Abstract
Effects of Co+2, Zn+2, Ca+2, Fe+2, Mn+2, Cr+3, Sn+2 and Mg+2 exposure of rainbow trout liver on the enzymatic activity of glutathione reductase (GR) were investigated. GR was purified from rainbow trout liver 1419-fold with a yield of 38.41% and a specific activity of 22.846 U/mg protein. Following enzyme isolation, inhibitory effects of Co+2, Zn+2, Ca+2, Fe+2, Mn+2, Cr+3 and Sn+2 were analysed. IC50 values of the metal ions ranged from 42.2 to 657 µM, and the Ki constants ranged from 24.2 to 567µ. Co+2, Zn+2, Fe+2 and Cr+3 exhibited competitive inhibition whereas others inhibited the enzyme in non-competitive manner. Cobalt was the most powerful inhibitor among others having the lowest IC50 and Ki values. Magnesium exhibited activatory effect on the enzyme.
Introduction
The undesirable biologic effects of oxidative agents such as free radical and reactive oxygen species are eliminated by enzymatic and non-enzymatic antioxidant defence systems. Enzymatic defence is provided by many enzyme systems, such as glutathione reductase, glutathione peroxidase, glutathione S-transferase, superoxide dismutase, catalase and DNA repair enzymes. Particularly, glutathione reductase (Glutathione: NADP+ oxidoreductase, EC 1.8.1.7; GR) is the key enzyme in the glutathione metabolism. Glutathione reductase is essential for the maintenance of cellular glutathione in its reduced form, which is highly nucleophilic for many reactive electrophilsCitation1,Citation2. This flavin enzyme is essential for reduction of glutathione disulfide (GSSG) to the reduced form (GSH), necessary for protection of the cells against oxidative stress as an antioxidant. GR catalyzes the reduction of glutathione disulfide (GSSG) at the expense of NADPH:
GSH is also a reaction partner for the detoxification of xenobiotics, it is a cofactor in isomerization reactions, and is a storage and transport form of cysteineCitation3,Citation4. It maintains the thiol redox potential in cells keeping sulfhydryl groups of intracellular proteins in the reduced formCitation5. Decreased GSH levels have been reported in several diseases, such as acquired immune deficiency syndrome (AIDSCitation6), adult respiratory distress syndrome,Citation7 Parkinson’s disease,Citation8 and diabetesCitation9. In addition, recent results suggest that GSH is essential for cell proliferation,Citation10 and it plays a role in the regulation of apoptosis.Citation11 Alternatively, high GSSG concentrations inhibit a number of important enzyme systems including protein synthesisCitation12.
Metals are notable for their wide environmental dispersion from such activity; their tendency to accumulate in selected tissues of the human body; and their overall potential to be toxic even at relatively minor levels of exposure. Some metals, such as copper and iron are essential for life and play irreplaceable roles in, for example, the functioning of critical enzyme systems. Other metals are xenobiotics, i.e. they have no useful role in human physiology (and most other living organisms) and, even worse, as in the case of lead and mercury, may be toxic even at trace levels of exposureCitation13. Exposure to heavy metals is an important problem of environmental toxicology. Most heavy metals are toxic to humans, animals and plants. Man is at great risk of suffering from health hazards associated with toxic metals because of bioaccumulationCitation14.
GR has been purified from different tissue cells, using different purification procedures. All reported purification procedures involve several chromatographic stepsCitation2,Citation15. Effects of many drugs, metal ions and chemicals on human and fish GR enzyme activities have been investigated so farCitation2,Citation16–19. However, no reports could be found in the literature on the effects of these metals on fish liver GR. Therefore, in the present study, we purified GR from rainbow trout and examined in vitro inhibition effects of some metal ions on the enzyme.
Materials and methods
Chemicals
Sephadex G-200, NADPH, GSSG, protein assay reagents and chemicals for electrophoresis were obtained from Sigma Chem. Co., Germany. 2′, 5′-ADP Sepharose-4B was obtained from GE Healthcare, Munich, Germany. All other chemicals used were of analytical grade and obtained from either Sigma-Aldrich or Merck, Germany.
Glutathione reductase enzyme activity
Glutathione reductase enzyme activity was measured by Beutler’s methodCitation20. One enzyme unit is defined as the oxidation of 1 µM NADPH per min under the assay condition (25°C, pH: 8.0).
Preparation of the homogenate
Rainbow trout (200 ± 20 g) were obtained from the Department of Fishery Sciences, Agriculture Faculty at Atatürk University. Liver samples were taken from each trout. The livers were washed three times with 50 mM Tris-HCl + 0.1 M Na2SO4 (pH 8.0) and homogenized by liquid nitrogen (approximately −163°C), transferred to the same buffer, and centrifuged at 4°C, 15,000 × g for 60 min. Supernatant was used in further studies.
Ammonium sulphate precipitation and dialysis
The homogenate was subjected to ammonium sulphate precipitation. For this aim, the homogenate was adjusted to 30–70% saline saturation with solid (NH4)2SO4, respectively. The precipitate was dissolved in a minimum amount of Tris-HCl buffer (50 mM; pH = 8.0). Then, it was dialyzed at 4°C in 1 mM EDTA + 10 mM Tris-HCl buffer (pH 8.0) for 2 h. Partially purified enzyme solution was kept at 4°C.
2′, 5′-ADP Sepharose-4B affinity chromatography
Two grams of dried 2′, 5′-ADP Sepharose-4B was used for a column (1 × 10 cm) of 10 mL bed volume. The gel was washed with 300 mL of distilled water to remove foreign bodies and air, suspended in 0.1 M K-acetate + 0.1 M K-phosphate buffer (pH 6.0), and packed in the column. The column was equilibrated with 50 mM K-phosphate buffer including 1 mM EDTA (pH 6.0). The flow rate was adjusted to 20 mL/h for washing and equilibration. Previously obtained dialyzed sample was loaded onto the 2′, 5′-ADP Sepharose-4B affinity column and the column was washed with 25 mL of 0.1 M K-acetate + 0.1 M K-phosphate, pH 6.0 and with 25 mL of 0.1 M K-acetate + 0.1 M K-phosphate, pH 7.85. Washing was continued with 50 mM K-phosphate buffer including 1 mM EDTA, pH 7.5, until the final difference in the absorbance reached 0.05 at 280 nm. The enzyme was eluted with a gradient of 0 to 0.5 mM GSH + 1 mM NADPH in 50 mM K-phosphate, containing 1 mM EDTA (pH 7.5). Active fractions were collected and dialyzed with equilibration buffer. All procedures were performed at 4°C.
Sephadex G-200 gel filtration chromatography
Dry Sephadex G-200 (3 g) was used for a 165 mL column (2 × 50 cm) bed volume. The gel was incubated in distilled water at 90°C for 5 h and loaded onto the column after removing the air. Flow rate was adjusted to 15 mL/h by means of a peristaltic pump. Then the column was equilibrated with 50 mM Tris-HCl + 50 mM KCl buffer, pH 8.0 until final absorbance difference reached zero at 280 nm. Dialyzed sample was mixed with 5% glycerol. Final sample was loaded onto the column and elutions were collected in 2 mL amounts. In each fraction, enzyme activity was determined at 340 nm. Active fractions were lyophilized and stored at −85°C in order to use in kinetic and electrophoretic studies.
Protein determination
The protein content was quantified spectrophotometrically at 595 nm according to Bradford’s methodCitation21 for all samples using bovine serum albumin as the standard.
SDS polyacrylamide gel electrophoresis (SDS-PAGE)
The control of enzyme purity, using Laemmli’s procedureCitation22, was carried out in 8% and 3% acrylamide concentrations for running and stacking gel, respectively. Ten percent SDS was added to the gel solution. The gel was stabilized in a solution containing 50% propanol +10% TCA +40% distilled water for 30 min. Staining was done for about 2 h in a solution of 0.1% Coommassie Brillant Blue R-250 + 50% methanol +10% acetic acid. Finally, washing was carried out in the solution of 50% methanol +10% acetic acid +40% distilled water until protein bands were cleared.
In vitro effects of metal ions
In order to determine the effects of the metal ions on fish liver GR, several concentrations of (0.02–1 mM) the metal ions were added into the reaction medium. The enzyme activity was measured and an experiment in the absence of metal ion was used as control (100% activity). IC50 values were obtained from activity (%)–metal ion concentration plots ().
Figure 1. Activity %-[Co+2] regression analysis graphs for fish liver GR in the presence of five different cobalt concentrations.
![Figure 1. Activity %-[Co+2] regression analysis graphs for fish liver GR in the presence of five different cobalt concentrations.](/cms/asset/d8ea9064-e4a5-4c3c-aa6e-304129b64dd7/ienz_a_615745_f0001_b.gif)
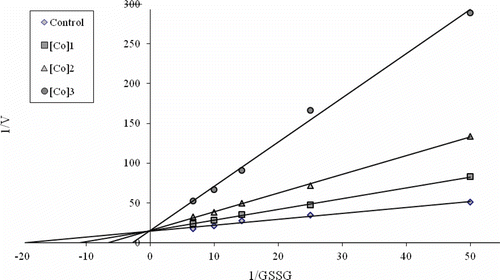
To determine Ki constants in the media with inhibitor, the substrate (GSSG) concentrations were 0.015, 0.04, 0.07, 0.10, and 0.15 mM. Inhibitor (metal ions) solutions were added to the reaction medium, 3 different inhibitor concentrations were used in 1 mL of total reaction volume. Lineweaver-Burk graphsCitation23 were drawn by using 1/V vs. 1/[S] values, and Ki constant were calculated from these graphs (). Regression analysis graphs were drawn for IC50 using inhibition % values by a statistical package (SPSS for windows, version 10.0) on a computer (Student’s t-test; n = 3).
Results and discussion
In this study, rainbow trout (Oncorhynchus mykiss) liver GR enzyme was purified using simple chromatographic methods. Purification procedure was carried out by the preparation of the homogenate, ammonium sulphate precipitation, and affinity chromatography on 2′, 5′-ADP Sepharose-4B, and gel filtration chromatography on Sephadex G-200. Pyridine nucleotides are able to bind not only glutathione reductase but also to several other proteins.Citation24 For that reason, after 2′, 5′-ADP Sepharose-4B affinity chromatography, Sephadex G-200 gel filtration chromatography was performed. As a result of the consecutive steps, the enzyme was purified up to 1419-fold with a recovery ratio of 38.41% compared to the homogenate (). After preparation of the homogenate, precipitate saturation of the enzyme was determined as 30–70% with solid (NH4)2SO4.
Table 1. Purification scheme of GR from rainbow trout (Oncorhynchus mykiss) liver.
Besides, Co+2, Zn+2, Ca+2, Fe+2, Mn+2, Cr+3, Sn+2 and Mg+2 were chosen to investigate their inhibitory effects on fish liver GR in this study. Heavy metals have various toxicological effects on living organisms. For example, it has been reported that heavy metals such as mercury and cadmium exist toxic action in a synergistic fashion with salinityCitation16,Citation25,Citation26. To the best of our knowledge, although the toxic effects of the metal ions have been described, their effects on rainbow trout liver GR have not been studied yet. Ki constants and IC50 values are the most suitable parameters for observing inhibitory effects. As shown in , IC50 values ranged from 42.2 to 657 µM showing that Co+2 was the most potent inhibitor although Zn+2 showed very similar action. However, other metals were effective in low millimolar concentrations. Ki constants for the metal ions ranged from 24.2 to 567 µM in accordance with the IC50 values. Co+2, Zn+2, Fe+2 and Cr+3 exhibited competitive inhibition whereas others inhibited the enzyme in non-competitive manner. Similar results were obtained in both in vitro and in vivo studies for various enzymes, such as human carbonic anhydrase isozymes (CA-I and CA-II), human erythrocyte GR, and following enzymes of sparus aurata fish; liver GR, blood catalase, liver catalase, glutathione peroxidaseCitation16,Citation25,Citation27. Contrarily, Mg+2 showed activatory effect in the concentration range of 0.05–5 mM.
Table 2. Ki and IC50 values obtained from regression analysis graphs for fish liver GR in the presence of different metal ion concentrations.
Studies regarding undesirable effects of cobalt, zinc or calcium ions on various components of the metabolism have gained particular attention over the recent years. For instance, Ekinci et al (2011) demonstrated that micromolar cobalt and zinc exposure attenuates the expression of insulin-like growth factors and growth hormone in rainbow trout. The study proposed that IGF/GH axis may be affected by micromolar exposure to these metalsCitation28.
Nemery et al (2003) carried out several experiments in which they measured indices of oxidant stress, mainly changes in the oxidation state of glutathione and in the activity of the pentose phosphate pathway, upon exposure of hamster pulmonary tissue to CoCl2 in vivo by intra-tracheal instillation or in vitro by incubating lung slices. Their experiments indicated that cobalt ions are capable of causing thiol oxidation in lung tissue as an early manifestation of oxidant stress, but more studies are needed to establish the relevance of this mechanism in the causation of lung disease in subjects exposed to cobalt-containing dusts.Citation29
There are many studies concerning the effects of environmental conditions on the activity of antioxidant enzymes and oxidative stress. For instance, two common rubber additives, 2-mercaptobenzothiazole (MBT) and diphenylamine (DPA) were reported to cause an increase in hepatic GR activity as well indicating that GR is very susceptible to environmental conditions.Citation30 Additionally, increasing stocking density was demonstrated to inhibit the antioxidant enzyme GR in rainbow trout recently.Citation31 Cobalt and zinc exposure of rainbow trout was also showed to increase the expression of metallothioneins and cytocrome P450.Citation32
In this investigation, metal ions showed high inhibitory effects on rainbow trout liver GR enzyme activity. Nowadays, the number of industry wastes and metal refuses are rising in rivers, lakes and seas in the world. Thus, undesirable side effects of these metal ions on GR activity, body metabolism and GSH synthesis can be reduced for fish and fish eaters by concerning these obtained Ki and IC50 values.
Declaration of interest
The authors report no conflicts of interest.
References
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 1975;250:5475–5480.
- Tekman B, Ozdemir H, Senturk M, Ciftci M. Purification and characterization of glutathione reductase from rainbow trout (Oncorhynchus mykiss) liver and inhibition effects of metal ions on enzyme activity. Comp Biochem Physiol C Toxicol Pharmacol 2008;148:117–121.
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711–760.
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem 1997;378:793–802.
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry—glutathione-protein interactions: A molecular link between oxidative stress and cell proliferation? Biochem Biophys Res Commun 1998;247:481–486.
- Akerlund B, Tynell E, Bratt G, Bielenstein M, Lidman C. N-acetylcysteine treatment and the risk of toxic reactions to trimethoprim-sulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIV-infected patients. J Infect 1997;35:143–147.
- Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest 1991;100:1397–1403.
- Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol 1998;44:S72–S84.
- Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: Regulation of glutathione synthesis and efflux. Diabetologia 1995;38:201–210.
- Poot M, Teubert H, Rabinovitch PS, Kavanagh TJ. De novo synthesis of glutathione is required for both entry into and progression through the cell cycle. J Cell Physiol 1995;163:555–560.
- van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem 1996;271:15420–15427.
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol 1989;257:L163–L173.
- Hu H, McCally M. (ed), Life Support: The Environment and Human Health-Book Review J Sociol Social Welfare 2002;4:1–9.
- Hura C, Hura BA. Assessment of the heavy metals in the food from Romania. Toxicol Lett 2005;164:S270–S270.
- Scott EM, Duncan IW, Ekstrand V. Purification and properties of glutathione reductase of human erythrocytes. J Biol Chem 1963;238:3928–3933.
- Coban TA, Senturk M, Ciftci M, Kufrevioglu OI. Effects of some metal ions on human erythrocyte glutathione reductase: An in vitro study. Protein Pept Lett 2007;14:1027–1030.
- Senturk M, Kufrevioglu OI, Ciftci M. Effects of some antibiotics on human erythrocyte glutathione reductase: An in vitro study. J Enzyme Inhib Med Chem 2008;23:144–148.
- Senturk M, Irfan Kufrevioglu O, Ciftci M. Effects of some analgesic anaesthetic drugs on human erythrocyte glutathione reductase: An in vitro study. J Enzyme Inhib Med Chem 2009;24:420–424.
- Sentürk M, Talaz O, Ekinci D, Cavdar H, Küfrevioglu OI. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3663.
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. Academic press, London. 1971;12:68–70.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–683.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–666.
- Le Trang N, Bhargava KK, Cerami A. Purification of glutathione reductase from gerbil liver in two steps. Anal Biochem 1983;133:94–99.
- Abdülkadir Coban T, Beydemir S, Gülcin I, Gücin I, Ekinci D, Innocenti A et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg Med Chem 2009;17:5791–5795.
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–120.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Akkemik E, Senturk M, Ozgeris FB, Taser P, Ciftci M. In vitro effects of some drugs on human erythrocyte glutathione reductase. Turk J Med Sci 2011;41:235–241.
- Stephensen E, Adolfsson-Erici M, Hulander M, Parkkonen J, Förlin L. Rubber additives induce oxidative stress in rainbow trout. Aquat Toxicol 2005;75:136–143.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011; doi:10.1016/j.livsci.2011.07.006.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res 2011; doi: 10.1007/s12011-011–9068-z.