Abstract
A new bis schiffbases, 3 a-b were synthesized compound 2 with various bis aldehydes. Compounds 3 a-b have been reduced with NaBH4 to afford the corresponding bis amino triazole compounds 4 a-b. The obtained products 3 a-b and 4 a-b were identified by FTIR, 1H-NMR, 13C-NMR. A series of triazol derivatives were evaluated for their antibacterial, antioxidant, antiurease and antielastase activities. The results showed that the synthesized new bis-1,2,4-triazole derivatives had effective antioxidant, antiurease and antielastase activities.
Introduction
The synthesis of bis schiffbases has been attracting increasing interest in a number of areas in chemistry as well as biochemistryCitation1. Schiff bases have played an important role in the development of coordination chemistry as they readily form stable complexes with most transition metalsCitation2. They are also important in diverse field’s chemistry has been owing to their biological activitiesCitation3. There has been a remarkable interest in the synthesis and study of schiff bases physical properties. Furthermore, in recent years, some Schiff base derivatives of 1,2,4-triazoles and their reduced derivatives have also been found to possess pharmacological activitiesCitation4.
Elastases are potent serin protease of the chymotrpsin family that hydrolytically degrades extracellular matrix components such as elastin, proteoglycans, fibronectin and collagenCitation5. Inhibition of the elastase activity could also be used method to protect against skin agingCitation6.
In plants, urease probably participates in systemic nitrogen transport pathways and possibly acts a toxic defense proteinCitation7. In agriculture, high urease activity causes significant environmental and economic problems by releasing abnormally large amounts of ammonia into the atmosphere during urea fertilization. Plant damage and ammonia toxicity can be eliminated by amending the fertilizer with a small amount of a urease inhibitor. It is shown that these inhibitors can induce some phytotoxicity at higher concentrationsCitation8. Urease is directly involved in the formation of infection stones and contributes to the pathogenesis of urolithiasis, pyelonephritis, hepatic encephalopathy, hepatic coma and urinary catheter encrustationCitation7. In the near past, a number of compounds have been proposed as urease inhibitors to reduce environmental problems and enhance the uptake of urea nitrogen by plants and healthy problemsCitation7.
Free radical oxidative processes also play a significant pathological role in causing human disease. Many disease manifestations have been correlated with oxidative tissue damage. Antioxidants are widely studied for their capacity to protect organisms and cells from damage induced by oxidative stress during metabolism.
In this present study, we have synthesized some new 1,2,4-bis triazole compounds and radical scavenging and antioxidant activities for the newly synthesized compounds are evaluated using four antioxidant methodologies. Moreover, the new synthesized compounds were screened as urease-elastase inhibitors and antibacterial activity.
Materials and methods
General
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Mp: cap. melting-point apparatus (Barnstead-Electrothermal 9200, Iowa USA); uncorrected. IR Spectra: solns in KBr pellets with a Perkin-Elmer 100 FTIR spectrometer (Cambridge, England). 1H- and 13C- NMR spectra (in DMSO): 200 (50) MHz Varian spectrometer (Danbury, CT); δ in ppm; Me4Si as the internal standard. Mass spectra: Agilent 6230 TOF (ESI-MS) (CA, USA). Antioxidant activities of samples were determined in a spectrophotometer (UV-1240, Shimadzu, Japan).
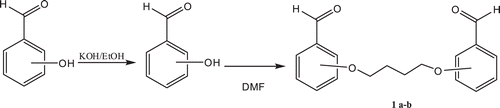
Synthesis of bis-aldehydes 1a-b and amino compound 2
Bis-aldehydes 1a-bCitation9 and 4-Amino-3,5-diethyl-4H-1,2,4-triazole(2)Citation10 were prepared by using literature procedures.
Synthesis of bis-schiff bases 3a-b
The corresponding bis-aldehyde (0.01 mol) was added to a solution of compound 3 (0.005 mol) in glacial acetic acid (20 mL) and the mixture was refluxed for 16 h. After cooling, the mixture was poured into a beaker containing ice-water (100 mL). The precipitate formed was filtered. After drying in vacuo, the product was recrystallized from 1:2 benzene-petroleum ether to give the desired compound.
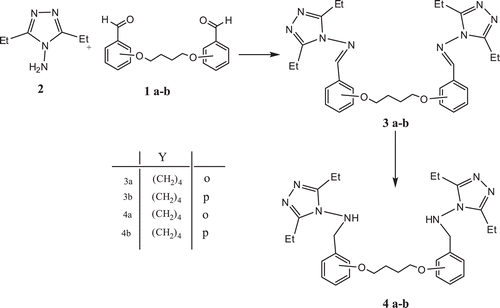
(N,N’,N,N’)-N,N’-(2,2′-(butane-1,4-diylbis(oxy))bis(2,1-phenylene)bis(methan-1-yl-1-ylidene)bis (3,5-diethyl-4H-1,2,4-triazol-4-amine) (3a): Yield (0,93g, 65%); m.p. 100–101 °C; IR: 1600 (C=N), 1249 (C-O), 751 cm−1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.18 (t, 12H, CH3), 1.98 (bs, 8H, CH2), 2.65–2.69 (m, 4H, OCH2), 4.20 (bs, 4H, OCH2), Ar-H: [7.02–7.06 (m, 2H), 7.18–7.22 (m, 2H), 7.59–7.66 (m, 4H)], 8.89 (s, 2H, N=CH); 13C-NMR (DMSO-d6) δ (ppm): 166.31 (2C, N=CH), 152.26 (4C, triazole C3, C5), Ar-C: [137.05 (2CH), 135.68 (2C), 128.36 (2CH), 125.18 (2C), 121.25 (2CH), 114.20 (2CH)], 68.68 (2C, OCH2), 23.94 (2C, OCH2), 18.92 (4C, CH2), 11.84 (4C, CH3). ESI-MS(TOF) (M+H)+:543.3646 Anal. Calc. For (C30H38N8O2):542.7082.
(N,N’,N,N’)-N,N’-(4,4′-(butane-1,4-diylbis(oxy))bis(4,1-phenylene)bis(methan-1-yl-1-ylidene)bis (3,5-diethyl-4H-1,2,4-triazol-4-amine) (3b): Yield (0.79, 55%); m.p. 101–102°C IR: 1605 (C=N), 1251 (C-O), 831 cm−1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.19 (t, 12H, CH3), 1.92 (bs, 8H, CH2), 2.68–2.73 (m, 4H, OCH2), 4.16 (bs, 4H, OCH2), Ar-H: [7.09–7.12 (m, 4H), 7.83–7.88 (m, 4H)], (8.73 (s, 2H, N=CH); 13C-NMR (DMSO-d6) δ (ppm): 166.87 (2C, N=CH), 152.11 (4C, triazole C3, C5), Ar-C: [132.49 (4CH), 131.55 (4CH), 125.08(2C), 115.82 (2C)], 68.26 (2C, OCH2), 25.85 (2C, OCH2), 18.73 (4C, CH2), 11.68 (4C, CH3). ESI-MS(TOF) (M+H)+:543.3313 Anal. Calc. For (C30H38N8O2):542.7082.
Synthesis of reduced compounds 4a-b
The corresponding compound 3a-b (0.005 mol) was dissolved in dried methanol (50 mL) and NaBH4 (0.01 mol) was added in small portions to this solution. The mixture was refluxed for 20 min and then allowed to cool. After evaporation at 30–35°C under reduced pressure, the solid residue was washed with cold water. After drying in vacuo, the solid product was recrystallized from an appropriate solvent (1:1 ethanol-water, unless otherwise noted) to afford the desired compound.
N,N’-(2,2′-(butane-1,4-diylbis(oxy))bis(2,1-phenylene)bis(methylene)bis(3,5-diethyl-4H-1,2,4-triazol-4-amine) (4a):Yield (0.39 g, 50%); m.p. 102–103°C; IR: 3239 (NH), 1600 (C=N), 1237 (C-O), 752 cm−1 (aromatic ring); 1H-NMR (DMSOd6) δ (ppm): 1.16 (t, 12H, CH3), 1.88 (s, 8H, CH2), 4.02 (s, 4H, OCH2), 4.52 (s, 4H, OCH2), 4.78 (bs, 4H, NH-CH2), 6.45 (t, 2H, NH), Ar-H: [6.89–6.93 (m, 4H), 7.15–7.19 (m, 2H), 7.37 (d, 2H)]; 13C-NMR (DMSO-d6) δ (ppm): 155.99 (4C, triazole C3, C5), Ar-C: [130.84 (2CH), 130.43 (2CH), 129.25 (2CH), 128.65 (2C), 121.67 (2C), 115.83 (2CH)], 67.85 (2C, OCH2), 54.10 (2C, NH-CH2), 26.28 (2C, OCH2), 23.70 (4C, CH2), 11.96 (4C, CH3). ESI-MS(TOF) (M+H)+:547.3521 Anal. Calc. For (C30H42N8O2):546.7398.
N,N’-(4,4′-(butane-1,4-diylbis(oxy))bis(4,1-phenylene)) bis (methylene) bis (3,5-diethyl-4H-1,2,4-triazol-4-amine) (4b): Yield (0.25 g, 49.5%); m.p. 130–131°C; IR: 3193 (NH), 1611 (C=N), 1249 (C-O), 830 cm−1 (aromatic ring); 1H-NMR (DMSOd6) δ (ppm): 1.16 (t, 12H, CH3), 1.84 (s, 8H, CH2), 3.96 (s, 4H, OCH2), 4.00 (s, 4H, OCH2), 4.92 (bs, 4H, NH-CH2), 6.62 (bs, 2H, NH), Ar-H: [6.85–6.88 (m, 4H), 7.12–7.21 (m, 4H)]; 13C-NMR (DMSO-d6) δ (ppm): 151.46 (4C, triazole C3, C5), Ar-C: [135.87 (2C), 130.59 (2CH), 129.76 (2CH), 128.83 (2CH), 122.04 (2C), 114.21 (2CH)], 68.10 (2C, OCH2), 55.77 (2C, NH-CH2), 27.09 (2C, OCH2), 24.13 (4C, CH2), 11.76 (4C, CH3). ESI-MS(TOF) (M+H)+:547.3533 Anal. Calc. For (C30H42N8O2):546.7398.
Antibacterial activity was measured using the standard method of diffusion disc plates on agarCitation11. Elastase activity was examined by using N-succinyl-Ala-Ala-Ala-p-nitroanilide (STANA) as a substrate and by the measuring the release of p-nitroaniline at 410 nm. Urease inhibitory activity was determined according to Van Slyke and ArchilbaldCitation12. The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of the triazole derivatives was measured according to the procedure described by Brand-WilliamsCitation13. The ABTS radical scavenging activity of the triazole derivatives was measured according to the procedure described by Arnao, Cano and AcostaCitation14. The reducing power of the of the triazole derivatives was measured according to the method of OyaizuCitation15. For the reducing ability of triazole derivatives, the cupric ions reducing power capacity was also usedCitation16 with slight modifications.
Results and discussion
In this study, a convenient method was established for the synthesis in good yields of new bis triazole schiff bases 3a-b and corresponding bis amino triazole compounds 4a-b. Four new bis-(4H-1,2,4-triazole) derivatives synthesized in the study are exhibit some biological activities and these results are reported.
The syntheses of bis triazole schiff bases 3a-b were accomplished according to the reactions shown in schemes. First, bis-aldehydes 1 were synthesized using a published methodCitation9, as indicated in schemes. 3,5-di-ethyl-4-amino-4H-1,2,4-triazole 2 was prepared by using literature proceduresCitation10. Finally reactions of compounds 1 and 2 afforded the desired compounds 3a-b (). In general, reduction of imine type compounds is possible, but attempts to reduce imines such as 3a-b may also lead to a reduction of the heterocyclic ring. For this reason, the selective reduction of the imino group present in compounds 3a-b without affecting the heterocyclic ring was another aim of the study. Thus, a general and convenient method using NaBH4 as a selective reducing agent was employed for the synthesis in good yields of the corresponding bis amino triazole compounds 4a-b ().
In the IR spectra of compounds 3a-b the characteristic C=N absorption bands appeared at around 1600–1605 cm−1. The 1H-NMR signals for the -N=CH group were observed between δ 8.73–8.89 ppm. The 13C-NMR signals for the−N=CH- group were recorded at δ 161–163 ppm. Reduced compounds 4a-b showed IR absorption bands around 3194–3239 cm−1 (υNH). The 1H-NMR signals for the -NH-CH2- group of these compounds were observed as a doublet at around δ 3.96–4.02 ppm and the proton signals of−NH-CH2- groups were recorded as a triplet or strong singlet between δ 6.45–6.62 ppm. In the 13C-NMR the triazole C3 and C5 of the bis-schiff base derivatives 3a-b were observed between δ 152.11–152.26 ppm and the triazole C3 and C5 signals of the reduced compounds 4a-b were observed between δ 155.46–155.99 ppm.
Antibacterial activity was measured using the standard method of diffusion disc plates on agar, but unfortunately, a few ligands compounds do show biological activity against the studied microorganisms. Antibacterial activity results were given in Table (in supp. File).
The inhibition effect of elastase activity is shown in . We have found that all concentrations exerted inhibitory effects on elastase in a dose-dependent manner. The inhibition was increased with increasing triazole concentration (). Compound 3b proved to be the most potent showing an enzyme inhibitory activity with an IC50 = 0.00207 µM. All of the compounds showed high potent activity (IC50= 0.00207, 0.00212, 2.52, 2.56 µM) than that of standard ursolic acid (IC50 = 3.31 ± 0.141 µM).
Table 1. The elastase and urease inhibitory activity of different concentrations of triazole derivatives.
Almost all compounds showed moderate to good urease inhibitory activity (). The inhibition was increased with increasing triazole concentration. IC50 values for triazole compounds were found to be 2.44, 2.51, 2.57 and 2.62 µM. Compound 3a proved to be the most potent showing an enzyme inhibition activity with an IC50 = 2.44 ± 0.049 µM. The least active compound 3b had an IC50 = 2.62 ± 0.049 µM. All of the compounds showed high potent activity (IC50= 2.44, 2.51, 2.57, and 2.62 µM) than that of standard hydroxy urea (IC50 = 14.29 ± 0.06 µM).
Triazoles have been regarded as structural type inhibitors of urease. 1,2,3 - Triazole and 1,3,4-thiadiazole derivatives were synthesized by Khan et al.Citation17. These synthesized compounds showed potent urease inhibitor activity between IC50 = 45.60 - 459.56 µM. This compounds had a lower urease inhibitor activity than our triazole compounds (IC50 = 2.44–2.62 µM). In the other studyCitation18, all synthesized triazol compounds (IC50 = 22–43.8 µM) had a lower urease inhibitor activity than our study. The triazole derivatives may be regarded as substrate like inhibitors on the basis of their structural similarity with the natural substrate of urease i.e. urea. Since all the synthesized triazole compound promising urease inhibitor activity, this may be due to their basic skeleton.
In the present study, the antioxidant activities of the synthesized compounds (3a, 3b, 4a, 4b) were determined in vitro with different bioanalytical methodologies. Antioxidant capacity is widely used as a parameter for medicinal bioactive components. The antioxidant activity of the compounds was compared with BHT. These comparisons were performed using a series of in vitro tests including DPPH, ABTS radical scavenging activities and reducing power by two methods (Fe3+- Fe2+ transformation and cuprac assays).
DPPH is used as a free radical to evaluate the antioxidative activity of some natural and synthetic sources. The scavenging of the stable DPPH radical model is a widely used method to evaluate antioxidant activities in a relatively short time compared with other methods. The inhibitory effects of different concentrations of synthesized compounds on DPPH radical are presented in . Their comparable scavenging activities were also expressed in IC50 (the effective concentration at which the DPPH radicals were scavenged by 50%) value (). All the tested compounds showed lower free radical scavenging activities when compared BHT (IC50 = 1.43 ± 0.01 mM). Compound 3a showed potent DPPH free radical activity (IC50 = 5.72 ± 0.32 mM). Compounds 4a exhibited moderate effect on DPPH radical with (IC50 = 7.30 ± 0.50 mM). The least active compound 3b had an IC50 = 16.41 ± 0.19 mM. It has been reported that 1,2,4 triazole derivatives showed a high DPPH radical scavenging effect (IC50 = 242.49–292.38 µM)Citation17. It was evident that 1,2,4 triazole derivatives had a stronger effect on the DPPH radical compared to our compounds (IC50 = 5.72–16.41 mM).
Table 2. DPPH and ABTS radical scavenging activity of different concentrations of triazole derivatives.
ABTS radical can directly react with antioxidants. DPPH and ABTS radical scavenging assays have been used to evaluate the antioxidant activity of compounds due to the simple, rapid, and reproducible procedures of compounds. shows the ABTS radical scavenging activity of triazole derivatives and standard. Lower IC50 values indicate higher ABTS radical scavenging ability. All of the compounds (6.91–9.01 mM) showed lower ABTS radical scavenging activity than BHT (1.30 ± 0.09 mM). ABTS radical scavenging activity of triazole compounds and standard compounds exhibited the following order: BHT > 3a > 4b > 3b > 4a ().
The reducing powers of the compounds were observed at different concentrations, and results were compared with BHT (). The reducing capacity of a compound may serve as a significant indicator for its potential antioxidant activityCitation19. In this study, the reducing ability of compounds synthesized augmented with increasing concentration of samples. Compounds 3a, 3b, 4a, 4b were given similar results. The higher activity was found at compound 4a. Tested compounds showed lower activity than BHT at 1000 µg/mL concentration. There was a correlation found between the reducing capabilities and substituents. Reducing power of triazole compounds and standard compounds exhibited the following order: BHT> 3a > 4a > 3b > 4b ().
Table 3. Reducing power and Cupric ions reducing antioxidant capacity of different concentrations of triazole derivatives.
The reducing power associated with antioxidant activity reflects the electron donating capacity of bioactive compounds. Antioxidants can be reductants and inactivators of oxidants. The cupric ion (Cu2+) reducing ability (Cuprac method) of triazole derivatives is shown in . The cupric ion (Cu2+) reducing capability of triazole derivatives by the cuprac method was found to be concentration dependent (25–100 μg/mL). The cupric ion reducing power of triazole derivatives and BHT is as follows at the same concentration (100 μg/mL): BHT > 4a > 3a > 4b > 3b ().
In this study, the results showed that the synthesized new bis-1,2,4- triazole derivatives had antioxidant, antibacterial, antiurease and antielastase activities. For reason, new bis-1,2,4- triazole derivatives may be considered as a main elastase and urease inhibitory and free radical scavenger. Therefore, these compounds could be used as a source of antioxidant, antielastase, antibacterial and antiurease in pharmaceutical, cosmetic and agriculture industries.
Acknowledgments
The author thanks to the Zihni Demirbag for his contribution.
Declaration of interest
The authors report no conflicts of interest.
References
- Lehn JM. Supramolecular Chemistry. VCH, Weinheim, 1995.
- Fenton DE., Vigato P.A. Macrocyclic schiff base complexes of lanthanides and actinides. Chem Soc Rev 1988;17:69–90.
- Walsh CT, Orme-Johnson WH. Nickel enzymes. Biochemistry 1987;26:4901–4906.
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enzyme Inhib Med Chem 2006;21:749–755.
- Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: A novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol 1995;131:775–789.
- Wiedow O, Schröder JM, Gregory H, Young JA, Christophers E. Elafin: An elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem 1990;265:14791–14795.
- Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev 1995;59:451–480.
- Krogmeier MJ, McCarty GW, Bremner JM. Potential phytotoxicity associated with the use of soil urease inhibitors. Proc Natl Acad Sci USA 1989;86:1110–1112.
- Karadeniz F. PhD Thesis in Turkish. Dicle University, Diyarbakir. Turkey. 2000.
- Sahin O, Büyükgüngör O, Sasmaz S, Gümrükçüoglu N, Kantar C. 4-Amino-3,5-diethyl-4H-1,2,4-triazole at 100 K: Chains of edge-fused R(4)(4)(10) and R(4)(4)(20) rings. Acta Crystallogr C 2007;63:o431–o433.
- Demirbag Z, Belduz A.O, Sezen K, Nalcacioglu R. Bazı bitki özütlerinin antibakteriyel etkilerinin araştırılması. Kukem 1997;20:47–53.
- Van Slyke DD, Archibald RM. Manometric, titrimetric and colometric methods for measurements of urease activity. J Biol Chem 1944;154:623–642.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 1995;28:25–30.
- Arnao MB, Cano A, Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 2001;73:239–244.
- Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Japan J Nutr 1986;44:307–315.
- Güngör N, Ozyürek M, Güçlü K, Cekiç SD, Apak R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011;83:1650–1658.
- Khan I, Ali S, Hameed S, Rama NH, Hussain MT, Wadood A et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur J Med Chem 2010;45:5200–5207.
- Akhtar T, Hameed S, Khan KM, Choudhary MI. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H,4H-1,2,4-triazoles. Med Chem 2008;4:539–543.
- Meir S, Kanner J, Akiri B, Hadas SP. Determinating and involvement of aqueus reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 1995;43:1813–1819.