to P2 position; Bip is biphenylalanine) showed the highest inhibitory activity against MMP-9, and this Regasepin 1 sequence was included in the present analysis ()Citation12. It is interesting to notice that some peptides in library B also showed relatively strong inhibition of both MMP-9 and TACE (e.g. Pro-Arg-D-Cys-Bip-Arg-Gly-Glu, Pro-D-Pyr-D-Cys-Bip-Arg-Gly-Glu and Pro-D-Pyr-D-Cys-Bip-Lys-Gly-Glu). Five sequences (Pro-Arg-Cys-D-Bip-D-Arg-Gly-Glu, Pro-Arg-D-Cys-Bip-Arg-Gly-Glu, Pro-D-Pyr-D-Cys-Bip-D-Cys-Gly-Glu, Pro-D-Pyr-D-Cys-Bip-Arg-Gly-Glu and Pro-D-Pyr-D-Cys-Bip-Lys-Gly-Glu) were selected for further investigation. This selection was based on their inhibitory activity on MMP-9 and TACE, and the relative selectivity between the two target enzymes.
Inhibitory activity
The inhibitory activities of the five selected peptide inhibitors against stromelysin-1/MMP-3, neutrophil collagenase/MMP-8, gelatinase B/MMP-9 and TACE/ADAM-17 were detected by conversion of fluorogenic peptide substratesCitation21. The IC50 of the five peptide inhibitors on the target enzymes were presented in . As expected, the peptide with sequence Pro-Arg-Cys-D-Bip-D-Arg-Gly-Glu inhibited MMP-9 activity with an IC50 value of 0.75 µM, whereas it inhibited only 50% TACE activity at the concentration of 20 µM. On the other hand, the peptide with sequence Pro-D-Pyr-D-Cys-Bip-D-Cys-Gly-Glu showed selectivity for TACE. The other three inhibitors showed relatively strong inhibition of both MMP-9 and TACE. Therefore, the aim of the library screening to obtain selective inhibitor for MMP-9 and TACE was fulfilled.
Table 4. IC50 values of the selected peptide inhibitors on the target enzymes.
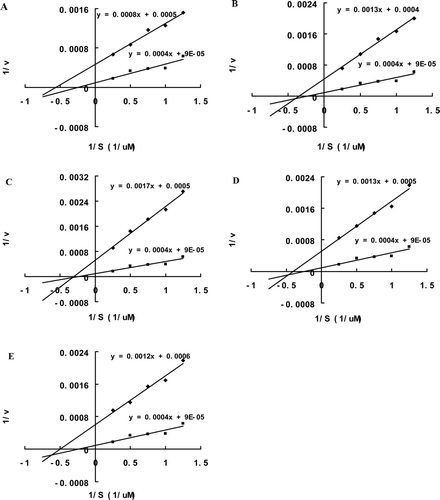
In order to know the kinetic mode by which the inhibitory peptides interact with MMP-9, Lineweaver-Burk plots for cleavage of fluorogenic peptide substrate by MMP-9 in the presence or absence of each peptide inhibitor were generated. As shown in , all the five peptide inhibitors showed mixed non-competitive inhibition of MMP-9. The KI and KI′ values for each inhibitor were calculated from the plots and were presented in .
Table 5. KI or KI′ values of the selected peptide inhibitors on MMP-9.
Figure 2. Lineweaver-Burk plots of inhibition of MMP-9 by the selected peptide inhibitors. Enzyme cleavage of the fluorogenic peptide substrate was performed with the substrate concentration of 4, 2, 1.33, 1 and 0.8 uM in the presence or absence of a peptide inhibitor. Both intercepts and the slope changed in the presence of each inhibitor, which indicated the mode of mixed non-competitive inhibition.
Discussion
Inflammatory cytokines, including TNF-α, and matrix metalloproteinases, such as MMP-9 and MMP-8 have been proposed as targets for the therapy of shock syndromes, including endotoxin shockCitation11. In a previous report, we targeted MMP-8 and MMP-9 as neutrophil-derived proteases and showed that a peptide inhibitor protected against endotoxin shockCitation22. In view of the fact that TNF-α is processed by TACE/ADAM-17, we double-selected peptide inhibitors that target both MMP-9 and TACE. Incorporation of unnatural amino acids, including D-form amino acids, into designed peptide sequences and use of validated biochemical technology enabled us to identify new MMP-9 and/or TACE inhibitors from synthetic peptide libraries. The first finding was that the D-form amino acids incorporated well into the active site of the enzyme. For peptide inhibitor (Pro-D-Pyr-D-Cys-Bip-Lys-Gly-Glu), D-Cys functioned as a zinc-binding group. The spherical shape of the thiol group generates the same low dissolution cost and easy ionization, as observed in the L-formCitation18.
Peptide inhibitors with sequence Pro-Arg-D-Cys-Bip-Arg-Gly-Glu, Pro-D-Pyr-D-Cys-Bip-Arg-Gly-Glu or Pro-D-Pyr-D-Cys-Bip-Lys-Gly-Glu, identified as an MMP-9 inhibitor, possessed relative selectivity against TACE (2 to 8 μM). The latter values are comparable to that of Regasepin2Citation19. These peptide inhibitors were also good inhibitors of MMP-8, another MMP that is abundantly released during neutrophil degranulation. The peptide inhibitors were found to have a good solubility (267 mM in 100% DMSO). The latter value is better than that of Regasepin1Citation22 and comparable to that of the hydroxamate GM-6001Citation23. However, the potency of GM-6001 (low nanomolar activity) is higher than the peptide inhibitors here. As a critical notice, peptide inhibitors of Regasepin or those presented here are active in the micromolar range and need to be parenterally administered. This implies that these are merely biochemical probes, rather than end products. However, the establishment of good methodologies to test inhibitors and techniques, toward probing selectivity within enzyme families, constitutes a way to define better and hopefully useful new moleculesCitation11.
Another way to obtain peptide inhibitors for MMPs is by the phage display techniqueCitation24. In this work, up to 5 × 107 combinations were generated and a gelatinase recognizing motif–HWGF was found de novo. By the operation limitation, a combinatory peptide library contains peptides on a 103 level. However, in the design of our peptide library, the amino acid building blocks were not randomly selected, but on the basis of known informationCitation12, e.g. collagen II digestionCitation6, a phage display screening results, to find suitable substrate sequence for all MMPsCitation25 and another combinatorial peptide libraryCitation26, etc. Therefore, the designed peptide library was a target-focused and degenerated library toward ligand identification. In the present work, the library was designed based on the backbone of Regasepin1/2Citation12,Citation19, and by incorporating D-form amino acids and suitable substrate sequence for TACE. This library was deconvoluted with two aims: to obtain a peptide inhibitor with prolonged in vivo half-life, and with selectivity for MMP-9 or TACE. A peptide inhibitor with selectivity only for TACE was obtained. Previous work showed that the Pro was preferred in P3 position, Cys was preferred at P1 position, and Bip was preferred at P1′position. These principles were well followed in the present work. The peptide D-Pyr-D-Cys-Bip-D-Cys (from P2 to P2′) showed a strong inhibition of TACE activity, which means that the amino acids in P2 and P2′ may confer selectivity without losing inhibitory efficiency. Preliminary in vivo work was performed to evaluate the roles of the peptide inhibitor (with sequence Pro-D-Pyr-D-Cys-Bip-Lys-Gly-Glu) in acute inflammation with an endotoxin shock model. Regasepin2 was used as a positive control peptide, and it protected mice against lethal endotoxin shock in Kunming strain mice (data not shown). This effect of Regasepin2 confirmed its efficiency in different mouse strains. However, the tested peptide failed to protect the mice from lethal endotoxin shock (data not shown), although it has a similar in vitro inhibitory profile as that for Regasepin1/2. The peptide inhibitor with sequence Pro-Arg-Cys-D-Bip-D-Arg-Gly-Glu and Pro-D-Pyr-D-Cys-Bip-D-Cys-Gly-Glu showed selectivity for MMP-9 and TACE (see ). As has been shown before, Regasepin2, which showed inhibition of both, MMP-8, MMP-9 and TACE, can protect mice from lethal endotoxin shockCitation19. It is interesting to identify the inhibition of which enzyme is more important in this case, and this part of the work is going on.
The peptide libraries designed in this and the previous studies were based on a suitable peptide substrate of MMP-9 or the peptide inhibitor derived from it. It is reasonable to think that the peptide inhibitors obtained from the libraries were competitive inhibitors of MMP-9. Enzyme kinetic work was performed and it was found that all the five selected peptide inhibitors showed mixed non-competitive inhibition of MMP-9. A reasonable explanation is that inhibitor binding has altered the conformation of the enzyme active site. MMP-9 is one of the “deep pocket” MMPs, and the biphenyl site group of Bip at P1′ position incorporates well into this “pocket”Citation12, and also, the side chain of Cys or D-Cys at P1 position acts as a zinc-binding group. The fluorogenic peptide substrate does not have these characters. It is possible that the conformation of the enzyme active site interacting with peptide inhibitors is different from the conformation interacting with the fluorogenic peptide substrates.
The peptide inhibitors developed in the previousCitation12,Citation19 and present work can be applied not only based on their inhibition of relevant enzymes, but also on their “targeting” potential. Being a peptide, its sequence can be readily incorporated into a peptide/protein with known function and may target the new peptide/protein where it is needed in vivo. For example, endostatin is a well-known endogenous anti-angiogenic protein composed of 183 amino acids. Its fragment (amino acid 60 to 70), named ES-2, retains its anti-angiogenic effect in an in vitro CAM assay, while it has no such effect in an in vivo murine tumor model in which angiogenesis develops aggressively within the tumor and for the growth of the tumorCitation27. By incorporating a RGD-4C (a polypeptide that recognizes integrin αvβ3, which is highly expressed by endothelial cells within the new blood vessels) sequence, the modified RGD-4C-ES-2 peptide accumulates in the tumor and the angiogenesis and tumor growth were inhibitedCitation28. Phages which displayed the sequence CTTHWGFTLC (which is a micromolar peptide inhibitor for MMP-2 and MMP-9) accumulated within Kaposi sarcoma, on a comparable and even higher level than RGD-4C phagesCitation24. This inspires us to make use of the molecules as MMP inhibitors and at the same time as “targeting” motifs.
Conclusions
We developed a combined method to define a novel peptide gelatinase B and TACE inhibitor out of a peptide library. The building-block character of enzyme substrates and the power of the screening technique make this method an ideal tool to select peptide inhibitors for an endopeptidase, providing a sensitive activity assay exists. Peptide inhibitors were found to inhibit neutrophil collagenase/MMP-8, gelatinase B/MMP-9 and TNF-α converting enzyme/ADAM-17.
Declaration of interest
The present study was supported by the Fund for Scientific Research (FWO-Vlaanderen), and the “Geconcerteerde OnderzoeksActies” (2007–2011). The authors announce that there are no actual or potential conflicts of interest of this article.
References
- Barrett AJ, Rawlings ND, Woessner JF. Handbook of Proteolytic enzymes. London: Academic Press, 2003.
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516.
- Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002;2:657–672.
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA 1990;87:5578–5582.
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 2002;37:375–536.
- Van den Steen PE, Proost P, Grillet B, Brand DD, Kang AH, Van Damme J et al. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. Faseb J 2002;16:379–389.
- Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 2002;169:2643–2647.
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000;96:2673–2681.
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA et al. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000;102:647–655.
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol 2007;42:113–185.
- Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 2007;6:480–498.
- Hu J, Fiten P, Van den Steen PE, Chaltin P, Opdenakker G. Simulation of evolution-selected propeptide by high-throughput selection of a peptidomimetic inhibitor on a capillary DNA sequencer platform. Anal Chem 2005;77:2116–2124.
- Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993;328:1471–1477.
- Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol 2002;9:2–10.
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–376.
- Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem 1991;198:391–398.
- Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J Biol Chem 2006;281:18626–18637.
- Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev 1999;99:2735–2776.
- Hu J, Dubois V, Chaltin P, Fiten P, Dillen C, Van den Steen PE et al. Inhibition of lethal endotoxin shock with an L-pyridylalanine containing metalloproteinase inhibitor selected by high-throughput screening of a new peptide library. Comb Chem High Throughput Screen 2006;9:599–611.
- Konings DA, Wyatt JR, Ecker DJ, Freier SM. Strategies for rapid deconvolution of combinational libraries: comparative evaluation using a model system. J Med Chem 1997;40:4386–4395.
- Knight CG, Willenbrock F, Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett 1992;296:263–266.
- Hu J, Van den Steen PE, Dillen C, Opdenakker G. Targeting neutrophil collagenase/matrix metalloproteinase-8 and gelatinase B/matrix metalloproteinase-9 with a peptidomimetic inhibitor protects against endotoxin shock. Biochem Pharmacol 2005;70:535–544.
- Solorzano CC, Ksontini R, Pruitt JH, Auffenberg T, Tannahill C, Galardy RE et al. A matrix metalloproteinase inhibitor prevents processing of tumor necrosis factor alpha (TNF alpha) and abrogates endotoxin-induced lethality. Shock 1997;7:427–431.
- Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkilä P et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 1999;17:768–774.
- Kridel SJ, Chen E, Kotra LP, Howard EW, Mobashery S, Smith JW. Substrate hydrolysis by matrix metalloproteinase-9. J Biol Chem 2001;276:20572–20578.
- McGeehan GM, Bickett DM, Green M, Kassel D, Wiseman JS, Berman J. Characterization of the peptide substrate specificities of interstitial collagenase and 92-kDa gelatinase. Implications for substrate optimization. J Biol Chem 1994;269:32814–32820.
- Xu HM, Yin R, Chen L, Siraj S, Huang X, Wang M et al. An RGD-modified endostatin-derived synthetic peptide shows antitumor activity in vivo. Bioconjug Chem 2008;19:1980–1986.
- Yin R, Zheng H, Xi T, Xu HM. Effect of RGD-4C position is more important than disulfide bonds on antiangiogenic activity of RGD-4C modified endostatin derived synthetic polypeptide. Bioconjug Chem 2010;21:1142–1147.
