Abstract
Coumarins have attracted intense interest in recent years because of their diverse pharmacological properties. According to our continuing investigations of biological effects of several coumarins, the structure–antioxidant activity relationships (SARs) of six naturally occurring coumarins and their 16 synthesized analogues were established. For this purpose, the very reliable colorimetric assay (ferric reducing antioxidant power) modified to be used in 96-well microplates was used. This approach, which determines the reducing capacity of tested compounds directly, has previously been used for the determination of SARs of flavonoids, but has not been used for SAR determination of coumarins. It is known that the biological properties and consequently, therapeutic application of simple coumarins depends upon the pattern of substitution. It was established that 7,8-dihydroxy- and 6,7-dihydroxy-4-methylcoumarins have shown excellent ferric-reducing properties.
Introduction
In recent years, considerable effort has been directed towards identifying naturally occurring substances and their synthesized derivatives that can protect against oxidative stress. There is convincing evidence that oxidative stress and reactive oxygen species play an important role in the aetiology or progression of a number of human diseasesCitation1–4. This knowledge has led to the suggestion that antioxidants can have health benefits as prophylactic agentsCitation5.
Natural phenolic compounds (e.g. phenolic acids, flavonoids, coumarins and tannins) have been reported to exhibit a wide range of biological effects, including antibacterial, antiviral, antioxidant, anti-inflammatory, antiallergic and vasodilatory actionsCitation6,Citation7. The health benefit activity of phenolic compounds is often derived from their antioxidant action. Their protective effect against oxidative damage depends on the hydrogen-donating capacity of a hydroxyl group in each moleculeCitation5,Citation8. Coumarins, compounds derived from benzopyran, are quite similar to well-known antioxidants–flavonoids, which have been recently studied extensively for their antioxidant propertiesCitation9. Many of them have been isolated and identified from natural sources, especially green plants, and many others have been synthesizedCitation10,Citation11. The antioxidant activities of coumarins have been previously evaluated against various radicals such as, e.g. 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicalCitation12, hydroxyl radicalCitation13, superoxideCitation14 and peroxyl radicalCitation15.
Another commonly used method for the determination of antioxidant capacity is “ferric reducing antioxidant power” (FRAP) assay. FRAP assay is a simple, reliable colorimetric method, based on ability of the antioxidants to reduce Fe3+ to Fe2+. FRAP assay measures directly the reducing capacity of the substance, which is an important parameter for compounds to be a good antioxidantCitation16.
In our previous study, antioxidant potency of 22 coumarins (6 natural and 16 synthetic coumarins) against DPPH radical using the sequential injection analysis method was evaluatedCitation17. The structure–activity relationship (SAR) results showed high DPPH radical scavenging activity, especially in 7,8-dihydroxy-4-methylcoumarins. In this study the reducing effect of the same group of coumarins was examined by the FRAP test modified to be used in 96-well microplates. Results of FRAP assay are demonstrated as FRAP values at 4 and 60 min according to the study of Firuzi et al.Citation18. Obtained results were compared with known antioxidants trolox, quercetin and (+)-catechin. According to the results, the SARs of the tested compounds were evaluated.
Materials and methods
Chemicals
Coumarins () nos (5–22) were obtained from Prof. Saso (Department of Human Pharmacology and Physiology, University La Sapienza, Roma, Italy), scopoletin (1), umbelliferon (3) and scopolin (4) were earlier isolated from Evolvulus alsinoides Linn in our department. Trolox (23), (+)-catechin (24), 4-hydroxycoumarin (2), quercetin (25), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), dimethylsulphoxide (DMSO), ferrous sulphate heptahydrate (FeSO4·7H2O), ferrous chloride hexahydrate (FeCl3·6H2O), acetic acid 99% (CH3COOH), hydrochloric acid 1000 mM (HCl) and natrium acetate trihydrate (CH3COONa·3H2O) were obtained from Sigma-Aldrich (Prague, Czech Republic). DMSO was used as a solvent. Stock solutions of the tested compounds in DMSO 1:1 (v/v) were prepared. Less concentrated solutions (200, 80, 60, 40 and 10 µM) were prepared by diluting the stock solutions with acetate buffer (pH = 3.6).
Table 1. Structures and FRAP values at 4 and 60 min (±SD) of tested compounds.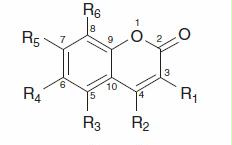 .
.
Apparatus
All measurements were carried out by using 96-well microplates P (Brand 400 µl; Fisher Scientific, Pardubice, Czech Republic) and Anthos 2010 Microplate Reader (Biochrom Ltd, Cambridge, UK) with program Anthos WinRead working in Windows system.
FRAP assay
FRAP assay is a simple, reliable colorimetric method, based on ability of the antioxidants to reduce Fe3+ to Fe2+. Fe2+ is measured spectrophotometrically by the determination of blue colour complex with TPTZ, which has the maximum absorbance at 595 nmCitation19.
All experiments were carried out according to study of Firuzi et al.Citation18, which deals with the FRAP method modified to be performed in 96-well microplates. The FRAP value at time interval t (FRAP valuet) was calculated according to the following formula:
where Δat coumar is the absorbance change after the time interval t (4 and then 60 min) relative to the tested coumarins at the concentration of 1 × 10−5 M and Δat Fe2+ is the absorbance change at the same time interval (i.e. after 4 and then 60 min) relative to the ferrous sulphate at the same concentration. Well-known antioxidants (trolox, quercetin and (+)-catechin) have been used as standards.
Statistic analyses were performed using the software SigmaPlot 2002 for Windows version 8.0 (Systat Software Inc, Erkrath, Germany). All experiments were repeated at least three times.
Results
In this study, reducing power of 25 compounds presented by coumarins (both natural and synthesized) with various substituents on benzoid nucleus and other phenolic substances (quercetin, trolox and (+)-catechin) were tested.
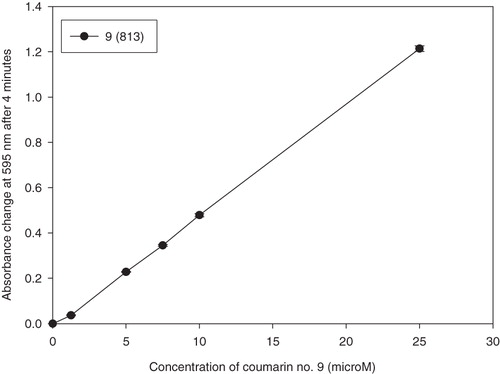
Results of the FRAP assay are demonstrated as FRAP values at 4 and 60 min and are outlined in and . The absorbance changes in time in the presence of reducing active coumarins and the absorbance change of Fe2+ in the same concentration are shown in . For comparison, results of FRAP values of known antioxidant compounds (quercetin, trolox and (+)-catechin) are also presented. Their reducing power decreases in this order: quercetin > (+)-catechin > trolox ( and ). These results correspond with data already publishedCitation18. For illustration, in , the effect of the concentration of the most reducing active coumarin 6,7-dihydroxy-4-methylcoumarin (9) on the change of absorbance at 4 min is shown.
Figure 1. The results of FRAP values at 4 and 60 min of tested coumarins (±SD). The measured values are presented in .
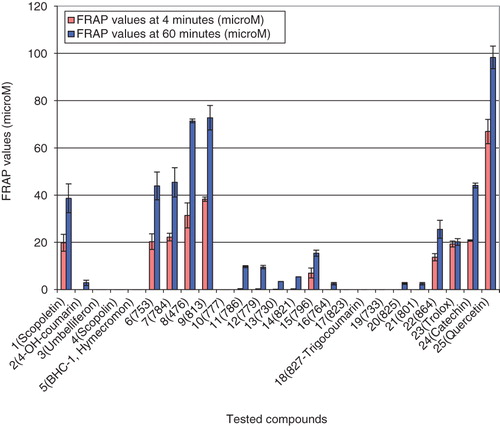
Figure 2. Absorbance changes at 595 nm at different time intervals in the presence of some active tested compounds (10 µM) and the absorbance change of Fe2+ (10 µM) in the FRAP assay.
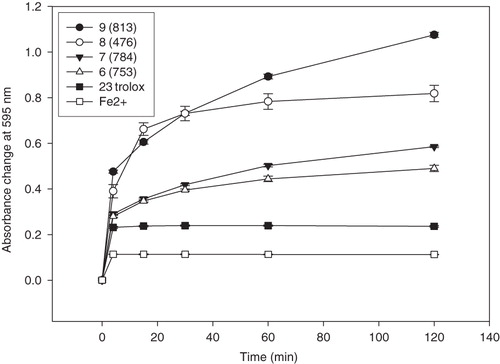
Figure 3. Dependence of absorbance change at 595 nm after 4 min on the concentration of the most reducing active compound 6,7-dihydroxy-4-methylcoumarin (9) assayed by the FRAP method.
The results demonstrated that monohydroxycoumarins presented by some naturally occurring coumarins such as 4-hydroxycoumarin (2), umbelliferon (3) and 4-methyl-7-hydroxycoumarin (hymecromon) (5) did not show any significant antioxidant activity in this test. Similarly, natural scopolin (glycoside of scopoletin and glucose) (4) was found as almost inactive. On the other hand, naturally occurring coumarin scopoletin (1) showed a significant ferric reducing activity, which was close to that of (+)-catechin (24).
Our results showed that some of the dihydroxy-4-methylcoumarins possessed excellent ferric reducing activity, which depend on the position of both hydroxyl groups on the benzoid nucleus. Ferric reducing activity of various position isomers of dihydroxy-4-methylcoumarins decreased in the following order: 6,7-dihydroxy-4-methylcoumarin (9) > 7,8-dihydroxy-4-methylcoumarin (6) > 5,7-dihydroxy-4-methylcoumarin (10) (). It was also observed that binding of propanoic acid ethylester at position C3 influenced the ferric reducing activity in a positive way: propanoic acid ethylester (8) > acetic acid ethylester (7) ∼ hydrogen (6). According to our study, the 6,7-dihydroxy-4-methylcoumarin (9), 7,8-dihydroxy-4-methylcoumarin (6), its C3 acetic acid ethylester (7) and propanoic acid ethylester (8) derivates are powerful ferric reducing agents; the FRAP values of 7,8-dihydroxy-4-methylcoumarin (6) and its C3 acetic acid ethylester (7) are comparable with that of (+)-catechin (24). The FRAP values of 6,7-dihydroxy-4-methylcoumarin (9) and propanoic acid ethylester of 7,8-dihydroxy-4-methylcoumarin (8) were even higher, nevertheless not as high as that of the most powerful reducing agent quercetin (25). Contrariwise, 5,7-dihydroxy-4-methylcoumarin (10) and its acetic acid ethylester (11) or propanoic acid ethyl ester (12) derivatives did not show any significant antioxidant activity in this test. These results confirmed the importance of the ortho-position of hydroxyl groups to each other to reach maximal ferric reducing activity.
According to our results, the ferric reducing activity of diacetoxy-4-methylcoumarins (13–16) is much lower than the activity of dihydroxy-4-methylcoumarins (6–9). Nevertheless, the FRAP values of propanoic acid ethylester of 7,8-diacetoxy-4-methylcoumarin (15) were significantly higher than those of acetic acid ethylester of 7,8-diacetoxy-4-methylcoumarin (14). Obtained results showed diacetoxy-4-methylcoumarins (13 and 14) with the acetoxy groups at the ortho-position as weak ferric reducing agents. Similarly, diacetoxy-4-methylcoumarin (16) with the acetyoxy groups at the meta-position was found to be almost inactive. No significant ferric reducing activity was also found among ortho- or meta-dimethoxy-4-methylcoumarins (17–21). In addition, 1,4-dihydroxyanthron (22) was tested. This compound showed a significant reducing activity approximately close to that of trolox.
Discussion
The antioxidant activities of a series of structurally different coumarins (both natural and synthesized) were analysed by a modified FRAP assay, which facilitate fast analysisCitation18. The FRAP assay gives reproducible results with single antioxidants. This method is based on the measurement of ability of the substance to reduce Fe3+ to Fe2+ and was initially proposed to measure the total antioxidant activity of plasma and then applied by the same authors to other substratesCitation16,Citation20. Fe2+ is measured spectrophotometrically via determination of its coloured complex with TPTZ, which has a high absorbance at 595 nm. However, the absorption (A595 nm) does not stop at 4 min, instead it slowly increased at several minutes. This feature is typical for phenolic compounds, including trolox and also coumarins. Hence, the FRAP value of these compounds could not be obtained accurately just at 4 minCitation8. In our study, the absorbance was measured in time interval 0–120 min and the FRAP value was determined in two time intervals (4 and 60 min).
Antioxidants with different ferric reducing abilities were characterized by evaluation of the dependence of the absorbance change at 595 nm on the concentration of the tested compounds (µM; ) and results facilitated fast evaluation of SAR. On the other hand, this method determinates only the reducing activity; hence, perspective samples have to be further analysed using other in vitro and in vivo antioxidant methodsCitation1,Citation21–23.
In this study, using the FRAP method, 25 compounds were tested. The group included 6 naturally occurring coumarins (scopoletin, umbelliferon, scopolin, 4-hydroxycoumarin, hymecromon and trigocoumarin), 16 synthesized analogues and 3 known antioxidants (quercetin, trolox and (+)-catechin). The various substituents on coumarin nucleus have been used – one or two hydroxyl, acetoxy and methoxy groups have been used alone or in different combinations. Moreover, the effect of the presence of acetic acid ethylester or propanoic acid ethyl ester at position C3 has been examined.
As can be observed from the results, the excellent antioxidant activity of dihydroxy derivatives of 4-methylcoumarins depends on the ortho-position of both hydroxyl groups on the benzoid nucleus, while the coumarins with the hydroxyl groups in the meta-position are weak to be an antioxidant. Generally, ferric reducing activity of dihydroxy-4-methylcoumarins follows this order: 6,7-dihydroxy-4-methylcoumarins > 7,8-dihydroxy-4-methylcoumarins > 5,7-dihydroxy-4-methylcoumarins. On the other hand, monomethoxy-4-coumarins, monohydroxy-4-coumarins and dimethoxy-4-coumarins did not show any significant effect. The binding of acetic acid ethylester, and especially propanoic acid ethyl ester at position C3 significantly influenced the ferric reducing activity in a positive way. This results suggest new antioxidant active compound, C3 propanoic acid ethyl ester of 6,7-dihydroxy-4-methylcoumarin, as the most perspective compound for further study.
In the case of diacetoxy-4-methylcoumarins, according to our study, the FRAP values at 4 and 60 min of ortho-diacetoxy-4-methylcoumarins were significantly weaker compared to those of ortho-dihydroxy-4-methylcoumarins. In addition, 1,4-dihydroxyanthron has been tested to find out whether the more modified structure also possesses ferric reducing activity. Tested 1,4-dihydroxyanthron showed the significant ferric reducing activity. Obtained result is promising for further evaluation of SAR of this type of compounds.
In conclusion, we have established FRAP method as an excellent method for fast and sensitive determination of ferric reducing activity of various types of compounds. Using this method, we have evaluated the SAR of 22 potential antioxidants. Our results confirmed the 6,7-dihydroxy-4-methylcoumarin and the 7,8-dihydroxy-4-methylcoumarins as excellent ferric reducing agents.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
This study was supported by long-term development plan of University Hospital, Hradec Kralove (Czech Republic) and by the European Social Fund and the state budget of the Czech Republic, TEAB, project no. CZ.1.07/2.3.00/20.0235.
References
- Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 2003;523–24:9–20
- Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Neurobehavioral aspects of antioxidants in aging. Int J Dev Neurosci 2000;18:367–81
- Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res 2001;480–81:243–68
- Vaya J, Aviram M. Nutritional antioxidants mechanisms of action, analyses of activities and medical applications. Curr Med Chem 2001;1:99–117
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, et al. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res 2005;579:200–13
- Koleckar V, Brojerova E, Rehakova Z, et al. In vitro antiplatelet activity of flavonoids from Leuzea carthamoides. Drug Chem Toxicol 2008;31:27–35
- Koleckar V, Kubikova K, Rehakova Z, et al. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev Med Chem 2008;8:436–47
- Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345–96
- Koleckar V, Opletal L, Brojerova E, et al. Evaluation of natural antioxidants of Leuzea carthamoides as a result of a screening study of 88 plant extracts from the European Asteraceae and Cichoriaceae. J Enzyme Inhib Med Chem 2008;23:218–24
- Kostova I. Synthetic and natural coumarins as antioxidants. Mini Rev Med Chem 2006;6:365–74
- Cervenka F, Koleckar V, Rehakova Z, et al. Evaluation of natural substances from Evolvulus alsinoides L. with the purpose of determining their antioxidant potency. J Enzyme Inhib Med Chem 2008;23:574–8
- Raj HG, Parmar VS, Jain SC, et al. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part I: dioxygenated 4-methyl coumarins as superb antioxidant and radical scavenging agents. Bioorgan Med Chem 1998;6:833–9
- Chakrabarti S, Makrigiorgos GM, O’Brien K, et al. Measurement of hydroxyl radicals catalyzed in the immediate vicinity of DNA by metal-bleomycin complexes. Free Radic Biol Med 1996;20:777–83
- Fylaktakidou KC, Gautam DR, Hadjipavlou-Litina DJ, et al. Reactions of 4-methylchromene-2,7,8-trione with phosphonium ylides. Synthesis and evaluation of fused 1,3-dioxolocoumarins as antioxidants and antiinflammatories. J Chem Soc Perkin Trans 2001;1:3073–9
- Arora A, Nair MG, Strasburg GM. Structure–activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic Biol Med 1998;24:1355–63
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6
- Rehakova Z, Koleckar V, Cervenka F, et al. DPPH radical scavenging activity of several naturally occurring coumarins and their synthesized analogues measured by SIA method. Toxicol Mech Meth 2008;18:413–18
- Firuzi O, Lacanna A, Petrucci R, et al. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 2005;1721:174–84
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005;53:1841–56
- Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth Enzymol 1999;299:15–27
- Koleckar V, Jun D, Opletal L, et al. Assay of radical scavenging activity of antidotes against chemical warfare agents by DPPH test using sequential injection technique. J Appl Biomed 2007;5:81–4
- Roginsky V, Lissi EA. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 2005;92:235–54
- Paulova H, Bochorakova H, Taborska E. In vitro methods for estimation of the antioxidant activity of natural compounds. Chem Listy 2004;98:174–9
