Abstract
Lippia alba (Miller) N.E. Brown is an aromatic plant known locally as “Erva-cidreira-do-campo” that has great importance in Brazilian folk medicine. The aim of our study was to evaluate the antidermatophytic potential of linalool-rich essential oil (EO) from L. alba and analyze the ability of this EO to inhibit peptidase and keratinase activities, which are important virulence factors in dermatophytes. The minimum inhibitory concentrations (MICs) of L. alba EO were 39, 156 and 312 µg/mL against Trichophyton rubrum, Epidermophyton floccosum and Microsporum gypseum, respectively. To evaluate the influence of L. alba EO on the proteolytic and keratinolytic activities of these dermatophytes, specific inhibitory assays were performed. The results indicated that linalool-rich EO from L. alba inhibited the activity of proteases and keratinases secreted from dermatophytes, and this inhibition could be a possible mechanism of action against dermatophytes. Due to the effective antidermatophytic activity of L. alba EO, further experiments should be performed to explore the potential of this linalool-rich EO as an alternative antifungal therapy.
Introduction
Lippia alba (Miller) N.E. Brown belongs to the Verbenaceae family, which includes many important medicinal plants used all over the world. L. alba occurs in nearly all regions of Brazil and has great importance in Brazilian folk medicine. The most common popular name of this plant is “erva-cidreira”; however, there are many popular names represented by over 25 synonymies, including “carmelitana”, “erva-cidreira-do-campo” and “falsa-melissa”Citation1,Citation2.
The pharmacologic properties of L. alba have been extensively explored in scientific research. This aromatic plant is used in many ways: as a sudorific and an expectorant; for the treatment of colds, cough and bronchitis; as a cathartic, antidysenteric, stimulant and antirheumatic; in the treatment of hypertension; as a febrifuge; to aid wound healing and to treat hepatic diseasesCitation3,Citation4. Additionally, the antimicrobial activity of L. alba has been demonstrated by several authorsCitation1,Citation5,Citation6, and its use in popular medicine can be explained, at least in part, by its volatile bioactive constituents. However, large variations in the composition of L. alba essential oils (EOs) have been observed that depend on the origin of the plant material, the growth stage and the specific plant part selected for distillation of the EOCitation7. This chemical variability is rather common, and it was suggested that L. alba EOs be divided into at least 12 chemotypes, including linalool, carvone and citralCitation8,Citation9. The extensive biological activity of L. alba is likely due to the great diversity of these bioactive constituents.
Dermatophytosis involves superficial mycoses and is restricted to the stratum corneum in the epidermisCitation10. Dermatophytes have the capacity to invade keratinized tissues and cause lesions in the skin as well as other areas of the body. Trichophyton rubrum, Microsporum gypseum and Epidermophyton floccosum are examples of important dermatophyte species. During the initial stages of infection, dermatophytes induce the expression of adhesins, non-specific peptidases and keratinases that act on keratinous and non-keratinous substrates. The activity of these enzymes is likely to be an important factor in fungal pathogenicity and virulence. Currently, the antifungal substances used in the treatment of dermatophytosis can induce adverse effects such as gastrointestinal disturbances, hepatotoxicity and leucopeniaCitation11,Citation12. Thus, the development of antifungal agents that are more effective and less toxic is desirableCitation13,Citation14.
Plant compounds are promising sources of new antifungals, and several EOs and their constituents have antifungal capabilitiesCitation13,Citation15,Citation16. However, the effect of EOs on fungal virulence factors is still poorly understoodCitation17.
The aim of this study was to evaluate the in vitro inhibitory effect of a linalool-rich EO from L. alba on three species of dermatophytes: T. rubrum, M. gypseum and E. floccosum. This study focused on growth inhibition and the effect of the EO on the proteolytic profile.
Methods
Extraction and analysis of L. alba EO
To obtain the EO of L. alba, leaves of this species were collected from the Active Germplasm Bank located on the experimental farm at the rural campus of the Federal University of Sergipe, São Cristovão, Sergipe, Brazil. The plant material was dried in an oven at 40 °C for five days and subjected to steam distillation using a clevenger extractorCitation18 for a period of two hours after boiling. The EO was stored in a freezer in an amber glass bottle until analysis. Chemical analysis of EO was performed by gas chromatography coupled with mass spectrometry (GC/MS) using a Shimadzu QP5050A (Shimadzu do Brazil, SP, Brazil) equipped with a DB-5 capillary column (Agilent technologies, SP, Brazil) (30 m × 0.25 mm × 0.25 µm). The GC/MS conditions were as follows: helium carrier gas at a flow rate of 1.0 mL/min; split injection (1:20) at 250 °C; detector maintained at 280 °C; oven program, 80 °C for 1.5 min with an increase of 4 °C per minute to 180 °C followed by 10 °C per minute to 300 °C; and a final step of 10 min to 300 °C (isothermal). The mass spectra were obtained by electron impact at 70 eV with a scan speed of 1000 and a scan interval of 0.50 pieces/fragments detected in the range 40–350 Da. Identification of the components was determined based on a comparison of the retention ratios with (1) a series of n-alkane homologs obtained by co-injection of oil samples with a mixture of linear hydrocarbons and (2) the NIST21 and NIST107 mass spectral libraries of the GC/MS data system and published mass spectraCitation19.
Microorganisms, growth conditions and cell-free culture supernatants
Three pathogenic dermatophyte strains, T. rubrum, M. gypseum and E. floccosum, were obtained from human isolates at the University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro (Brazil).
For conidium formation, dermatophyte isolates were inoculated onto potato dextrose agar (PDA; Difco, Franklin Lakes, NJ) in petri plates at 30 °C for four to seven days or until conidial growth was observed. The fungal growth on solid medium was covered with approximately 1 mL of sterile saline (0.85%), and a conidia suspension was prepared by gently probing the colonies.
For enzyme secretion, conidia were inoculated in a 1000 mL Erlenmeyer flask containing 500 mL of potato dextrose broth (PDB; Difco), and the cultures were incubated for seven days at room temperature. To obtain cell-free supernatants, the cultures were strained and the supernatants were filtered through a 0.22 µm membrane (Millipore, Billerica, MA). The cell-free supernatants were concentrated 100-fold in an Amicon micropartition device (Beverly, MA) with a molecular weight cut-off of 10 kDaCitation20.
Determination of minimal inhibitory concentration in vitro
Minimal inhibitory concentrations (MICs) of L. alba EO against T. rubrum, M. gypseum and E. floccosum were determined using the broth microdilution method recommended by the Clinical and Laboratory Standards InstituteCitation21. MICs were determined for all species with the adjusted inoculum suspension of 3 × 103 cells/mL (counted with a hemocytometer) in RPMI 1640 medium (Sigma, St Louis, MO) containing various concentrations of EO (2 µg/mL–5 mg/mL) directly diluted in culture medium. Cell growth was determined visually in 96-well plates by turbidity and concomitant color change of resazurin used as indicator of viability (blue indicates inhibition of microorganisms growth and pink indicates microorganisms growth). The MIC was defined as the concentration required to inhibit the growth of the strains relative to the controls, which were grown in the absence of antimicrobial agents. To evaluate the fungicidal or fungistatic effect of EO, microorganisms treated with the MIC of the EO were inoculated onto PDA and observed for possible growth. All the measurements were performed in triplicate, and two independent experiments were performed with similar results.
Detection of peptidase/keratinase activities
Peptidase and keratinase activities in 100-fold concentrated cell-free supernatants from T. rubrum, M. gypseum and E. floccosum cultures were determined by the method of Buroker-Kilgore and WangCitation22 with modifications using bovine serum albumin (BSA) or keratin (Sigma) as a substrate. The influence of pH on the peptidase activity was also determinedCitation22 using BSA as a substrate and 100-fold concentrated cell-free supernatants in buffers adjusted to pH values ranging from 1.0 to 11.0 (100 mM KCl–HCl buffer, pH 1.0; 50 mM sodium citrate buffer, pH 2.0–4.0; 100 mM sodium phosphate buffer, pH 5.0–7.0; 100 mM glycine–NaOH buffer, pH 8.0–11.0).
Effect of EO on peptidase/keratinase activities
The ability of L. alba EO to inhibit peptidase/keratinase activity was evaluated using the method of Buroker-Kilgore and WangCitation22 with modifications. Briefly, 20 μL of cell-free supernatant from T. rubrum, M. gypseum, or E. floccosum (concentrated 100-fold) was mixed with 1 μL of inhibitor (EO at a concentration of 1 mg/mL) and incubated for five minutes at 37 °C. After the incubation, 20 μL of substrate (BSA or keratin at 0.1 mg/mL) was added, and the samples were incubated for one hour at 37 °C. Subsequently, 50 μL of distilled water and 50 μL of a Coomassie solution (0.025% Coomassie brilliant blue G-250, 11.75% ethanol and 21.25% phosphoric acid) were added. A control, where the substrate was added just after the reactions were stopped, was used as blank. After 10 min to allow dye binding, the samples were read at 595 nm using a Stat Fax 2100 microplate reader (Awareness Technology Inc, Palm City, FL). One unit of enzyme activity was defined as the amount of enzyme that caused an increase of 0.001 in absorbance unit, under standard assay conditionsCitation20. The control reactions did not contain EO and were performed exactly the same as the experimental reactions. All the reactions were performed in triplicate, and the mean control absorbance value was subtracted from the mean experimental absorbance value to obtain the percent reduction in enzymatic activity.
Evaluation of BSA cleavage by sodium dodecyl sulfate polyacrylamide gel electrophoresis
Fifty microliters of 100-fold concentrated supernatants from T. rubrum, M. gypseum and E. floccosum cultures were mixed with 20 μL of BSA (0.1 mg/mL) diluted in 50 mM sodium citrate buffer (pH 3.0). These preparations were incubated in the absence or presence of 1 μL of EO (1 mg/mL) for 6 and 24 h at 37 °C. A negative control for degradation was also performed (T0 = 0 hour). The reactions were terminated by freezing the samples, which were maintained at −20 °C until lyophilization and further analysis. To each reaction, 10 μL of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (125 mM Tris, pH 6.8, 4% SDS, 20% glycerol) supplemented with 5% (v/v) β-mercaptoethanol was added, followed by boiling at 100 °C for five minute. The protein degradation profiles were analyzed by SDS-PAGE (10%) following the method described by LaemmliCitation23 to determine the hydrolysis of BSA. Electrophoresis was carried out at 100 V for 90 min at 4 °C, and the gels were stained overnight with 0.2% (w/v) Coomassie brilliant blue R-250 in methanol:acetic acid:water (50:10:40) and destained in the same solvent solution. Additionally, a control for BSA substrate was made by replacing concentrated supernatant with the same volume of citrate bufferCitation20. The presence of BSA bands in the gels was indicative of intact proteins in the reactions. The gels were scanned and digitally processed.
Evaluation of keratin cleavage by SDS-PAGE
Feather keratin powder was obtained as described by Mazotto et al.Citation24. Aliquots (20 μL) of 100-fold concentrated culture supernatants from T. rubrum, M. gypseum and E. floccosum were mixed with an equal volume of feather keratin substrate diluted in 10 mM sodium phosphate buffer (pH 7.0) to a concentration of 0.15 mg/mL. These preparations were incubated in the absence or presence of 1 μL of EO (1 mg/mL) for six hours at 37 °C. Keratin hydrolysis was then analyzed by 12.5% SDS-PAGE. Electrophoresis was carried out at 100 V for two hours at 4 °C. The gels were stained with 0.2% Coomassie brilliant blue R-250 in methanol:acetic acid:water (50:10:40) and destained in the same solvent. A negative control for degradation was also performed (T0 = 0 hour).
Results
Antidermatophyte activity of linalool-rich EO from L. alba
Lippia alba EO contains 66% linalool, and its full composition is presented in . The antifungal activity of this linalool-rich EO was confirmed (). The minimum inhibitory concentration (MIC) values were 39, 156 and 312 µg/mL against T. rubrum, E. floccosum and M. gypseum, respectively. Lippia alba EO was fungicidal against T. rubrum and fungistatic against M. gypseum and E. flocossum.
Table 1. Chemical composition of L. alba EO characterized by GC/MS.
Table 2. MIC of L. alba linalool-rich EO against various dermatophyte strains by the microdilution method.
The effect of pH on the peptidase activities of dermatophytes
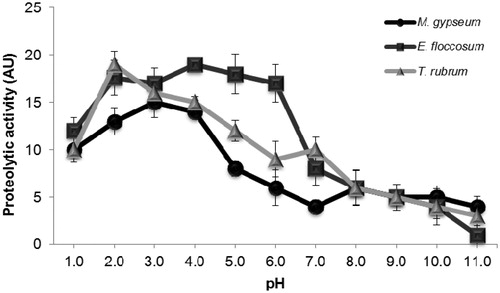
Peptidase activity was evaluated at various pH levels using BSA as a substrate (). For all the dermatophytes tested, significant BSA hydrolysis was detected above pH 2.0 and below pH 5.0. For M. gypseum, T. rubrum and E. floccosum, the highest activities were observed at pH 3.0, 2.0 and 5.0, respectively. Based on the high level of enzymatic activity observed at acidic pH, we performed all the BSA cleavage experiments at pH 3.0.
Figure 1. Effect of pH on the extracellular proteolytic activity of enzymes released from M. gypseum, E. floccosum and T. rubrum. Cells were grown in PDA medium for five days at room temperature. Subsequently, the culture supernatant was filtered, concentrated and tested for its ability to degrade soluble BSA at distinct pH values ranging from 1.0 to 11.0. The values represent the mean (± standard deviation) of three independent experiments, which were performed in triplicate.
Effect of a linalool-rich EO from L. alba on enzymatic activities
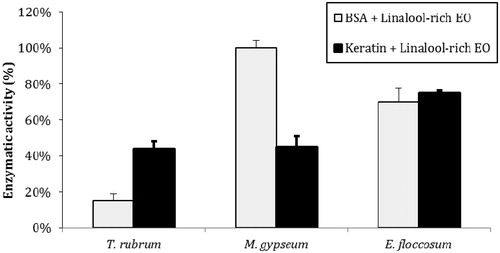
Trichophyton rubrum, M. gypseum and E. floccosum were cultivated in PDB, and all species were able to produce extracellular peptidases and keratinases in a complex medium. Dermatophyte culture supernatants (concentrated 100-fold) contained enzymes capable of cleaving BSA at pH 3.0 and keratin at pH 5.0 (). A linalool-rich EO from L. alba inhibited the enzymatic activities of all the dermatophytes tested; specifically, it inhibited 25% and 85% of the peptidase activities of E. floccosum and T. rubrum, respectively. However, this EO was not able to inhibit peptidases produced by M. gypseum. Additionally, this linalool-rich EO inhibited 30%, 55% and 56% of the keratinase activities of E. floccosum, M. gypseum and T. rubrum, respectively.
Figure 2. Inhibition of peptidase and keratinase activities by L. alba EO. The control activities (171 AU/mL for BSA and 196 AU/mL for keratin) are each set as 100%. The values represent the means of three independent experiments performed in triplicate. The error bars represent the standard error of the mean.
Cleavage of soluble proteins and detection by SDS-PAGE
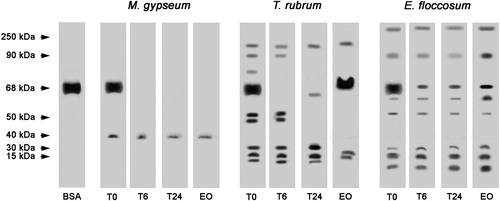
Proteins with various molecular weights were detected in the concentrated supernatants of T. rubrum, M. gypseum and E. floccosum cultures (). The peptidases of E. floccosum and T. rubrum were partially and completely inhibited, respectively, when incubated in the presence of a linalool-rich EO from L. alba, illustrating the inhibitory effect of this EO. However, this EO did not inhibit the ability of concentrated supernatant of M. gypseum culture to cleave BSA.
Figure 3. Effects of L. alba EO on the peptidase activity of T. rubrum, M. gypseum and E. floccosum culture supernatant extracts. BSA was used as a control. T0: control in which the reaction mixture (containing enzyme extract and keratin) was subjected to SDS-PAGE without incubation. T6: reaction mixture after six hours of incubation at 37 °C. T24: reaction mixture after overnight incubation at 37 °C. EO: reaction mixture containing L. alba EO after overnight incubation at 37 °C. The molecular weights of the proteins (in kDa) are provided at the left.
Evaluation of keratin cleavage by SDS-PAGE
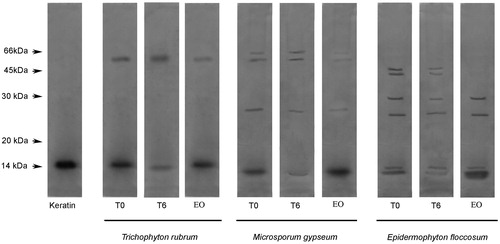
The keratinase activities of concentrated supernatants of T. rubrum, M. gypseum and E. floccosum cultures were similar. After incubating the dermatophyte culture supernatants with feather keratin for six hours, the keratin bands were weaker when compared with bands of the keratin-only control (without dermatophyte culture supernatants), thus indicating keratin degradation (). Keratinase activities detected in concentrated supernatants from T. rubrum, M. gypseum and E. floccosum cultures were completely inhibited when the reactions were incubated in the presence of L. alba linalool-rich EO, illustrating that this EO is capable of enzymatic inhibition.
Figure 4. Enzymatic activity analysis using feather keratin as a substrate. T0: control in which the reaction mixture (containing dermatophyte culture supernatant enzyme extract and keratin) were subjected to SDS-PAGE without incubation. T6: reaction mixture after six hours of incubation. EO: reaction mixtures containing L. alba EO after six hours of incubation. The degradation profile was analyzed by 12% SDS-PAGE. The molecular weights of the proteins (in kDa) are provided at the left.
Discussion
Dermatophytes cause superficial mycoses that are generally confined to the stractum corneum in the epidermis and cutaneous appendagesCitation10. According to an estimative from the World Health Organization, dermatophytic fungi affect approximately 25% of the human population and are found in many different regions of the worldCitation14. In general, the establishment of a fungal infection depends on the interaction of a host cell with secreted virulence factors like proteinases. These extracellular enzymes are likely to be essential for these microorganisms to obtain nutrients and establish infectionsCitation17.
During infection, the dermatophyte–host interaction triggers specific metabolic adaptations, which enable pathogens to adhere and penetrate host tissue, by remodeling the metabolism to capture nutrients and overcome the defense mechanisms of the hostCitation14. Furthermore, dermatophytes obtain nutrients for their growth and survival using macromolecules present in host tissue. For this reason, the secretion of a wide variety of enzymes by dermatophytes such as proteases, lipases, elastases, collagenases, phosphatases and esterases, that promote the breakdown of host substrates, are important factors during the infectious processCitation25–27. This enzymatic machinery is one of the virulence factors of dermatophytes most well characterized, allowing these pathogens to become invasive, especially in immunocompromised patientsCitation14,Citation28–30. Among this wide variety of enzymes secreted by dermatophytes, proteolytic enzymes are the most studied, and the importance of keratinolytic proteases for pathogenicity is well established in the literatureCitation14,Citation31,Citation32. Enzymes play an important role in the pathogenesis of many fungiCitation17,Citation33,Citation34, and newly discovered enzyme inhibitors are being explored as potential alternative antifungal agentsCitation17,Citation35.
Several medicinal plants are popularly used to treat infectious diseases, and some biological assays corroborate these antimicrobial activitiesCitation36–38. Among diferent targets in the cell, some studies describe the inhibitory effect of natural products in enzymatic activity of several microorganisms. EOs from plants such as Cymbopogon citratus, Cymbopogon martini, Rosmarinus officinalis, Mentha piperita, Pelargonium odoratissimum and Vitex negundo are effectively used to treat various types of diseases and different natural compounds such as citral, geraniol, thymol and linalool were used to evaluate their protease inhibitory propertiesCitation35. Khan and AhmadCitation17 demonstrated that EOs of different plant species inhibited the activity of elastases and keratinases of Aspergillus fumigatus and T. rubrum. However, the influence of EOs on fungal pathogenesis are not yet explored or still poorly known. Thus, in this study, we evaluated the antimicrobial activity of L. alba EO against dermatophytes and also whether the mechanism of action of L. alba involves the inhibition of proteolytic and keratinolytic activities of these fungi.
Dermatophytes used in this study were able to produce extracellular enzymes ( and ). Moreover, our results show peaks of enzyme activity at acidic pH values (). Silveira et al.Citation39 related that acidic peptidases of T. rubrum are important virulence factors. In the early stages of infection and in response to the pH of the skin, the dermatophytes produce various non-specific proteases with optimum activity at acidic pH values, allowing the pathogen to adhere and penetrate the host tissue. During this process, amino acids are liberated and used by the fungi as a source of nutrients such as carbon and nitrogenCitation14,Citation39,Citation40. SDS-PAGE analysis of the extracellular proteolytic enzymes obtained from the dermatophytes cultures showed peptidases with molecular masses ranging from 30 to 150 kDa for T. rubrum, from 30 to 40 kDa for M. gypseum and from 10 kDa to 260 kDa for E. floccosum (). External digestion of protein substrates by secreted proteases is required for survival and growth of both saprophytic and pathogenic speciesCitation41, and the inhibition of these enzymes difficult the infectious process.
Keratinase activity was demonstrated by keratin cleavage by SDS-PAGE and our results showed enzymes with molecular masses ranging from 15 to 66 kDa (). Previous studies reported that keratinases have molecular masses ranging from 15 to 240 kDa, although the majority of keratinases presented molecular masses varying from 20 to 50 kDaCitation42–44. Keratinases mostly belong to the class of serine or metalloproteases irrespective of the microorganismCitation42,Citation43. The linalool-rich EO derived from L. alba caused high reduction in keratinase activities of all dermatophytes tested, suggesting that these specific enzymatic classes have been inhibited by EO. Considering that enzymes produced by fungi contribute to pathogenicity by degradation of proteins in host tissuesCitation45, the inhibition of these enzymes by L alba EO may reduce fungal virulence.
Lippia alba EO was active against the cellular growth of all dermatophytes evaluated in this study. Therefore, L. alba EO antimicrobial, antipeptidase and antikeratinase activities are indicative of its potential as antifungal drug. However, further studies would be necessary to elucidate L. alba EO mechanisms of action against dermatophytes. In conclusion, our findings highlight antipeptidase and antikeratinase activities of L. alba EO to be exploited as alternative therapy for the treatment of dermatophytosis.
Conclusions
In this study, we described the antifungal potential of L. alba EO and its influence on the peptidases and keratinases of dermatophytes. The EO-induced inhibition of peptidases and keratinases in dermatophytes indicates one potential mechanism of antifungal action. However, further experiments are needed to elucidate the additional mechanisms of action of L. alba EO and to explore its suitability as an antifungal drug for the treatment of mycoses.
Acknowledgements
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro.
Declaration of interest
The authors report no declarations of interest.
References
- Oliveira DR, Leitão GG, Santos SS, et al. Ethnopharmacological study of two Lippia species from Oriximiná, Brazil. J Ethnopharmacol 2006;108:103–8
- Hennebelle T, Sahpaz S, Joseph H, Bailleul F. Ethnopharmacology of Lippia alba. J Ethopharmacol 2008;116:211–22
- Correa CBV. Anatomical and histochemical study of Lippia alba (Mill.) N.E. Br. ex Britt. And Wilson-known as erva-cidreira. Revista Brasileira de Farmacia 1992;73:57–64
- Di Stasi LC, Oliveira GP, Carvalhaes MA, et al. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 2002;73:69–91
- Alanís AD, Calzada F, Cervantes JA, et al. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol 2005;100:153–7
- Duarte MCT, Figueira GM, Sartoratto A, et al. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol 2005;97:305–11
- Zoghbi MGB, Andrade EHA, Santos AS, et al. Essential oils of Lippia alba (Mill.) N.E. Brown growing wild in Brazilian Amazon. Flavour Fragr J 1998;14:411–14
- Matos FJA, Machado MIL, Craveiro AA, Alencar JW. The essential oil composition of two chemotypes of Lippia alba grown in Northeast Brazil. J Essen Oil Res 1996;8:695–8
- Frighetto N, Oliveira JG, Siani AC, Chagas KC. Lippia alba (Mill.) N.E. Br (Verbenaceae) as a source of linalool. J Essen Oil Res 1998;10:578–80
- Tani K, Adachi M, Nakamura Y, et al. The effect of dermatophytes on cytokine production by human keratinocites. Arch Dermatol Res 2007;299:381–7
- Gupta AK, Lynde CW, Lauzon GJ. Cutaneous adverse effects associated with terbinafine therapy: 10 case reports and a review of the literature. Br J Dermatol 1998;138:529–32
- Carazo JLS, Losada LO, Sanjuan VP. Tratamiento actual de las micosis superficiales. Rev Iberoam Micol 1999;16:26–30
- Silva MRR, Oliveira Jr. JG, Fernandes OFL, et al. Antifungal activity of Ocimum gratissimum towards dermatophytes. Mycoses 2005;48:172–5
- Peres NTA, Rossi A, Maranhão FCA, et al. Dermatophytes: host-pathogen interaction and antifungal resistance. Na Bras Dermatol 2010;85:657–67
- Cavaleiro C, Pinto E, Gonçalves MJ, Salgueiro L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J Appl Microbiol 2006;100:1333–8
- Bajpai VK, Yoon JI, Kang SC. Antifungal potential of essential oil and various organic extracts of Nandina domestica Thun against skin infections fungal pathogens. Appl Microbiol Biotechnol 2009;83:1127–33
- Khan MAS, Ahmad I. In vitro antifungal, anti-elastase and anti-keratinase activity of essetial oils of Cinnamomum-, Syzygium- and Cympopoon species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine 2011;15:48–55
- Guenther E. Individual essential oils of the plant families Rutaceae and Labiatae. In: Van Nostrand D, ed. The essential oils. Vol. 3. Malabar (FL): Krieger Publishing Company; 1972:1–777
- Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream (IL): Allured Publishing Corporation; 2007:1–804
- Palmeira VF, Kneipp LF, Alviano CS, Santos ALS. The major chromoblastomycosis fungal pathogen Fonsecaea pedrosoi extracellularly releases proteolytic enzymes whose expression is modulated by culture medium composition: implications on the fungal development and cleavage of key’s host structures. FEMS Immunol Med Microbiol 2006;46:21–9
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests. 4th ed. Approved standards, M38-A2. Wayne (PA): CLSI; 2008
- Buroker-Kilgore M, Wang KKW. A Coomassie brilliant blue G-250- based colorimetric assay for measuring activity of calpain and other proteases. Anal Biochem 1993;208:387–92
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Mazotto AMA, Melo CAN, Macrae A, et al. Biodegradation of feather waste by extracellular keratinases and gelatinases from Bacillus spp. World J Microbiol Biotechnol 2011;27:1355–65
- Brouta F, Descamps F, Monod M, et al. Secreted metalloprotease gene family of Microsporum canis. Infect Immun 2002;70:5676–83
- Vermout S, Tabart J, Baldo A, et al. Pathogenesis of dermatophytosis. Mycopathologia 2008;166:267–75
- Leng W, Liu T, Wang J, et al. Expression dynamics of secreted protease genes in Trichophyton rubrum induced by key host’s proteinaceous components. Med Mycol 2009;47:759–65
- Tejasvi T, Sharma VK, Sethuraman G, et al. Invasive dermatophytosis with lymph node involvement in an immunocompetent patient. Clin Exp Dermatol 2005;30:506–8
- Gong JQ, Liu XQ, Xu HB, et al. Deep dermatophytosis caused by Trichophyton rubrum: report of two cases. Mycoses 2007;50:102–8
- Warycha MA, Leger M, Tzu J, et al. Deep dermatophytosis caused by Trichophyton rubrum. Dermatol Online J 2011;17:21
- Viani FC, Dos Santos JI, Paula CR, et al. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med Mycol 2001;39:463–8
- Monod M. Secreted proteases from dermatophytes. Mycopathologia 2008;166:285–94
- Okumura Y, Ogawa K, Uchgiya K, Nikai T. Characterization and primary structure of elastase inhibitor, AFLEI from Aspergillus flavus. Jpn J Med Mycol 2007;18:13–18
- Vermount S, Tabart J, Baldo A, et al. Pathogenesis of dermatophytosis. Mycopathologia 2008;166:267–75
- Sivamani P, Singaravelu G, Thiagarajan V, et al. Comparative molecular docking analysis of essential oil constituents as elastase inhibitors. Bioinformation 2012;8:457–60
- Bastos GM, Nogueira NAP, Soares CL, et al. In vitro determination of the antimicrobial potential of homemade preparations based on medicinal plants used to treat infectious diseases. J Basic Appl Sci 2011;32:113–20
- Watkins F, Pendry B, Sanchez-Medina A, Corcoran O. Antimicrobial assays of three native British plants used in Anglo-Saxon medicine for wound healing formulations in 10th century England. J Ethnopharmacol 2012;144:408–15
- Karunai Raj M, Balachandran C, Duraipandiyan V, et al. Antimicrobial activity of Ulopterol isolated from Toddalia asiatica (L.) Lam.: a traditional medicinal plant. J Ethnopharmacol. 2012;140:161–5
- Silveira HCS, Gras DE, Cazzaniga RA, et al. Transcriptional profiling reveals genes in the human pathogen Trichophyton rubrum that are expressed in response to pH signaling. Microb Pathog 2010;48:91–6
- Maranhao FCA, Paiao FG, Martinez-Rossi NM. Isolation of transcripts over-expressed in human pathogen Trichophyton rubrum during growth in keratin. Microb Pathog 2007;43:166–72
- Yike I. Fungal proteases and their pathophysiological effects. Mycopathologia 2011;171:299–323
- Gupta R, Ramnani P. Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 2006;70:21–33
- Prakash P, Jayalakshmi SK, Sreeramulu K. Purification and characterization of extreme alkaline, thermostable keratinase, and keratin disulfide reductase produced by Bacillus halodurans PPKS-2. Appl Microbiol Biotechnol 2010;87:625–33
- Mazotto AMA, Coelho RRR, Cedrola SLM, et al. Keratinase production by three Bacillus spp. using feather meal and whole feather as substrate in a submerged fermentation. Enz Res 2011. [Epub ahead of print]. doi: 10.4061/2011/523780
- Santos ALS, Palmeira VF, Rozental S, et al. Biology and pathogenesis of Fonsecaea pedrosoi, the major etiologic agent of chromoblastomycosis. FEMS Microbiol Rev 2007;31:570–91
