Abstract
Serum paraoxonase 1 (PON1) was purified from bovine serum using hydrophobic interaction chromotography on Sepharose 4B-coupled l-tyrosine 1-naphthylamine gel, and monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Paraoxonase enzyme was immobilized using different ratios of glutaraldehyde and the maximum activity was observed with 7% glutaraldehyde. The effects of inhibition by Mn+2, Co+2 and Cu+2 heavy metals on the immobilized and free enzyme activities were studied. At the optimum pH and temperature, the Km and Vmax kinetic values for bovine serum paraoxonase and immobilized paraoxonase towards paraoxon substrate were determined as 0.296 × 10−3 M & 37.04 EU vs. 0.727–10−3 M & 36.36 EU, respectively.
| Abbreviations | ||
| ApoA1, | = | apoenzyme A1 |
| BChE, | = | butyrylcholinesterase |
| CHD, | = | coronary heart disease |
| CPG, | = | controlled pore glass |
| EC, | = | enzyme code |
| EDTA, | = | ethylenediaminetetraacetic acid |
| EU, | = | enzyme unit |
| GA, | = | glutaraldehyde |
| HDL, | = | high-density lipoprotein |
| HIC, | = | hydrophobic interaction chromatography |
| IC50, | = | 50% inhibitor concentration |
| LDL, | = | low-density lipoprotein |
| PON, | = | paraoxonase |
| SDS-PAGE, | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TEMED, | = | tetramethylethylenediamine |
| Tris-Base, | = | tris (hydroxymethyl)-aminomethane |
Introduction
Human plasma contains four different esterases, butyrylcholinesterase (EC 3.1.1.8), paraoxonase (EC 3.1.8.1), albumin esterase, and acetylcholinesterase (EC 3.1.1.7), although the last binds to the membrane of erythrocytes, which contain additional cholinesterasesCitation1 and in mammalian serum, butyrylcholinesterase, paraoxonase (PON), and albumin are in high enough concentrations to contribute significantly to ester hydrolysis. In addition to these esterases, only under in vitro conditions, the esterase activity of carbonic anhydrase (CA) was determinedCitation2. CA enzyme activity was assayed both with respect to its hydratase activity (i.e. hydration of CO2)Citation3,Citation4, and to its esterase activity (i.e. hydrolysis of an ester of p-nitrophenylacetate)
PON, a calcium-dependent antioxidant glycoprotein, is synthesized in the liver and secreted into the plasmaCitation5. PON is a polymorphic enzyme of many tissues as well as serum, where it is associated with high-density lipoprotein (HDL)Citation6. PON’s Ca2+ dependency suggests a model of metal-catalyzed hydrolysis, such as that proposed for phospholipase A2, in which Ca2+ is thought to act as an electrophilic catalystCitation7. Purified arylesterase/PON is a glycoprotein with a minimal molecular weight of about 43,000 daltons. It has up to three sugar chains per molecule, and carbohydrate represents about 15.8% of the total weight. The enzyme has an isoelectric point of 5.1Citation8.
PON enzyme has detoxification and antioxidant features as well as hydrophobic character and capability of hydrogen peroxide hydrolysisCitation9. PON acts as an antioxidant to prevent low-density lipoprotein (LDL) oxidation by hydrolyzing lipid peroxides, cholesterol linoleate hydroperoxides, and hydrogen peroxide. PON also renders HDL resistant to oxidation, thereby maintaining the capacity of HDL to induce reverse cholesterol transportCitation10. The existence of two or more enzyme forms with PON activity has been reported in sheep, rabbit, human, rat, and bovine serumCitation11. The N-terminal domain of PON is associated with apolipoprotein ApoA1 of HDL. HDL paraoxonase retards the oxidation of LDL and is a major antiatherosclerotic component of HDL. HDL is present in the artery wall at a much higher concentration than LDL and should, therefore, be ideally placed to protect LDL from oxidative modificationCitation12. The oxidation of LDL is centrally involved in the initiation and progression of atherosclerosisCitation6.
The expanded number of PON genes has important implications for future experiments designed to discover the individual functions, catalytic properties, and physiological roles of the PONsCitation13. For example, the PON1 gene contains a number of functional polymorphisms in the coding and the promoter regions, which affect either the level or the substrate specificity of PON1Citation14, This difference is related to the position-192 polymorphism of the PON gene. The PON A-phenotype is associated with low PON activity and the PON B-phenotype with high PON activityCitation15. The enzyme was originally characterized as an organophosphate hydrolase, thus its name derived from the commonly used substrate, paraoxonCitation16. By hydrolyzing paraoxon, PON1 provides protection against exogenous organophosphate poisoningCitation17. Moreover, PON1 status has relevance beyond the toxicology community, since low PON1 status has been associated with cardiovascular and other diseases. PON1 is also involved in the activation and inactivation of specific drugs and the inactivation of bacterial quorum-sensing factorsCitation18.
The PON1 gene, as well as the other members of the gene family PON2 and PON3 (both of which also have antioxidant propertiesCitation19, and are 65 and 70% similar at the nucleotide levelCitation20, are located on chromosome 7q21.3–q22.1Citation21 and linked with diabetic retinopathy. There are conflicting reports of an association between PON1 and Parkinson’s diseaseCitation22, and also PON1 status (i.e. activity or concentration) is more closely related to coronary heart disease (CHD), and indeed, PON1 has been shown to be an independent risk factor for CHD in a prospective study, compared to the genetic polymorphismsCitation14. For any given LDL concentration, the HDL-cholesterol concentration is inversely correlated with the risk of CHD and strokeCitation23.
PON has been shown to play an important role in lipid metabolism as an antioxidant–antiatherosclerotic molecule through (i) hydrolysis of active oxidized phospholipids (phospholipase A2-like activity), (ii) destruction of platelet-activating factor, lipid hydroperoxides and H2O2 (peroxidase-like activity), (iii) reduction of monocyte chemotaxis and adhesion to endothelial cells, (iv) preservation of HDL integrity and functionCitation13, and (v) protection of macrophages from oxidative stress and inhibition of macrophage cholesterol biosynthesisCitation24. In diabetic patients, PON1 activity was shown to be decreased under oxidative stress conditionsCitation25, so oxidative stress might be an important predictor of the development of complications in type 2 diabetic individualsCitation26. Moreover, PON contributes to detoxification of several organophosphorus compounds, such as pesticides, neurotoxins and arylestersCitation27. In terms of these properties of PON, purification and immobilization of PON is very important.
Materials and methods
Materials
Sepharose 4B, l-tyrosine, 1-naphthylamine, paraoxon, and all other chemicals were obtained from Merck (Milan, Italy).
Purification of PON1 enzyme from bovine serum
Ammonium sulfate precipitation
In order to purify PON from bovine serum, ammonium sulphate precipitation and hydrophobic interaction chromatography (HIC) methods were used. Ammonium sulfate has been the most widely used protein precipitant, because it has high solubility and is relatively inexpensiveCitation28. It is used early in a protein purification procedure to precipitate out contaminants. Bovine serum was centrifuged at 2208g for 15 min before precipitation with 60% ammonium sulfate to remove the bulk of contaminants. PON1 was then precipitated from the supernatant with 80% ammonium sulfate, and collected by centrifugation at 19,872g for 30 min.
Hydrophobic interaction chromatography
Sepharose-4B gel (10 mL) was washed with distilled water and then CNBr (4 g) was added to equal volumes of Sepharose 4B and water. The mixture was titrated to pH 11 with 4 M NaOH in an ice bath. The reaction was stopped by filtering the gel on a Buchner funnel and washing with cold 0.1 M NaHCO3 pH 10 giving CNBr-activated Sepharose-4B. Tyrosine (15 mg) was added to the activated matrix in 0.1 M NaHCO3 buffer, pH 10 and stirred with a magnet for 90 min. After this, the suspension was kept at 4°C for 16 h, and then washed with distilled water followed by 100 mL of 0.2 M NaHCO3 (pH 8.8). The hydrophobic gel was then completed by diazotization of 40 mL of the Sepharose-4B-l-tyrosine with 1-naphthylamine. The pH was adjusted to 9.5 with 1 M NaOH and, after gentle stirring for 3 h at room temperature, the coupled red Sepharose derivative was washed with 1 L of water and then 200 mL of 0.01 M Na2HPO4 (pH 6.0)Citation29,Citation30.
Hydrophobic interaction chromatography (HIC) is ideal as the next step, as the protein of interest is present in the supernatant at a high ammonium sulphate concentration, and the sample can be directly applied to the HIC column. Purification occurs concomitant with a reduction in volumeCitation31. HIC is a technique used to separate peptides, proteins, and other biological molecules based on their degree of hydrophobicityCitation32. HIC requires a minimum of sample pre-treatment and can thus be used effectively in combination with traditional protein precipitation techniques. Protein binding to HIC adsorbents is promoted by moderately high concentrations of anti-chaotropic salts (e.g. ammonium sulphate), which also have a stabilizing influence on protein structure. Elution is achieved by a linear or stepwise decrease in the concentration of salt in the adsorption buffer. Recoveries are often very satisfactoryCitation28.
The hydrophobic interaction column was equilibrated with 0.1 M Tris-HCl pH 8.0 including 1 M (NH4)2SO4. A linear, decreasing, salt gradient was applied from 1 M to zero (NH4)2SO4 in 0.1 M Tris-HCl pH 8.0. Fractions of 1.5 mL were collected in eppendorf tubes and was followed for 2 min at 37°C at 412 nm in CARY 1E, UV-Visible SpectrophotometerCitation29.
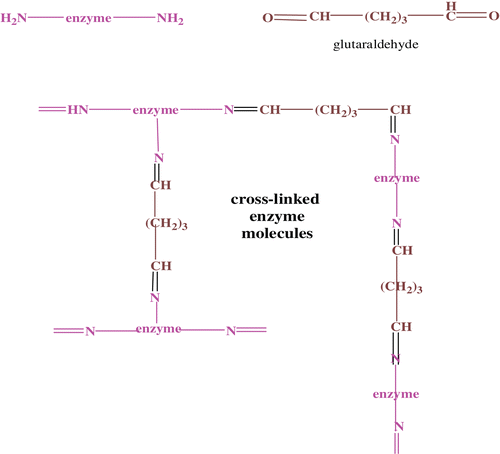
A large number of techniques and supports are now available for the immobilization of enzymes or cells onto a variety of natural and synthetic supports. The choice of the support as well as the technique depends on the nature of the enzyme and the substrate, and its ultimate application. In this study, a cross-linking immobilization method is applied for paraoxanase enzyme using glutaraldehyde as the cross-linker.
Cross-linked enzyme aggregates were constituted between free enzyme molecules and glutaraldehyde (GA), which is a homo cross-link reagent (). Because GA is bivalent, it can cross-link soluble proteins into insoluble aggregates comprised of many protein molecules. The advantages of this method include simplicity, ease of use with no carrier required, and cheapness. Glutaraldehyde at concentrations varying from 1–10% (w/w), relative to protein, was added to different portions of free enzyme molecules. The mixtures of GA and PON were incubated with shaking at 1 g, 25°C to form cross-linked aggregates. At first, the free enzyme was colourless, but with time the immobilized enzyme turned brown. After 24 h, the immobilized PON was centrifuged at 2208g (4°C, 5 min.) and recovered in the solid phase, which was then reconstituted in 100 mM Tris-HCl buffer, included 2 mM CaCl2. Enzyme activities of free and immobilized enzymes were compared by spectrophotometric assay.
Paraoxonase enzyme activity method
PON activity towards paraoxon, either as a free enzyme or cross-linked by GA, was determined spectrophotometrically at 412 nmCitation33 using 850 µL 100 mM Tris-HCl, pH 8 + 100 µL 2 mM paraoxon, 2 mM CaCl2 + 100 µL enzyme solution. The assay components were mixed at 37°C and the change at absorbance during a minute was read by spectrophotomoter, as the paraoxon was converted to p-nitrophenol. Enzyme units were calculated from the absorbance change according to the formula belowCitation29,Citation30:
Comparison of IC50 values for different heavy metal inhibitors of PON activity
Many functional assays seek the total inhibitor concentration that reduces these activities by 50% (i.e. IC50)Citation34,Citation35. The heavy metal ions Mn+2, Co+2 and Cu+2 were tested over a range of concentrations using the spectrophotometric assay at 412 nm to determine their respective IC50 values upon PON activity.
Results and discussion
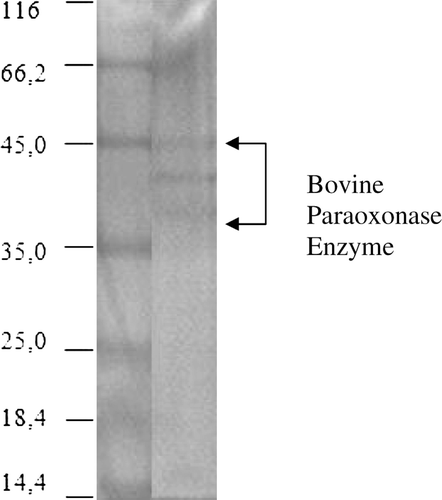
For this study, PON1 was purified from bovine serum on Sepharose 4B-coupled l-tyrosine 1-naphthylamine gel (), and monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis (). Paraoxonase enzyme was immobilized using different ratios of glutaraldehyde and the maximum activity was observed with 7% glutaraldehyde ().
Figure 2. Purification of bovine PON monitored by SDS-PAGE. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction chromatography were analyzed by SDS-PAGE (10% separating gel and 3% stacking gel) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 1 contained 3 µg of various molecular-mass standards: Bovine serum albumin (66.7 kDa), ovalbumin (45.0 kDa), lactate dehydrogenase (35 kDa), β-lactoglobulin (18.4 kDa), lysozyme (14.4 kDa). Thirty microgram of purified bovine serum PON (lane 2) migrated as a series of three bands with mobilities corresponding to apparent masses of between 38–45 kDa.
PON was subsequently immobilized by cross-linking into protein aggregates using GA, which reacts with several functional groups of proteins, such as amine, thiol, phenol, and imidazole groupsCitation36 and maximum activity was observed at the ratio of cross-linker concentration 7% GA. Consequently, this ratio was used in all subsequent assays. Glutaraldehyde has been previously and successfully used for various other enzymes. For instance, trypsin immobilization with GA, either by covalent attachment to aminopropyl controlled pore glass (CPG) particles, or by cross-linking of the enzyme in solution, gave a product that showed an increase in the apparent Michaelis constant, Km, app (i.e. a decrease in enzyme-substrate affinity) relative to free trypsin. This effect was more pronounced for the GA-crosslinked trypsin than for the CPG-coupled trypsinCitation37. Similarly, in another study, the Bacillus licheniformis ATCC 21415 alkaline protease was immobilized by covalent binding through a spacer group of GA giving a good loading efficiency and immobilization yield. Summary of the activity of bovine serum PON1 immobilized using different concentrations of GA is seen in .
Table 1. Summary of the activity of bovine serum PON1 immobilized using different concentrations of glutaraldehyde.
This good loading efficiency for the immobilization by covalent binding might have been due to the formation of stable cross-linking between the carrier and the enzyme through a spacer group;Citation38 but, in the present study bovine serum paraoxonase enzyme was immobilized with glutaraldehyde without any carrier or a spacer group.
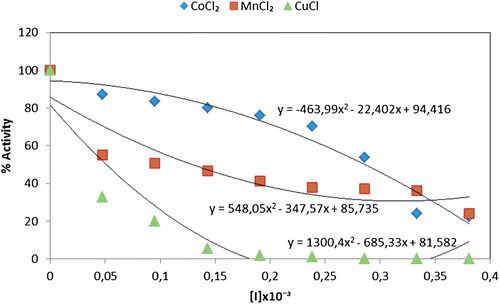
In a previously published study, EDTA and also barium, lanthanum and copper compounds gave rise to competitive inhibition of PON, whereas zinc ions showed noncompetetive inhibitionCitation39. Later studies also demonstrated that some metal ions (cobalt, nickel, cadmium, etc.) displayed an inhibitory effect upon human serum PON1Citation40,Citation41. Here, we specifically investigated and compared the activities of Co+2, Mn+2, and Cu+2 upon both free and immobilized PON activity. By comparison, Cu+2 was a significantly less effective inhibitor of the immobilized enzyme: IC50 value of 0.171 × 10−3 M (). With free enzyme, the observed IC50 values were 0.286 × 10−3 M for Co+2, 0.129 × 10−3 M for Mn+2 and 0.031 × 10−3 M for Cu+2 (), respectively, making Cu+2 the most effective inhibitor. In contrast, Co+2 and Mn+2 proved to be activators of the immobilized enzyme. The comparison of CoCl2, MnCl2 and CuCl heavy metals effect upon the immobilized paraoxonase and free paraoxonase enzyme activities are seen in and . In literature, Ekinci and Beydemir also investigated the in vitro effects of some heavy metals (Pb, Cr, Fe, and Zn) on the only purified human serum PON1. In that study, Pb+2 was found to be more effective inhibitor on purified human serum PON1. IC50 value was 0.838 mMCitation42.
Figure 4. Comparison of CoCl2, MnCl2 and CuCl heavy metals effect upon the immobilized paraoxonase enzyme activity.
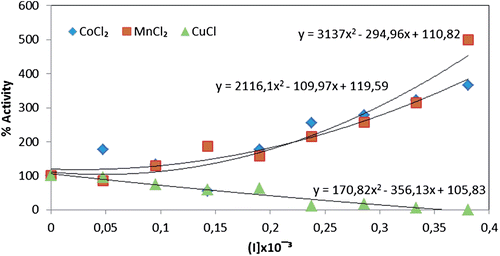
Figure 5. Comparison of CoCl2, MnCl2 and CuCl heavy metals effects upon the free paraoxonase enzyme activity.
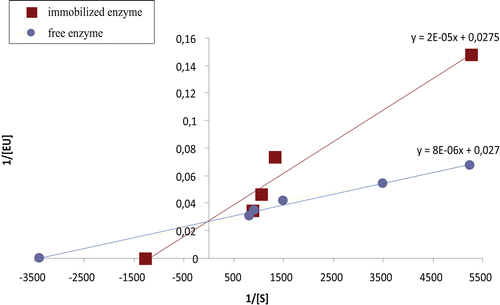
Catalytic efficiencies (Vmax/Km) and Kcat/Km ranges were compared for immobilized and free enzymes as shown in . As the Vmax value of immobilized PON was slightly lower than that of free PON, the affinity of the immobilized PON for the substrate was lower than that for free PON.
Table 2. Influence of immobilization process on kinetic constants.
Conclusions
Bovine paraoxonase enzyme activity was studied as free and immobilized enzyme. The latter was obtained by cross-linking with glutaraldehyde through enzyme amino groups (as seen in ) to form cross-linked enzyme aggregates. Among the advantages of this approach are: (i) an easy immobilization procedure, (ii) high immobilization capacity, and (iii) the substrate affinity of the immobilized enzyme was lower than for the free enzyme.
In addition, our experiments have shown that the bovine serum paraoxonase, when immobilized using glutaraldehyde, demonstrates different activation/inhibition properties in the presence of some heavy metals. From this point of view, the benefit of paraoxonase immobilization with glutaraldehyde is that it provides simplicity in stabilization of enzyme, being quick and low cost. The increased activity of the immobilized paraoxonase, together with its greater tolerance for heavy metals, compared to the free enzyme, is also advantageous, and could prove beneficial in potential biotechnological and pharmaceutical applications, such as a new approach for the treatment of organophosphorus poisoning, or as a candidate catalytic bioscavenger.
Acknowledgment
This work was carried out in the Balikesir University Research Center of Applied Sciences (BURCAS). The authors would like to thank to Dr. Malcolm Lyon for his invaluable contribution to this paper.
Declaration of interest
The authors report no conflicts of interest.
References
- Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol 2005;70:1673–1684.
- Polat MF, Nalbantoğlu B. In vitro esterase activity of carbonic anhydrase on total esterase activity level in serum. Turk J Med Sci 2002;32:299–302.
- Basaran I, Sinan S, Cakir U, Bulut M, Arslan O, Ozensoy O. In vitro inhibition of cytosolic carbonic anhydrases I and II by some new dihydroxycoumarin compounds. J Enzym Inhib Med Chem 2007;23:1,32–36.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Pat 2006;16:1627–1644.
- Agrawal S, Tripathi G, Prajnya R, Sinha N, Gilmour A, Bush L et al. Paraoxonase 1 gene polymorphisms contribute to coronary artery disease risk among north Indians. Indian J Med Sci 2009;63:335–344.
- Turk R, Juretic D, Geres D, Turk N, Rekic B, Simeon-Rudolf V et al. Serum paraoxonase activity and lipid parameters in the early postpartum period of dairy cows. Res Vet Sci 2004;76:57–61.
- Sorenson RC, Primo-Parmo SL, Kuo CL, Adkins S, Lockridge O, La Du BN. Reconsideration of the catalytic center and mechanism of mammalian paraoxonase/arylesterase. Proc Natl Acad Sci USA 1995;92:7187–7191.
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–106.
- Parthasarathy S, Morales AJ, Murphy AA. Antioxidant: a new role for RU-486 and related compounds. J Clin Invest 1994;94:1990–1995.
- Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. Belgium: Center for Experimental Surgery and Anesthesiology, Katholieke Universiteit Leuven.
- Rodrigo L, Gil F, Hernández AF, Pla A. Identification of two rat liver proteins with paraoxonase activity: biochemical evidence for the identity of paraoxonase and arylesterase. Chem Biol Interact 1999;119-120:263–275.
- Selek S, Cosar N, Kocyigit A, Erel O, Aksoy N, Gencer M et al. PON1 activity and total oxidant status in patients with active pulmonary tuberculosis. Clin Biochem 2008;41:140–144.
- Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genom 1996;33:498–507.
- Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: is the gene or the protein more important? Free Radic Biol Med 2004;37:1317–1323.
- Karakaya A, Ibis S, Kural T, Köse SK, Karakaya AE. Serum paraoxonase activity and phenotype distribution in Turkish subjects with coronary heart disease and its relationship to serum lipids and lipoproteins. Chem Biol Interact 1999;118:193–200.
- Akçay YD, Sagin FG, Sendag F, Oztekin K, Sozmen EY. Effects of estrogen-only therapy on LDL oxidation in women with hysterectomy: does paraoxonase genotype play a role? Maturitas 2006;53:325–332.
- Pola R, Flex A, Ciaburri M, Rovella E, Valiani A, Reali G et al. Responsiveness to cholinesterase inhibitors in Alzheimer’s disease: a possible role for the 192 Q/R polymorphism of the PON-1 gene. Neurosci Lett 2005;382:338–341.
- Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol Appl Pharmacol 2009;235:1–9.
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 2005;69:541–550.
- Alici HA, Ekinci D, Beydemir S. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem 2008;41:1384–1390.
- Ikedaa Y, Suehiroa T, Ariia K, Kumonb Y, Hashimotoa K. High glucose induces transactivation of the human paraoxonase 1 gene in hepatocytes. Metabolism 2008;57:1725–1732.
- Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscl Throm Vas 2001;21:473–480.
- Mackness MI, Mackness B, Durrington PN. Paraoxonase and coronary heart disease. Atheroscler Suppl 2002;3:49–55.
- Efrat M, Aviram M. Macrophage paraoxonase 1 (PON1) binding sites. Biochem Biophys Res Commun 2008;376:105–110.
- Rozenberg O, Shiner M, Aviram M, Hayek T. Paraoxonase 1 (PON1) attenuates diabetes development in mice through its antioxidative properties. Free Radic Biol Med 2008;44:1951–1959.
- Tiwari AK, Prasad P, B K T, Kumar KM, Ammini AC, Gupta A et al. Oxidative stress pathway genes and chronic renal insufficiency in Asian Indians with Type 2 diabetes. J Diabetes Complicat 2009;23:102–111.
- Clarimon J, Eerola J, Hellström O, Tienari PJ, Singleton A. Paraoxonase 1 (PON1) gene polymorphisms and Parkinson’s disease in a Finnish population. Neurosci Lett 2004;367:168–170.
- Jakoby WB. Crystallization as a purification technique. In: Jakoby, WB. (ed.), Enzyme Purification and Related Techniques Method Enzymol 1971;Vol. 22, New York: Academic Press.
- Arslan O, Beyaztaş S, Sinan S. Inhibition of plant growth regulators on paraoxonase activity. FEB 2008;17:1994–1999.
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–574.
- Builder SE. Hydrophobic interaction chromatography, principles and methods. San Francisco: Amersham Pharmacia Biotech, So; 1993.
- Warren BS, Kusk P, Wolford RG, Hager GL. Purification and stabilization of transcriptionally active glucocorticoid receptor. J Biol Chem 1996;271:11434–11440.
- Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis 2006;187:74–81.
- Munson PJ, Rodbard D. An exact correction to the “Cheng-Prusoff” correction. J Recept Res 1988;8:533–546.
- Huang X. Fluorescence polarization competition assay: the range of resolvable inhibitor potency is limited by the affinity of the fluorescent ligand. J Biomol Screen 2003;8:34–38.
- Habeeb AJ, Hiramoto R. Reaction of proteins with glutaraldehyde. Arch Biochem Biophys 1968;126:16–26.
- Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004;37:790–6, 798.
- Ahmed SA, Saleh SA, Abdel-Fattah AF. Stabilization of Bacillus licheniformis ATCC 21415 alkaline protease by immobilization and modification. Australian J Basic Appl Sci 2007;1:313–322.
- Pellin MC, Moretto A, Lotti M, Vilanova E. Distribution and some biochemical properties of rat paraoxonase activity. Neurotoxicol Teratol 1990;12:611–614.
- Debord J, Dantoine T, Bollinger JC, Abraham MH, Verneuil B, Merle L. Inhibition of aryesterase by aliphatic alcohols. Chem-Biol Interact 1998;113:105–115.
- Debord J, Bollinger JC, Merle L, Dantoine T. Inhibition of human serum arylesterase by metal chlorides. J Inorg Biochem 2003;94:1–4.
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–120.