Abstract
The methoxy substitued two novel bis triazole-schiff bases (6 a–b) were synthesized with 4-amino-3,5-diethyl-4H-1,2,4-triazole and various bis-aldehydes. Their amine derivatives prepared by reduced with NaBH4 (5 a–b). The obtained products 6 a–b and 7 a–b were identified by FT-IR, 1H-NMR, 13C-NMR. The bis triazole-schiff bases and amine derivatives were tested for antimicrobial activity using the agar diffusion technique against 11 bacteria. The synthesized compounds (6 a–b and 7 a–b) were screened for their antielastase, antiurease and antioxidant activities. The resuts showed that the synthesized compounds (6 a–b and 7 a–b) had effective antielastase and antiurease activities.
Introduction
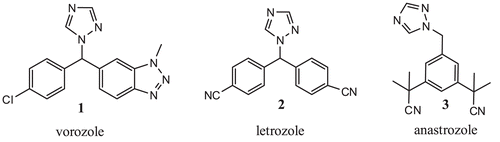
Triazole derivatives have been reported to have pharmacological, insecticidal, fungicidal, and herbicidal activitiesCitation1. In addition, it was reported that compounds having triazole moieties, such as Vorozole (1), Letrozole (2) and Anastrozole (3), (in ) have been used as nonsteroidal aromatase inhibitors in medicine for treating breast cancerCitation2. 1,2,4-triazoles are also important in diverse field’s chemistry has been owing to their biological activitiesCitation3. Schiff bases comprise a group of both cyclic and acyclic chemical compounds containing -C=N- moieties. They are made from the condensation of an amine and a molecule bearing an active carbonyl function. The presence of potentially donating atoms in their structure makes these molecules an important class of ionophore, which are widely used in metal ion complexation studiesCitation4.
Neutrophils, which are the most abundant leukocytes in the circulation, constitute the first line of defense against microorganisms, virally infected cells and tumor cellsCitation5. Neutrophil activation by soluble or particulate stimuli leads to generation of reactive oxygen species (ROS) through the oxidative metabolism, which involves activation of the NADPH oxidase enzymatic complex and increase in cellular oxygen consumptionCitation6. In addition, these cells release proteolytic enzymes, such as elastase and cathepsins, that act as microbicidal agents, degrade the extracellular matrix and contribute to cellular migration at the inflammatory siteCitation7. This potent serine proteinase is capable of digesting a panoply of matrix proteins and is involved in numerous inflammatory respiratory diseases including emphysema, cystic fibrosis, chronic obstructive pulmonary disease, pulmonary fibrosis and asthmaCitation8.
A variety of ureases (E.C.3.5.1.5) are found in bacteria, fungi, higher plants and in soil as soil enzymes. Medically, bacterial ureases are important virulence factors implicated in the pathogenesis of many clinical conditions such as pyelonephritis, hepatic coma, peptic ulcerationn and the formation of injection-induced urinary stonesCitation9 and stomach cancerCitation10. In agriculture, high urease activity causes significant environmental and economic problems by releasing abnormally large amounts of ammonia into the atmosphere during urea fertilization. This further induces damage to germinating seeds, seedling and young plants primarily by depriving them of their nutrition by the essential nutritient and secondarily by ammonia toxicity, increasing the pH of the soil. In the near past, a number of compounds have been proposed as urease inhibitors to reduce environmental problems and enhance the uptake of urea nitrogen by plants.
Antioxidants are extensively studied for their capacity to protect organisms and cells from damage that is induced by oxidative stress. Nowadays antioxidants arouse researchers’ interest in both medical plants and synthetic compounds. Synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ), have been widely used in the food industry to prevent oxidative deterioration, but BHA and BHT are suspected of being responsible for liver damage and carcinogenesisCitation11. Scientists in various disciplines have become more interested in new compounds, either synthesized or obtained from natural sources that could provide active components to prevent or reduce the impact of oxidative stres on cells. Exogenous chemicals and endogenous metabolic processes in human body or in food system might produce highly reactive free radicals, especially oxygen derived radicals, which are capable of oxidizing biomolecules, resulting in cell death and tissue damage. ROS and free radicals are known to induce lipid peroxidation, the damage of lipids, proteins and nucleic acids in cells. In addition, there is much evidence that these molecules may be related to ageing and diseases, such as cancer, atherosclerosis, rheumatoid arthritis and emphysemaCitation12.
In this study, we have synthesized some new methoxy substitued bis-1,2,4-triazole derivatives. Their antioxidant activity were assessed by various in vitro assays and compared to the activities of synthetic standard antioxidant compound. Moreover, the newly synthesized compounds were screened as urease and elastase inhibitors and antimicrobial activities.
Materials and methods
General
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Mp: cap. Melting-point apparatus (Barnstead-Electrothermal 9200, Iowa USA ); uncorrected. IR Spectra: solns. in KBr pellets. with a Perkin-Elmer 100 FT-IR spectrometer (Cambridge, England). 1H- and Citation13C-NMR spectra: 200 (50) MHz Varian spectrometer (Danbury, CT); δ in ppm; Me4Si as the internal standard. Mass spectra: Agilent 6230 TOF (ESI-MS) (CA, USA). Antioxidant activities of samples were determined in a spectrophotometer (UV-1240, Shimadzu, Japan).
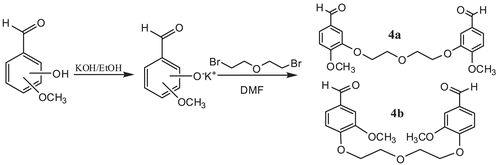
Synthesis of bis-aldehydes 1a–b and amino compound 3
Bis-aldehydes (4a–b)13–15 and 4-Amino-3,5-diethyl-4H-1,2,4-triazole (5)Citation15 were prepared by using literature procedures.
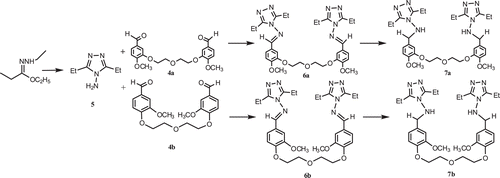
Synthesis of bis-schiff bases 6a–b
The corresponding bis-aldehyde (0.01 mol) was added to a solution of compound 5 (0.005 mol) in glacial acetic acid (20 mL) and the mixture was refluxed for 16 h. After cooling, the mixture was poured into a beaker containing ice–water (100 mL). The precipitate formed was filtered. After drying in vacuo, the product was recrystallized from 1:2 benzene:petroleum ether to give the desired compound.
Synthesis of reduced compounds 7a–b
The corresponding compound 6a–b (0.005 mol) was dissolved in dried methanol (50 mL) and NaBH4 (0.01 mol) was added in small portions to this solution. The mixture was refluxed for 20 min and then allowed to cool. After evaporation at 30–35°C under reduced pressure, the solid residue was washed with cold water. After drying in vacuo, the solid product was recrystallized from an appropriate solvent (1:1 ethanol:water, unless otherwise noted) to afford the desired compound.
N,N′-(2,2′-(2,2′-oxybis(ethane-2,1-diyl)bis(oxy))bis(4-methoxy-2,1-phenylene))bis (methylene) bis(3,5-diethyl-4H-1,2,4-triazol-4-amine) (7a). Yield (1.80 g, 71.43%); m.p. 116–117°C; IR: 3374 (NH), 1594 (C=N), 1261 (C-O), 640–738 cm−1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.08 (t, 12H, CH3), 1.78–1.92 (m, 4H, OCH2), 2,42 (g, 8H, CH2), 3.63 (d, 4H, NH-CH2), 3.67 (s, 6H, OCH3), 3.94–4.06 (m, 4H, OCH2), 6.19 (t, 2H, NH), Ar-H: [6.64 (d, 2H), 7.00–7.10 (m, 4H)]; 13C-NMR (DMSO-d6) δ (ppm): 155.00 (4C, triazole C3, C5), Ar-C: [148.18 (2C), 130.45 (2C), 129.25 (2CH), 128.65 (4CH), 121.52 (2C), 115.08 (4C)], 68.20 (2C, OCH2), 58.01 (2C, NH-CH2), 55.34 (2C, OCH3), 24.12 (4C, CH2), 18.11 (2C, OCH2), 11.13 (4C, CH3). ESI-MS(TOF) (M+H)+:623.3116, Anal. Calc. For (C32H46N8O5):622.7904.
Antibacterial activity was measured using the standard method of diffusion disc plates on agarCitation16. Elastase activity was examined by using N-succinyl-Ala-Ala-Ala-p-nitroanilide (STANA) as a substrate and by the measuring the release of p-nitroaniline at 410 nmCitation17. Urease inbitory activity was determined according to Van Slyke and ArchilbaldCitation18. The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of the triazole derivatives was measured according to the procedure described by Brand-Williams et alCitation19. The ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)diammonium salt) radical scavenging activity of the triazole derivatives was measured according to the procedure described by Arnao et alCitation20. The reducing power of the of the triazole derivatives was measured according to the method of OyaizuCitation21. For the reducing ability of triazole derivatives, the cupric ions reducing power capacity was also usedCitation22 with slight modifications.
Statistical analysis
Results were expressed as mean ± standart deviation (SD) of triplicate analyses. Statistical comparisons were performed with Student’s t-test. Differences were considered significant at p < 0.05.
Results and discussion
The syntheses of bis triazole-schiff bases 6 were accomplished according to the reactions shown in and . First, bis-aldehydes 4 were synthesized using a published methodCitation13,Citation14, as indicated in . ,5-di-ethyl-4-amino-4H-1,2,4-triazole 5 was obtained from the reaction of propionic acid with hydrazine using the published methods shown in Citation15. Finally reactions of compounds 1 and 3 afforded the desired compounds 6 (). In general, reduction of imine type compounds is possible, but attempts to reduce imines such as 6 may also lead to a reduction of the heterocyclic ring. For this reason, the selective reduction of the imino group present in compounds 6 without affecting the heterocyclic ring was another aim of the study. Thus, a general and convenient method using NaBH4 as a selective reducing agent was employed for the synthesis in good yields of the corresponding bis amino triazole compounds 7 ().
In the IR spectra of compounds 6, the characteristic C=N absorption bands appeared at around 1605–1607 cm−1. The 1H-NMR signals for the -N=CH group were observed between δ 8.73–8.76 ppm. The 13C-NMR signals for the−N=CH- group were recorded at δ 163–165 ppm. Reduced compounds 7 showed IR absorption bands around 3228–3230 cm−1 (υNH). The 1H-NMR signals for the -NH-CH2- group of these compounds were observed as a doublet at around δ 3.58–3.63 ppm and the proton signals of−NH- groups were recorded as a triplet or strong singlet between at δ 6.20 ppm. In the 13C-NMR spectra, the triazole C3 and C5 of the bis-schiff base derivatives 6 were observed between δ 150–152 ppm and the triazole C3 and C5 signals of the reduced compounds 7 were observed between δ 155 ppm.
Antibacterial activity was measured using the standard method of diffusion disc plates on agar, but unfortunately, a few ligands compounds do show biological activity against the studied microorganisms. 6a compound has a moderate activity against Enterococcus faecalis, Staphylococcus epidermitidis and Staphylococcus aureus. Antibacterial activity results were given in .
Table 1. Antimicrobial screening data for the synthesized compounds (6a–b,7a–b).
The inhibition effect of elastase activity is shown in . In this study, elastase inhibitor activity of triazole derivatives was found to increase dose dependently. The inhibition was increased with increasing triazole concentration. Triazole derivatives exhibited good elatase inhibitor activity. A high elastase inhibition (62.69 ± 0.73%) was seen in 1 µg/mL at 6a. Lower IC50 values indicate higher enzyme inhibitor activity. A low elastase inhibition (56.07 ± 0.15 %) was seen in 1 µg/mL at 7a. Compound 6a proved to be the most potent showing an enzyme inhibitory activity with an IC50 = 0.00095 µg/mL (). Previous studies have shown that the attachment of various leaving groups (halogen, carboxylate, heterocyclic sulfide, sulfone, methoxy) to the thiazole and triazole compound yields highly potent inhibitors of human leukocyte elastaseCitation23.
Table 2. The elastase and urease inhibitory activity of triazole derivatives.
The inhibition effect of urease activity is shown in . We found that all concentrations exerted inhibitor effects on urease activity in a dose dependent manner. The inhibition was increased with increasing triazole concentration. All the traizole derivatives exhibited good urease inhibition activity. The compound 6a proved to be most potent showing an enzyme inhibition activity with an IC50 = 0.99 ± 0.077 μg/mL. The least active compound 7a had an IC50 = 3.05 ± 2.17 μg/mL. The activity of the rest of the compounds falls in the range 1.15–1.41 μg/mL (). Since all the synthesized triazoles exhibited promising urease inhibitory activity, this may be due to their basic skeleton.
In the present study, antioxidant and radical scavenging effects of the synthesized triazol compounds (6a–b, 7a–b) were determined in vitro with different bioanalytical methodologies. The antioxidant and radical scavenging activities of the compounds were compared with BHT. These comparisons were performed using in vitro tests including DPPH, ABTS and reducing power (Fe+3→Fe+2 biotransformation and cuprac assay).
DPPH is a free radical compound that has been widely used to determine the free radical scavenging ability of various samples. DPPH decreases significantly upon exposure to proton radical scavengerCitation24. The DPPH free radical scavenging activities of triazole derivatives and BHT are presented in . For each compound, different concentrations (500–2000 µg/mL) were prepared. The DPPH scavenging activities of triazole derivatives were between 13.65–28.63 at 500 µg/mL and between 26.17–53.66 % at 2000 µg/mL. In this study, BHT showed a high radical scavenging ability. At 500–2000 µg/mL, the radical scavenging of BHT were between 79.18–92.34%. IC50 value is the effective concentration to inhibit 50% of DPPH radicals. A lower IC50 value is resulted in a stronger DPPH radical scavenging activity, with regard to IC50 values. BHT (315.75 ± 3.66 µg/mL) and 6b (1782.51 ± 56.09 µg/mL) had the highest radical scavenging abilities, whereas 6a (5809.65 ± 1639.21 µg/mL) had a lowest radical scavenging ability (). IC50 values, scavenging abilities on DDPH radicals, were significantly different (p < 0.05) from the IC50 values obtained for BHT. The presence of electrone donating methoxy substituent in the phenolic compounds is known to increase the stability of the free radical and hence the antioxidant activityCitation25. Thus, the compound 6b bearing a methoxy group (electron donating group) showed high DPPH activity.
Table 3. DPPH and ABTS radical scavenging activity of triazole derivatives.
showed the ABTS radical scavenging activity of triazole derivatives compared with BHT. All tested compounds showed some degree of ABTS radical scavenging activity. The scavenging effect of triazole compounds and BHT on ABTS decreased in the order: BHT > 7b > 6b > 7a > 6a at the concentration in 1000 µg/mL.
The reducing power of prepared triazole derivatives, which may serve as a significant reflection of the antioxidant activity, was determined using the iron (III) to iron (II) reduction assay. In this assay, the yellow colour of the test solution changes to various shades of green and blue, depending on the reducing power of compounds. The presence of reductants in the solution causes the reduction of the Fe+3/ferricyanide complex to the ferrous form. Therefore, the Fe+2 ion can be monitored by measurement of the formation of Perl’s Prussian blue at 700 nm. Table S1 showed the reducing power of triazole derivatives compared with BHT. All tested compounds showed some degree of reducing power; in the triazole derivatives, the reducing power followed the following order; BHT > 7b > 6b > 7a > 6a.
In this study, presence of methyl group and type of substitution affected decrease in reducing power. Similarly, the triazole derivatives showed marked cupric ion (Cu+2) reducing ability. Cupric ions (Cu+2) reducing ability of triazole derivatives is shown in Table 4. Cupric ions (Cu+2) reducing capability of triazole derivatives by cuprac method was found to be concentration-dependent (25–100 µg/mL). Cupric ions (Cu+2) reducing power of triazole derivatives and BHT at the same concentration (100 µg/mL) exhibited the following order: BHT > 6b = 7b >7a > 6a.
In this study, the results showed that the synthesized new methoxy substitued bis-1,2,4-triazole derivatives had antiurease and antielastase activities. For reason, new methoxy substitued bis-1,2,4-triazole derivatives may be considered as a main elastase and urease inhibitory. Therefore, these compounds could be used as a source of antielastase and antiurease in pharmaceutical, cosmetic and agriculture industries.
Declaration of interest
The authors declare no conflicts of interest.
References
- Joshi KC, Giri S, Bahel SC. Fungicidal & insecticidal activity of some organic fluorine compounds containing aryloxy, benzamido, acetamido & thiazole ring systems. J Sci Ind Res (C) 1962;21:315–318.
- Goss PE, Strasser-Weippl K. Aromatase inhibitors for chemoprevention. Best Pract Res Clin Endocrinol Metab 2004;18:113–130.
- Gumrukcuoglu N, Serdar M, Celik E, Sevim A, Demirbas NA. Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives. Turk J Chem 2007;31:335–348.
- Zolotove YA. (Ed) Macrocyclic compounds in analytical chemistry. New York: John Wiley and Sons; 1997.
- Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol 2006;534:1–11.
- Tauber AI, Fay JR, Marletta MA. Flavonoid inhibition of the human neutrophil NADPH-oxidase. Biochem Pharmacol 1984;33:1367–1369.
- Braga PC, Dal Sasso M, Culici M, Verducci P, Lo Verso R, Marabini L. Effect of metabolite I of erdosteine on the release of human neutrophil elastase. Pharmacology 2006;77:150–154.
- Guay C, Laviolette M, Tremblay GM. Targeting serine proteases in asthma. Curr Top Med Chem 2006;6:393–402.
- Krajewska B, Zaborska W. Jack bean urease: The effect of active-site binding inhibitors on the reactivity of enzyme thiol groups. Bioorg Chem 2007;35:355–365.
- Montecucco C, Rappuoli R. Living dangerously: How Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol 2001;2:457–466.
- Grice HP. Enhanced tumour development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 1988;26:717–723.
- Lee J, Koo N, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr Rev Food Sci F 2004;3:21–33.
- Tuncer H and Erk Ç. The synthesis and the cationic fluorescence role of glycols with aromatic end groups, Part III. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2003;45:271–274. citation.
- Zou Y, Tan S, Yuan Z, Yu Z. Synthesis, photo- and electro-luminescence properties of a PPV derivative with di(ethylene oxide) segment in the backone. Journal of Materials Scıence 2005;40:3569–3571.
- Herbest RM, Garrison JA. Studies on the formation of 4-aminotriazole derivatives from acyl hydrazides. J Org Chem 1953; 18: 872–877.
- Demirbag Z, Belduz A.O, Sezen K, Nalcacioglu R. Antibacterial activity studies of some plant extracts. Kukem 1997;20:47–53.
- James AE, Timothy DW, Gordon L. Inhibition of human leukocyte and pancreatic elastase by homologues of bovine pancreatic trypsin inhibitors. Biochemistry 1996;35:9090–9096.
- Van Slyke, DD, Archibald RM. Manometric, titrimetric and colometric methods for measurements of urease activity. J Biol Chem 1944;154:623–642.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Techn 1995;28:25–30.
- Arnao MB, Cano A, Acosta M. The hydrophilic,and lipophilic contribution to total antioxidant activity. Food Chem 2001;73:239–244.
- Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 1986;44:307–315.
- Güngör N, Ozyürek M, Güçlü K, Cekiç SD, Apak R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011;83:1650–1658.
- Arcadi A, Asti C, Brandolini L, Caselli G, Marinelli F, Ruggieri V. Synthesis and in vitro and in vivo evaluation of the 2-(6′methoxy-3′,4′-dihydro-1′-naphtyl)-4H-3,1-benzoxazin-4-one as a new potent substrate inhibitor of human leukocyte elastase. Bioorg Med Chem Lett 1999;9:1291–1294.
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem 1998;62:1201–1204.
- Naik N, Kumar HV, Shubvathi T. Synthesis and antioxidant evaluation of novel 5-methoxy indole analogues. Int J Curr Pharm Res 2011;3:109–113.