Abstract
A series of benzenesulfonamide derivatives, bearing benzimidazole moieties, were designed and synthesized as inhibitors of carbonic anhydrases (CAs). Their binding affinities to recombinant human CA isozymes I, II, VII, XII and XIII were determined by the thermal shift assay. A group of compounds containing a benzimidazole substituent in the para position of the benzenesulfonamide ring was found to exhibit higher binding potency toward tested CAs than meta-substituted benzenesulfonamides. Some of these compounds exhibited nanomolar affinities and selectivity toward the CA isozymes tested.
Introduction
Carbonic anhydrases (CAs) are widely investigated enzymes due to their involvement in various physiological and pathological processesCitation1–4. There are a total of 15 CA isoforms found in humans. However, three of them have no catalytic activity (CA VIII, X and XI). The 12 active isozymes (CA I–IV, VA, VB, VI, VII, IX, XII and XIV) are metalloenzymes, which participate in the regulation of acid–base balance and ion transport in many tissues and organs. The enhanced activity of CAs is often related to various diseases. The isoforms CA IX and CA XII were intensively investigated enzymes during the last decade due to their overexpression in various human cancersCitation5–9. Much work has been done to design selective CA IX and CA XII inhibitors. Some compounds such as glycosyl coumarins, N-β-glycosyl sulfamides and ureido-substituted sulfamates were found to be low nanomolar CA IX and XII inhibitors, while showing weak inhibition toward cytosolic isoforms CA I and CA IICitation10–12. Numerous inhibitors of various CA isoforms were reviewed by Alterio et al.Citation1. The design of CA inhibitors as drugs has been impeded by the large number of isoforms, their diffusion among many tissues/organs and the lack of isozyme-selective inhibitors among existing sulfonamidesCitation13. As the differences between the active sites of the 12 catalytically active human CAs are very subtle, CAs have proved to be a challenging drug target for the development of isozyme-specific inhibitorsCitation2,Citation14.
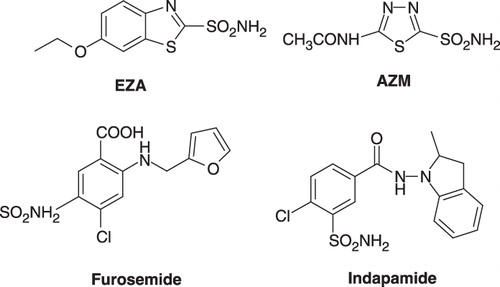
Primary sulfonamides present an important class of compounds which have been studied as drugs and drug candidates against diseases such as glaucoma, inflammation, edema, epilepsy, dandruff, cancer and as antiviral compoundsCitation15,Citation16. The majority of clinically used CA inhibitors are also primary sulfonamidesCitation1. To date, CA inhibitors such as acetazolamide (AZM), ethoxzolamide (EZA) and methazolamide are widely used as systemic antiglaucoma drugsCitation17; sultiame and zonisamide as antiepileptic drugsCitation18; and chlorthalidone, indapamide and furosemide as diureticsCitation19 (). The largest numbers of new CA inhibitors are benzenesulfonamidesCitation1,Citation14.
Benzenesulfonamides bind to the active site of CAs in their deprotonated form, with the nitrogen atom of the sulfonamide moiety coordinated to the Zn(II) ion. Depending on the nature of the substituent, additional interactions with hydrophobic and hydrophilic amino acids in the CA active center can be assigned. Therefore, a variety of substitutions on the benzenesulfonamide head group can be used for the development of novel inhibitors.
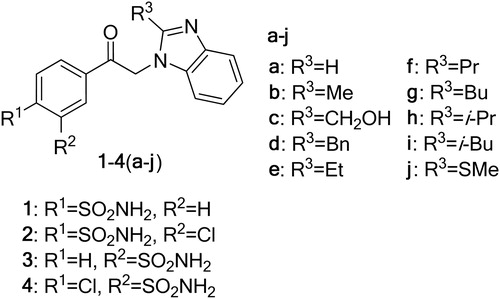
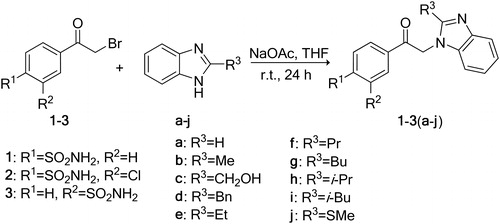
Recently, we have investigated the interaction of several CA isozymes with pyrimidine derivatives such as [(2-pyrimidinylthio)acetyl]benzenesulfonamidesCitation20, 4-[N-(substituted 4-pyrimidinyl)amino]benzenesulfonamidesCitation21 and thiadiazole derivativesCitation22. We have also reported on the synthesis of 2-substituted benzimidazoles N-alkylated with 2-chloro-5-bromoacetylbenzenesulfonamide (, compounds 4(a–j)) and their binding to four CA isozymes, namely CA I, CA II, CA VII and CA XIIICitation23. We extended these earlier investigations to new sulfonamides containing a benzimidazole group (compounds of series 1–3).
Here we present the binding data of 4(a–j) to the catalytic domain of CA XII, an anticancer drug target. The N-alkylated benzimidazoles 4(a–j) exhibited dissociation constants in the range of 0.3–10 µM against tested CAs. Furthermore, we compared series 4 to compounds of series 1–3, which are 4-[(2-substituted-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide 1(a–j), 2-chloro-4-[(2-substituted-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide 2(a–j) and 3-[(2-substituted-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide 3(a–j). Their binding was then assayed with five CA isozymes CA I, CA II, CA VII, CA XII and CA XII. The compounds 2(a–j) were found to be the most potent CA inhibitors exhibiting nanomolar affinities toward CA I, II, VII and XIII. Detailed structure–activity correlation could be drawn by comparing the affinities and selectivity of these compounds.
Materials and methods
Syntheses
Synthesis of 4-(bromoacetyl)benzenesulfonamide (1) and 3-(bromoacetyl)benzenesulfonamide (3) was achieved from commercially available 1-(4-aminophenyl)ethanone and 1-(3-aminophenyl)ethanone, respectively, as described by Fujikura et al.Citation24. Synthesis of 4-(bromoacetyl)-2-chlorobenzenesulfonamide (2) was accomplished from 1-(4-amino-3-chlorophenyl)ethanone as described by Fujikura et al.Citation24. 1-(4-Amino-3-chlorophenyl)ethanone was synthesized by the chlorination of 1-(3-aminophenyl)-N ethanone with N-chlorosuccinimide in acetonitrileCitation25,Citation26. 2-Substituted benzimidazoles (b–i) were prepared from 1,2-benzenediamine and the appropriate carboxylic acids according to Phillips’ procedureCitation27. Benzimidazole (a) and 2-methylthiobenzimidazole (j) are commercially available and were used without further purification. All ingredients were purchased from Sigma-Aldrich (Milwaukee, WI) and Alfa Aesar GmbH (Karlsruhe, Germany).
The melting points of the compounds were determined in open capillaries on a Thermo Scientific 9100 Series apparatus (Rochford, UK) without further correction. IR spectra were obtained on a Perkin-Elmer (Upplands Vasby, Sweden) FT-IR spectrophotometer Spectrum BX II in KBr. 1H and 13C NMR spectra were recorded on a Varian Unity Inova spectrometer (Palo Alto, CA) (300 and 75 MHz, respectively) in DMSO-d6 using residual DMSO signals (2.52 and 40.21 ppm for 1H and Citation13C NMR spectra, respectively) as the internal standard. Thin-layer chromatography was performed with silica gel 60 F254 aluminum plates (Merck, Darmstadt, Germany) and visualized with UV light. High-resolution mass spectra (HRMS) were recorded on a Dual-ESI Q-TOF 6520 mass spectrometer (Agilent Technologies, Santa Clara, CA). The purities of target compounds were analyzed using an HPLC system with UV detection.
General procedure for the synthesis of 1–3(a–j)
A mixture of the corresponding compounds 1–3 (0.360 mmol), appropriate 2-substituted benzimidazole (a–j) (0.540 mmol) and sodium acetate (33.9 mg, 0.414 mmol) in tetrahydrofuran (2 ml) were stirred at r.t. for 24 h. The reaction mixture was poured into water. The precipitate was filtered out and then washed with water and diethyl ether to yield 1–3(a–j).
4-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (1a). Yield 60%, m.p. 242–244 °C. IR (ν, cm−1): 3358 (NH2), 1701 (CO). 1H NMR δ ppm: 6.11 (2H, s, CH2CO), 7.24–7.27 (2H, m, C5′,6′–H), 7.57–7.60 (1H, m, C7′–H), 7.67 (2H, s, NH2), 7.70–7.73 (1H, m, C4′–H), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.24 (1H, s, C2′–H), 8.31 (2H, d, J = 8.1 Hz, C3,5–H). Citation13C NMR δ ppm: 51.82, 111.38, 119.97, 122.32, 123.14, 126.80, 129.66, 135.31, 137.46, 143.60, 145.57, 149.09, 193.67. HRMS Calcd for C15H13N3O3S ([M + H]+): 316.0750, Found: 316.0746.
4-(1H-benzimidazol-1-ylacetyl)-2-chlorobenzenesulfonamide (2a). Yield 72%, m.p. 234–236 °C. IR (ν, cm−1): 3379 (NH2), 1705 (CO). 1H NMR δ ppm: 6.10 (2H, s, CH2CO), 7.23–7.26 (2H, m, C5′,6′–H), 7.57–7.60 (1H, m, C7′–H), 7.70–7.72 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.19–8.23 (3H, m, C2′,5,6–H), 8.33 (1H, s, C3–H). Citation13C NMR δ ppm: 51.88, 111.37, 120.05, 122.27, 123.09, 127.72, 130.18, 131.75 (2C), 135.33, 138.83, 143.77, 145.50, 145.67, 192.98. HRMS Calcd for C15H12ClN3O3S ([M + H]+): 350.0361, Found: 350.0363.
3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (3a). Yield 83%, m.p. 247–249 °C. IR (ν, cm−1): 3270 (NH2), 1698 (CO). 1H NMR δ ppm: 6.13 (2H, s, CH2CO), 7.22–7.28 (2H, m, C5′,6′–H), 7.57–7.61 (1H, m, C7′–H), 7.64 (2H, s, NH2), 7.69–7.75 (1H, m, C4′–H), 7.88 (1H, t, J = 7.8 Hz, C5–H), 8.19 (1H, d, J = 7.8 Hz, C4–H), 8.22 (1H, s, C2′–H), 8.40 (1H, d, J = 7.8 Hz, C6–H), 8.50 (1H, s, C2–H). Citation13C NMR δ ppm: 15.71, 111.38, 120.04, 122.23, 123.08, 125.63, 130.60, 131.18, 132.26, 135.41, 135.74, 143.81, 145.60, 145.69, 193.48. HRMS Calcd for C15H13N3O3S ([M + H]+): 316.0750, Found: 316.0752.
4-[(2-Methyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1b). Yield 63%, m.p. 146–148 °C. IR (ν, cm−1): 3336 (NH2), 1698 (CO). 1H NMR δ ppm: 2.45 (3H, s, CH3), 6.06 (2H, s, CH2CO), 7.14–7.21 (2H, m, C5′,6′–H), 7.46–7.49 (1H, m, C7′–H), 7.57–7.60 (1H, m, C4′–H), 7.66 (2H, s, NH2), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.32 (2H, d, J = 8.1 Hz, C3,5–H). Citation13C NMR δ ppm: 14.00, 50.84, 110.60, 118.80, 122.06, 122.23, 126.74, 129.79, 136.53, 137.47, 142.88, 149.21, 153.31, 193.81. HRMS Calcd for C16H15N3O3S ([M + H]+): 330.0907, Found: 330.0909.
2-Chloro-4-[(2-methyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (2b). Yield 74%, m.p. 204–206 °C. IR (ν, cm−1): 3385, 3332 (NH2), 1707 (CO). 1H NMR δ ppm: 2.45 (3H, s, CH3), 6.09 (2H, s, CH2CO), 7.14–7.20 (2H, m, C5′,6′–H), 7.47–7.50 (1H, m, C7′–H), 7.57–7.61 (1H, m, C4′–H), 7.94 (2H, s, NH2), 8.20 (2H, s, C5,6–H), 8.37 (1H, s, C3–H). Citation13C NMR δ ppm: 13.97, 51.01, 110.75, 118.71, 122.19, 122.32, 127.80, 130.09, 131.71, 132.06, 136.41, 138.72, 142.59, 145.78, 153.29, 193.02. HRMS Calcd for C16H14ClN3O3S ([M + H]+): 364.0517, Found: 364.0517.
3-[(2-Methyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3b). Yield 62%, m.p. 295–297 °C. IR (ν, cm−1): 3280 (NH2), 1699 (CO). 1H NMR δ ppm: 2.46 (3H, s, CH3), 6.09 (2H, s, CH2CO), 7.14–7.21 (2H, m, C5′,6′–H), 7.47–7.51 (1H, m, C7′–H), 7.57–7.60 (1H, m, C4′–H), 7.63 (2H, s, NH2), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 8.1 Hz, C4–H), 8.44 (1H, d, J = 7.8 Hz, C6–H), 8.51 (1H, s, C2–H). Citation13C NMR δ ppm: 14.03, 50.78, 110.62, 118.83, 122.03, 122.21, 125.70, 130.57, 131.31, 132.51, 135.66, 136.58, 142.96, 145.66, 153.33, 193.59. HRMS Calcd for C16H15N3O3S ([M + H]+): 330.0907, Found: 330.0903.
4-{[2-(Hydroxymethyl)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (1c). Yield 57%, m.p. 200–202 °C. IR (ν, cm−1): 3375, 3314 (NH2, OH), 1702 (CO). 1H NMR δ ppm: 4.70 (2H, s, CH2OH), 5.64 (2H, br s, OH), 6.09 (2H, s, CH2CO), 7.20–7.26 (2H, m, C5′,6′–H), 7.53–7.56 (1H, m, C7′–H), 7.64–7.68 (3H, m, C4′–H, NH2), 8.06 (2H, d, J = 8.4 Hz, C2,6–H), 8.31 (2H, d, J = 8.4 Hz, C3,5–H). Citation13C NMR δ ppm: 51.04, 57.59, 111.02, 119.59, 122.31, 123.01, 126.76, 129.72, 137.00, 137.54, 142.33, 149.04, 154.76, 193.49. HRMS Calcd for C16H15N3O4S([M + H]+): 346.0856, Found: 346.0859.
2-Chloro-4-{[2-(hydroxymethyl)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (2c). Yield 52%, m.p. 195–197 °C. IR (ν, cm−1): 3370, 3267 (NH2, OH), 1707 (CO). 1H NMR δ ppm: 4.69 (2H, d, J = 4.8 Hz, CH2OH), 5.60 (1H, br s, OH), 6.08 (2H, s, CH2CO), 7.20–7.25 (2H, m, C5′,6′–H), 7.50–7.57 (1H, m, C7′–H), 7.60–7.65 (1H, m, C4′–H), 7.92 (2H, s, NH2), 8.19 (2H, s, C5,6–H), 8.33 (1H, s, C3–H). Citation13C NMR δ ppm: 51.11, 57.54, 110.97, 119.66, 122.27, 122.33, 127.74, 130.15, 131.05, 131.81, 137.01, 138.88, 142.50, 145.63, 154.71, 192.80. HRMS Calcd for C16H14ClN3O4S ([M + H]+): 380.0466, Found: 380.0463.
3-{[2-(Hydroxymethyl)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (3c). Yield 60%, m.p. 220–222 °C. IR (ν, cm−1): 3364, 3272 (NH2, OH), 1703 (CO). 1H NMR δ ppm: 4.69 (2H, d, J = 3.3 Hz, CH2OH), 5.63 (1H, br s, OH), 6.12 (2H, s, CH2CO), 7.21–7.24 (2H, m, C5′,6′–H), 7.54–7.57 (1H, m, C7′–H), 7.62–7.67 (3H, m, C4′–H, NH2), 7.87 (1H, t, J = 7.8 Hz, C5–H), 8.18 (1H, d, J = 7.5 Hz, C4–H), 8.40 (1H, d, J = 7.8 Hz, C6–H), 8.49 (1H, s, C2–H). Citation13C NMR δ ppm: 50.98, 57.64, 111.02, 119.63, 122.26, 122.97, 125.65, 130.59, 131.16, 132.33, 135.74, 137.07, 142.45, 145.63, 154.75, 193.20. HRMS Calcd for C16H15N3O4S ([M + H]+): 346.0856, Found: 346.0857.
4-[(2-Benzyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1d). Yield 62%, m.p. 241–243 °C. IR (ν, cm−1): 3284 (NH2), 1703 (CO). 1H NMR δ ppm: 4.26 (2H, s, CH2Ph), 6.06 (2H, s, CH2CO), 7.15–7.28 (7H, m, C5′,6′–H, Ph–H), 7.44–7.47 (1H, m, C7′–H), 7.62–7.65 (1H, m, C4′–H), 7.68 (2H, s, NH2), 8.04 (2H, d, J = 8.4 Hz, C2,6–H), 8.22 (2H, d, J = 8.7 Hz, C3,5–H). Citation13C NMR δ ppm: 33.42, 50.83, 111.03, 119.17, 122.28, 122.59, 126.63, 127.19, 129.06, 129.60 (2C), 129.70, 136.60, 137.36, 142.80, 149.03, 154.83, 193.15. HRMS Calcd for C22H19N3O3S ([M + H]+): 406.1220, Found: 406.1217.
4-[(2-Benzyl-1H-benzimidazol-1-yl)acetyl]-2-chlorobenzenesulfonamide (2d). Yield 71%, m.p. 210–212 °C. IR (ν, cm−1): 3382, 3294 (NH2), 1705 (CO). 1H NMR δ ppm: 4.27 (2H, s, CH2Ph), 6.07 (2H, s, CH2CO), 7.19–7.26 (7H, m, C5′,6′–H, Ph–H), 7.45–7.49 (1H, m, C7′–H), 7.61–7.64 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.07 (1H, d, J = 8.1 Hz, C5–H), 8.18 (1H, d, J = 8.1 Hz, C6–H), 8.25 (1H, s, C3–H). Citation13C NMR δ ppm: 33.40, 50.91, 111.01, 119.21, 122.25, 122.56, 127.15, 127.66, 129.03, 129.62, 129.98, 131.59, 131.87, 136.61, 137.41, 138.67, 142.91, 145.63, 154.77, 192.46. HRMS Calcd for C22H18ClN3O3S ([M + H]+): 440.0830, Found: 440.0836.
3-[(2-Benzyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3d). Yield 88%, m.p. 146–148 °C. IR (ν, cm−1): 3340 (NH2), 1702 (CO). 1H NMR δ ppm: 4.27 (2H, s, CH2Ph), 6.08 (2H, s, CH2CO), 7.17–7.36 (7H, m, C5′,6′–H, Ph–H), 7.45–7.52 (1H, m, C7′–H), 7.64–7.69 (1H, m, C4′–H, NH2), 7.85 (1H, t, J = 7.8 Hz, C5–H), 8.18 (1H, d, J = 7.8 Hz, C4–H), 8.34 (1H, d, J = 7.8 Hz, C6–H), 8.41 (1H, s, C2–H). Citation13C NMR δ ppm: 33.45, 50.76, 111.01, 119.22, 122.14, 122.22, 125.67, 127.18, 129.06, 129.63, 130.43, 131.16, 132.37, 135.63, 136.67, 137.44, 142.98, 145.55, 154.87, 193.01. HRMS Calcd for C22H19N3O3S ([M + H]+): 406.1220, Found: 406.1223.
4-[(2-Ethyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1e). Yield 71%, m.p. 236–238 °C. IR (ν, cm−1): 3334 (NH2), 1699 (CO). 1H NMR δ ppm: 1.31 (3H, t, J = 7.5 Hz, CH3), 2.79 (2H, q, J = 7.5 Hz, CH2), 6.07 (2H, s, CH2CO), 7.17–7.20 (2H, m, C5′,6′–H), 7.47–7.50 (1H, m, C7′–H), 7.61–7.64 (1H, m, C4′–H), 7.67 (2H, s, NH2), 8.07 (2H, d, J = 8.1 Hz, C2,6–H), 8.33 (2H, d, J = 8.1 Hz, C3,5–H). Citation13C NMR δ ppm: 12.16, 20.50, 50.63, 110.70, 118.91, 122.12, 122.37, 126.73, 129.82, 136.49, 137.44, 142.62, 149.21, 157.44, 193.89. HRMS Calcd for C17H17N3O3S ([M + H]+): 344.1063, Found: 344.1062.
2-Chloro-4-[(2-ethyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (2e). Yield 66%, m.p. 236–238 °C. IR (ν, cm−1): 3333 (NH2), 1706 (CO). 1H NMR δ ppm: 1.30 (3H, t, J = 7.5 Hz, CH3), 2.78 (2H, q, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.14–7.21 (2H, m, C5′,6′–H), 7.47–7.50 (1H, m, C7′–H), 7.60–7.63 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.17–8.23 (2H, m, C5,6–H), 8.38 (1H, s, C3–H). Citation13C NMR δ ppm: 12.19, 20.46, 50.76, 110.76, 118.90, 122.14, 122.36, 127.79, 130.06, 131.71, 132.10, 136.44, 138.70, 142.59, 145.78, 157.41, 193.16. HRMS Calcd for C17H16ClN3O3S ([M + H]+): 378.0674, Found: 378.0670.
3-[(2-Ethyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3e). Yield 66%, m.p. 292–294 °C. IR (ν, cm−1): 3288 (NH2), 1699 (CO). 1H NMR δ ppm: 1.31 (3H, t, J = 7.5 Hz, CH3), 2.79 (2H, q, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.14–7.21 (2H, m, C5′,6′–H), 7.47–7.50 (1H, m, C7′–H), 7.62–7.69 (1H, m, NH2, C4′–H), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 8.1 Hz, C4–H), 8.45 (1H, d, J = 7.8 Hz, C6–H), 8.51 (1H, s, C2–H). Citation13C NMR δ ppm: 12.20, 20.53, 50.53, 110.65, 119.01, 121.99, 122.27, 125.71, 130.56, 131.31, 132.54, 135.66, 136.60, 142.92, 145.66, 157.46, 193.72. HRMS Calcd for C17H17N3O3S ([M + H]+): 344.1063, Found: 344.1065.
4-[(2-Propyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1f). Yield 65%, m.p. 245–247 °C. IR (ν, cm−1): 3300 (NH2), 1700 (CO). 1H NMR δ ppm: 0.97 (3H t, J = 7.5 Hz, CH3), 1.78 (2H, sextet, J = 7,5 Hz, CH2), 2.75 (2H, t, J = 7.2 Hz, CH2), 6.08 (2H, s, CH2CO), 7.14–7.22 (2H, m, C5′,6′–H), 7.45–7.48 (1H, m, C7′–H), 7.60–7.63 (1H, m, C4′–H), 7.69 (2H, s, NH2), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.33 (2H, d, J = 8.7 Hz, C3,5–H). Citation13C NMR δ ppm: 14.47, 20.95, 28.84, 50.66, 110.75, 118.88, 122.12, 122.32, 126.71, 129.85, 136.38, 137.38, 142.66, 149.19, 156.32, 193.85. HRMS Calcdfor C18H19N3O3S([M + H]+): 358.1220, Found: 358.1222.
2-Chloro-4-[(2-propyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (2f). Yield 71%, m.p. 240–242 °C. IR (ν, cm−1): 3354 (NH2), 1702 (CO). 1H NMR δ ppm: 0.97 (3H, t, J = 7.5 Hz, CH3), 1.78 (2H, sextet, J = 7.5 Hz, CH2), 2.74 (2H, t, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.13–7.20 (2H, m, C5′,6′–H), 7.45–7.48 (1H, m, C7′–H), 7.59–7.62 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.20 (2H, s, C5,6–H), 8.39 (1H, s, C3–H). Citation13C NMR δ ppm: 14.49, 21.00, 28.87, 50.78, 110.74, 118.96, 122.05, 122.24, 127.79, 130.05, 131.71, 132.13, 136.41, 138.69, 142.90, 145.78, 156.31, 193.14. HRMS Calcd for C18H18ClN3O3S ([M + H]+): 392.0830, Found: 392.0827.
3-[(2-Propyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3f). Yield 87%, m.p. 275–277 °C. IR (ν, cm−1): 3314 (NH2), 1701 (CO). 1H NMR δ ppm: 0.97 (3H, t, J = 7.5 Hz, CH3), 1.79 (2H, sextet, J = 7.5 Hz, CH2), 2.75 (2H, t, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.13–7.21 (2H, m, C5′,6′–H), 7.46–7.49 (1H, m, C7′–H), 7.60–7.62 (3H, m, C4′–H, NH2), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 8.1 Hz, C4–H), 8.45 (1H, d, J = 7.8 Hz, C6–H), 8.50 (1H, s, C2–H). Citation13C NMR δ ppm: 14.50, 20.98, 28.91, 50.58, 110.69, 118.99, 122.00, 122.22, 125.71, 130.56, 131.30, 132.58, 135.64, 136.50, 142.97, 145.65, 156.35, 193.66. HRMS Calcd for C18H19N3O3S ([M + H]+): 358.1220, Found: 358.1219.
4-[(2-Butyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1 g). Yield 67%, m.p. 260–262 °C. IR (ν, cm−1): 3299 (NH2), 1699 (CO). 1H NMR δ ppm: 0.90 (3H, t, J = 7.5 Hz, CH3), 1.39 (2H, sextet, J = 7.5 Hz, CH2), 1.75 (2H, quintet, J = 7.5 Hz, CH2), 2.77 (2H, t, J = 7.5 Hz, CH2), 6.08 (2H, s, CH2CO), 7.14–7.21 (2H, m, C5′,6′–H), 7.45–7.48 (1H, m, C7′–H), 7.60–7.63 (1H, m, C4′–H), 7.69 (2H, s, NH2), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.34 (2H, d, J = 8.4 Hz, C3,3–H). Citation13C NMR δ ppm: 14.48, 22.50, 26.63, 29.65, 50.66, 110.70, 118.93, 122.06, 122.26, 126.73, 129.84, 136.45, 137.42, 142.82, 149.20, 156.46, 193.89. HRMS Calcd for C19H21N3O3S ([M + H]+): 372.1376, Found: 372.1376.
4-[(2-Butyl-1H-benzimidazol-1-yl)acetyl]-2-chlorobenzenesulfonamide (2 g). Yield 75%, m.p. 201–203 °C. IR (ν, cm−1): 3333 (NH2), 1708 (CO). 1H NMR δ ppm: 0.90 (3H, t, J = 7.2 Hz, CH3), 1.38 (2H, sextet, J = 7.5 Hz, CH2), 1.74 (2H, quintet, J = 7.5 Hz, CH2), 2.76 (2H, t, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.13–7.20 (2H, m, C5′,6′–H), 7.45–7.48 (1H, m, C7′–H), 7.59–7.61 (1H, m, C4′–H), 7.94 (2H, s, NH2), 8.20 (2H, s, C5,6–H), 8.39 (1H, s, C3–H). Citation13C NMR δ ppm: 14.47, 22.50, 26.60, 29.66, 50.78, 110.74, 118.93, 122.06, 122.24, 127.77, 130.06, 131.71, 132.12, 136.42, 138.69, 142.85, 145.78, 156.44, 193.15. HRMS Calcd. for C19H20ClN3O3S ([M + H]+): 406.0987, Found: 406.0981.
3-[(2-Butyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3 g). Yield 61%, m.p. 214–216 °C. IR (ν, cm−1): 3322 (NH2), 1702 (CO). 1H NMR δ ppm: 0.90 (3H, t, J = 7.2 Hz, CH3), 1.39 (2H, sextet, J = 7.2 Hz, CH2), 1.75 (2H, quintet, J = 7.5 Hz, CH2), 2.77 (2H, t, J = 7.5 Hz, CH2), 6.09 (2H, s, CH2CO), 7.13–7.20 (2H, m, C5′,6′–H), 7.45–7.48 (1H, m, C7′–H), 7.60–7.69 (3H, m, C4′–H, NH2), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 7.8 Hz, C4–H), 8.45 (1H, d, J = 8.1 Hz, C6–H), 8.50 (1H, s, C2–H). Citation13C NMR δ ppm: 14.47, 22.50, 26.64, 29.65, 50.57, 110.67, 118.98, 121.99, 122.20, 125.70, 130.56, 131.30, 132.56, 135.66, 136.52, 142.99, 145.65, 156.48, 193.67. HRMS Calcd for C19H21N3O3S ([M + H]+): 372.1376, Found: 372.1377.
4-[(2-Isopropyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1 h). Yield 65%, m.p. 258–260 °C. IR (ν, cm−1): 3341 (NH2), 1706 (CO). 1H NMR δ ppm: 1.30 (6H, d, J = 6.6 Hz, (CH3)2), 3.18 (1H, septet, J = 6.6 Hz, CH), 6.09 (2H, s, CH2CO), 7.13–7.22 (2H, m, C5′,6′–H), 7.44–7.47 (1H, m, C4′–H), 7.61–7.64 (1H, m, C7′–H), 7.66 (2H, s, NH2), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.34 (2H, d, J = 8.4 Hz, C3,5–H). Citation13C NMR δ ppm: 22.41, 26.20, 50.57, 110.82, 119.04, 122.12, 122.32, 126.72, 129.85, 136.28, 137.44, 142.71, 149.23, 161.05, 194.03. HRMS Calcd for C18H19N3O3S ([M + H]+): 358.1220, Found: 358.1218.
2-Chloro-4-[(2-isopropyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (2 h). Yield 57%, m.p. 149–151 °C. IR (ν, cm−1): 3375, 3278 (NH2), 1708 (CO). 1H NMR δ ppm: 1.29 (6H, d, J = 6.3 Hz, (CH3)2), 3.16–3.24 (1H, m, CH), 6.13 (2H, s, CH2CO), 7.10–7.26 (2H, m, C5′,6′–H), 7.48–7.50 (1H, m, C7′–H), 7.61–7.63 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.20 (2H, s, C5,6–H), 8.40 (1H, s, C3–H). Citation13C NMR δ ppm: 22.39, 26.11, 50.78, 111.00, 118.88, 122.33, 122.48, 127.81, 130.04, 131.71, 132.19, 136.08, 138.64, 142.25, 145.81, 161.03, 193.22. HRMS Calcd for C18H18ClN3O3S ([M + H]+): 392.0830, Found: 392.0827.
3-[(2-Isopropyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3 h). Yield 51%, m.p. 267–269 °C. IR (ν, cm−1): 3319 (NH2), 1706 (CO). 1H NMR δ ppm: 1.30 (6H, d, J = 6.6 Hz, (CH3)2), 3.19 (1H, septet, J = 6.9 Hz, CH), 6.12 (2H, s, CH2CO), 7.14–7.22 (2H, m, C5′,6′–H), 7.46–7.49 (1H, m, C7′–H), 7.62–7.63 (3H, m, C4′–H, NH2), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 7.8 Hz, C4–H), 8.47 (1H, d, J = 7.8 Hz, C6–H), 8.51 (1H, s, C2–H). Citation13C NMR δ ppm: 22.44, 26.19, 50.53, 110.87, 119.02, 122.13, 122.33, 125.72, 130.55, 131.32, 132.63, 135.61, 136.27, 142.67, 145.64, 161.07, 193.80. HRMS Calcd for C18H19N3O3S ([M + H]+): 358.1220, Found: 358.1221.
4-[(2-Isobutyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (1i). Yield 66%, m.p. 234–236 °C. IR (ν, cm−1): 3333 (NH2), 1703 (CO). 1H NMR δ ppm: 0.96 (6H, d, J = 6.6 Hz, (CH3)2), 2.12–2.25 (1H, m, CH), 2.68 (2H, d, J = 6.9 Hz, CH2), 6.07 (2H, s, CH2CO), 7.13–7.22 (2H, m, C5′,6′–H), 7.43–7.46 (1H, m, C4′–H), 7.61–7.64 (1H, m, C7′–H), 7.66 (2H, s, NH2), 8.07 (2H, d, J = 8.4 Hz, C2,6–H), 8.33 (2H, d, J = 8.4 Hz, C3,5–H). Citation13C NMR δ ppm: 23.08, 27.67, 35.76, 50.73, 110.82, 118.92, 122.12, 122.29, 126.72, 129.84, 136.34, 137.44, 142.77, 149.22, 155.71, 193.77. HRMS Calcd for C19H21N3O3S ([M + H]+): 372.1376, Found: 372.1377.
2-Chloro-4-[(2-isobutyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (2i). Yield 74%, m.p. 234–236 °C. IR (ν, cm−1): 3378 (NH2), 1706 (CO). 1H NMR δ ppm: 0.95 (6H, d, J = 6.3 Hz, (CH3)2), 2.12–2.24 (1H, m, CH), 2.67 (2H, d, J = 6.9 Hz, CH2), 6.10 (2H, s, CH2CO), 7.13–7.24 (2H, m, C5′,6′–H), 7.45–7.47 (1H, m, C7′–H), 7.60–7.62 (1H, m, C4′–H), 7.93 (2H, s, NH2), 8.20 (2H, s, C5,6–H), 8.39 (1H, s, C3–H). Citation13C NMR δ ppm: 23.07, 27.67, 35.66, 50.87, 110.88, 118.86, 122.17, 122.30, 127.79, 130.03, 131.69, 132.16, 136.24, 138.64, 142.67, 145.78, 155.69, 193.00. HRMS Calcd for C19H20ClN3O3S ([M + H]+): 406.0987, Found: 406.0989.
3-[(2-Isobutyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide (3i). Yield 68%, m.p. 216–218 °C. IR (ν, cm−1): 3312 (NH2), 1703 (CO). 1H NMR δ ppm: 0.96 (6H, d, J = 6.6 Hz, (CH3)2), 2.19 (1H, septet, J = 6.9 Hz, CH), 2.68 (2H, d, J = 7.2 Hz, CH2), 6.10 (2H, s, CH2CO), 7.13–7.21 (2H, m, C5′,6′–H), 7.45–7.47 (1H, m, C7′–H), 7.63–7.69 (3H, m, C4′–H, NH2), 7.89 (1H, t, J = 7.8 Hz, C5–H), 8.20 (1H, d, J = 7.5 Hz, C4–H), 8.46 (1H, d, J = 7.8 Hz, C6–H), 8.50 (1H, s, C2–H). Citation13C NMR δ ppm: 23.11, 27.65, 35.77, 50.65, 110.79, 118.98, 122.06, 122.23, 125.72, 130.56, 131.30, 132.62, 135.63, 136.40, 142.96, 145.65, 155.75, 193.56. HRMS Calcd for C19H21N3O3S ([M + H]+): 372.1376, Found: 372.1375.
4-{[2-(Methylsulfanyl)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (1j). Yield 62%, m.p. 213–215 °C. IR (ν, cm−1): 3319 (NH2), 1702 (CO). 1H NMR δ ppm: 2.72 (3H, s, CH3), 5.98 (2H, s, CH2CO), 7.15–7.23 (2H, m, C5′,6′–H), 7.50–7.52 (1H, m, C4′–H), 7.61–7.63 (1H, m, C7′–H), 7.68 (2H, s, NH2), 8.07 (2H, d, J = 8.1 Hz, C2,6–H), 8.34 (2H, d, J = 8.1 Hz, C3,5–H). Citation13C NMR δ ppm: 15.20, 50.96, 110.32, 118.26, 122.37(2C), 126.82, 129.82, 137.18, 173.72, 143.58, 149.29, 153.70, 193.05. HRMS Calcd for C16H15N3O3S2 ([M + H]+): 362.0628, Found: 362.0628.
2-Chloro-4-{[2-(methylthio)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (2j). Yield 53%, m.p. 195–197 °C. IR (ν, cm−1): 3362, 3256 (NH2), 1707 (CO). 1H NMR δ ppm: 2.72 (3H, s, CH3), 6.01 (2H, s, CH2CO), 7.14–7.23 (2H, m, C5′,6′–H), 7.48–7.51 (1H, m, C7′–H), 7.60–7.63 (1H, m, C4′–H), 7.92 (2H, s, NH2), 8.21 (2H, s, C5,6–H), 8.39 (1H, s, C3–H). Citation13C NMR δ ppm: 15.22, 51.09, 110.34, 118.26, 122.34, 122.38, 127.80, 130.17, 131.86, 132.04, 137.64, 138.43, 143.56, 145.91, 153.72, 192.37. HRMS Calcd for C16H14ClN3O3S2 ([M + H]+): 396.0238, Found: 396.0241.
3-{[2-(Methylthio)-1H-benzimidazol-1-yl]acetyl}benzenesulfonamide (3j). Yield 54%, m.p. 242–244 °C. IR (ν, cm−1): 3288 (NH2), 1706 (CO). 1H NMR δ ppm: 2.72 (3H, s, CH3), 6.99 (2H, s, CH2CO), 7.15–7.23 (2H, m, C5′,6′–H), 7.50–7.41(1H, m, C7′–H), 7.60–7.63 (3H, m, C4′–H, NH2), 7.88 (1H, t, J = 7.5 Hz, C5–H), 8.19 (1H, d, J = 7.5 Hz, C4–H), 8.45 (1H, d, J = 7.8 Hz, C6–H), 8.50 (1H, s, C2–H). Citation13C NMR δ ppm: 15.24, 50.90, 110.36, 118.28, 122.28, 122.37, 125.69, 130.69, 131.43, 132.47, 135.45, 137.76, 143.62, 145.72, 153.65, 192.83. HRMS Calcd for C16H15N3O3S2 ([M + H]+): 362.0628, Found: 362.0627.
Protein preparation
Expression and purification of CA I, II, VII, XII and XIII has been previously described: CA I by Baranauskiene et al.Citation28, CA II by Cimmperman et al.Citation29, CA VII and XIII by Sudzius et al.Citation21 and CA XII by Jogaite et al.Citation30.
Determination of compound binding to CAs by thermal shift assay
The thermal shift assay (TSA) measurements were performed in a Corbett Rotor-Gene 6000 (QIAGEN Rotor-Gene Q, Sydney, Australia) instrument using the blue channel (excitation 365 ± 20, detection 460 ± 15 nm). Samples contained 10 µM protein, 0–200 µM compound, 50 µM solvatochromic dye ANS (8-anilino-1-naphthalene sulfonate) and 50 mM phosphate buffer containing 100 mM NaCl at pH 7.0, with the final DMSO concentration at 2%. The applied heating rate was 1 °C/min. Data analysis was performed as previously describedCitation31.
Results
A series of benzenesulfonamides with a benzimidazole moiety 1–4(a–j) were designed as CA inhibitors (). The procedures for N-alkylation of 2-substituted benzimidazoles (a–j) with 5-(bromoacetyl)-2-chlorobenzenesulfonamide (4) have been described previouslyCitation23. N-substituted benzimidazole derivatives 1–3(a–j) were prepared by N-alkylation of benzimidazoles a–j with 4-(bromoacetyl)benzene-sulfonamide (1), 4-(bromoacetyl)-2-chlorobenzenesulfonamide (2) and 3-(bromoacetyl)benzenesulfonamide (3) ().
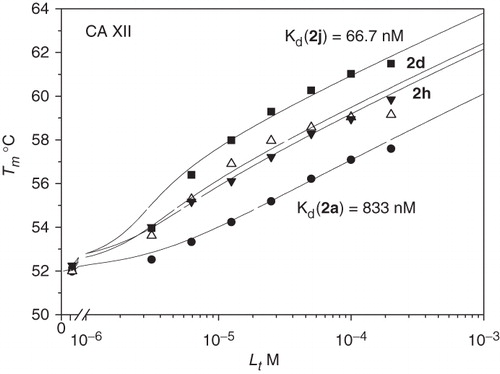
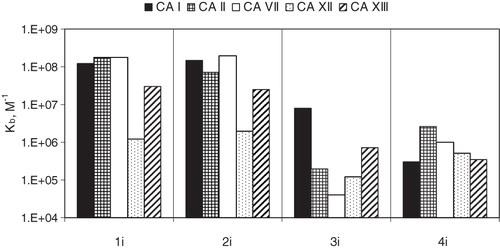
The dissociation constants for compounds 1–4(a–j) binding to five CA isozymes CA I, II, VII, XII and XIII are listed in . AZM and EZA were used as controls. The binding of these compounds was determined by the TSA. and show representative binding data obtained by TSA. The data in and show that the position of the sulfonamide group on the benzene ring significantly influences the binding strength to the various CA isoforms. In most cases, the para-substituted benzenesulfonamide (compounds 1–2(a–j)) had higher binding affinity than its meta-substituted benzenesulfonamide equivalent (compounds 3–4(a–j)) (). All 1–2(a–j) derivatives were nanomolar inhibitors of CA I, II, VII and XIII (dissociation constants (Kds) in the range of 1.6–286 nM). The binding of 1–2(a–j) to CA XII was weaker (Kd ranged from 62.5 nM to 8.3 µM). Most compounds of the 3–4(a–j) series were micromolar inhibitors of all investigated CA isoforms (Kds in the range of 0.1–100 µM). Compounds bearing a chlorine atom in the ortho position of the benzenesulfonamide ring were in most cases more effective CA inhibitors than compounds without a chlorine substitution in this position: (2(a–j) versus 1(a–j) and 4(a–j) versus 3(a–j)). For example, compound 2g exhibited 25 times greater affinity than 1g to CA XIII whereas compound 4h was 34 times more potent than 3h for CA VII. In contrast, CA I bound 4(a–j) weaker than 3(a–j). The highest difference was observed between compounds 4i and 3i, with the latter being 27 times stronger CA I inhibitor than 4i.
Figure 3. TSA data of 1–4d binding to CA XIII. The top panels compare denaturation curves observed by fluorescence at 0–200 µM added compound concentrations. The bottom panel shows the dependence of the protein melting temperatures Tm on compound concentrations.
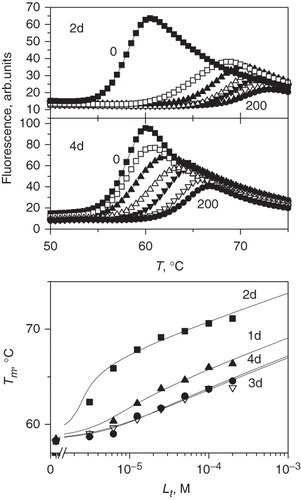
Table 1. Dissociation constants for compounds binding to five human recombinant CA isoforms, determined by TSA (37 °C, pH 7.0).
The binding of most benzenesulfonamides of types 1–4(a–j) to CA I, II, VII, XII and XIII did not strongly depend on the substituent (R3) on the benzimidazole ring. By comparing the series of compounds bearing methyl 1–4b, hydroxymethyl 1–4c, ethyl 1–4e, propyl 1–4f and butyl 1–4g substituents, we showed that the tail length did not affect the binding affinity by more than two times to any of the tested CAs. For example, the Kds for 1b, c and e–j for CA I varied only in the narrow nanomolar range (from 3.5 to 11.1 nM). The Kds for CA I were higher for compounds 1a and 1d, 33 and 143 nM, respectively. CA II was best inhibited (Kd = 4–100 nM), while CA XII (Kd = 0.48–8.33 μM) least inhibited. Compounds bearing iso-propyl 1 h and S-methyl 1j substitution on benzimidazole ring were good inhibitors of CA I and CA II (Kds were about 4–5 nM), whereas iso-butyl-substituted compound 1i potently bound CA VII (Kd = 5.56 nM). Benzyl-substituted 1d had the weakest binding affinity, especially to CA XII (Kd = 8.33 μM), but it was selective for CA VII (Kd = 20 nM).
N-alkylated benzimidazoles (2(a–j)) were the best inhibitors of CA I, II, VII and XIII exhibiting affinities in the nanomolar range. These compounds were weaker CA XII inhibitors by about one order of magnitude. Compounds with methyl, ethyl, propyl and butyl substituents had a high affinity for CA I (Kd ranged from 2.9 to 5.9 nM), CAII (Kd from 10.5 to 2.70 nM), CA VII (Kd from 11.1 to 3.33 nM) and CA XIII (Kd from 23.3 to 2.50 nM). Benzimidazoles that contained a branched chain aliphatic tail (compounds 2 h, 2i) had four to eight times reduced affinity for CA II (Kds were 14.3 nM for both compounds), CA XII (Kds were 333 and 500 nM, respectively) and CA XIII (Kds were 40 nM for both compounds), as compared to compounds 2f and 2g which contained an unbranched tail. Benzimidazole with an S-methyl substituent, 2j, bound to CA isoforms similarly as the propyl-substituted compound, 2f.
3(a–j) N-benzimidazoles were the weakest CA inhibitors (Kds in the range of 0.67–100 µM). Only three of them (3g, i, j) had a higher affinity for CA I and XIII, than for the other tested CAs. For example, 3i binds to CA I 40 times stronger than CA II, 200 times stronger than CA VII, 66 times stronger than CA XII and 10 times stronger than CA XIII.
4(a–j) N-substituted benzimidazoles (Kds in the range of 0.33–11.1 μM) were slightly better inhibitors than the 3(a–j) compounds. They behaved as weak inhibitors against CA I (Kds are in the micromolar range 3.3–11 μM). Six derivatives, namely 4a–c, e, g and h, were better inhibitors of CA XIII, with Kds in the range of 0.33–0.67 μM. Compound 4b, which is structurally the most similar to the clinically used indapamide, was slightly less potent. The binding affinity of 4b and indapamide to CA I was similar, but 4b bound about seven times weaker to CA II and CA VII and four times weaker to CA XII and CA XIII than indapamide.
Discussion
Indapamide, which is a clinically used diuretic, may act as an inhibitor of various CA isozymes present in the kidneys and blood vessels, thus lowering the blood pressure in patients with hypertension and type-2 diabetesCitation32. It has been reported previously by Supuran’s group that the inhibition constants (Kis) of indapamide for several CAs were: 51 900 nM for hCA I, 2520 nM for hCA II, 0.23 nM for hCA VII, 10 nM for CA XII and 13 nM for murine CA XIIICitation19. The TSA data confirmed that indapamide was a weak inhibitor of the isoform CA I (Kd = 10 000 nM). However, the Kd values toward other CAs differed from Ki values (Kds for CA II and VII were 300 nM, CA XII = 1400 nM and CA XIII = 100 nM). These discrepancies could be observed due to the different techniques utilized for compound binding and inhibition measurements. Usually, the TSA and other biophysical binding techniques, such as isothermal titration calorimetry, yield dissociation constants that are close to the inhibition constants determined by the stop-flow carbon dioxide hydration assayCitation28,Citation33.
Numerous indapamide analogues were designed in search of new, more potent and selective CA inhibitors. A diminished affinity of derivatives of both series 3(a–j) and 4(a–j), which are most structurally related to indapamide when compared with the corresponding inhibitors 1(a–j) and 2(a–j), towards isozyme I, II, VII, XII and XIII was observed. This is thought to occur due to the benzimidazole group in the meta position in relation to the benzenesulfonamide moiety, which is present in the first series of compounds. This could lead to steric hindrance when binding to the Zn ion within the enzyme active site.
It was interesting to notice that the chlorine atom in the ortho position of the sulfonamide group of the compounds 2(a–j) and 4(a–j) increased the affinity to all CAs, with the exception of CA I. All compounds of the series 4(a–j) with a chlorine substitution in the ortho position possessed a lower binding potency with CA I than compounds 3(a–j) without a chlorine substitution. Compound 3i, bearing a bulky iso-butyl substituent in the benzimidazole moiety, behaved as a selective CA I inhibitor, having more than 10 times higher affinity against CA I isozyme as compared to other CAs.
Conclusions
Benzenesulfonamides bearing benzimidazole substituents were synthesized and assayed as inhibitors of five CA isozymes, CA I, II, VII, XII and XIII. 4-[(2-Substituted-1H-benzimidazol-1-yl)acetyl]benzenesulfonamides 1(a–j) were more than two orders of magnitude stronger CA inhibitors than 3-[(2-substituted-1H-benzimidazol-1-yl)acetyl]benzenesulfonamides 3(a–j). Compounds of the series 1(a–j) and 2(a–j) were nanomolar inhibitors of CA I, II, VII and XIII (Kds in the range of 1.6–286 nM).
Declaration of interest
The authors report no conflicts of interest.
This research was funded by a grant (No. LIG-09/2012) from the Research Council of Lithuania. The authors also acknowledge FP7-REGPOT-2009-1 grant “MoBiLi”, agreement No.: 245721, and the COST projects TD0905 and CM0804. This work was partly supported by the Lithuanian Science Council Student Research Fellowship Award (No. SMT12P-079, student – MK).
References
- Alterio V, Fiore AD, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68
- Aggarwal M, Kondeti B, McKenna R. Insights towards sulfonamide drug specificity in α-carbonic anhydrases. Bioorg Med Chem 2012; doi: 10.1016/j.bmc.2012.08.019
- Supuran CT. Carbonic anhydrases as drug targets – an overview. Curr Top Med Chem 2007;7:825–33
- Supuran CT. Carbonic anhydrases – an overview. Curr Pharm Des 2008;14:603–14
- Nordfors K, Haapasalo J, Korja M, et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer 2010;10:148–58
- Chien MH, Ying TH, Hsieh YH, et al. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol 2012;48:417–23
- Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 2010;29:6509–21
- Said HM, Supuran CT, Hageman C, et al. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des 2010;16:3288–99
- Pastorekova S, Parkkila S, Zavada J. Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 2006;42:167–216
- Winum JY, Carta F, Ward C, et al. Ureido-substituted sulfamates show potent carbonic anhydrase IX inhibitory and antiproliferative activities against breast cancer cell lines. Bioorg Med Chem Lett 2012;22:4681–85
- Rodriguez OM, Maresca A, Tempera CA, et al. N-β-glycosyl sulfamides are selective inhibitors of the cancer associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem Lett 2011;21:4447–50
- Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem 2011;54:8271–7
- Supuran CT. Carbonic anhydrase inhibition with natural products: novel chemotypes and inhibition mechanisms. Mol Divers 2011;15:305–16
- Supuran CT, Scozzafava A, Conway J. Carbonic anhydrase – its inhibitors and activators. Boca Raton, FL: CRC Press; 2004:1–363
- Supuran CT, Innocenti A, Mastrolorenzo A, Scozzafava A. Antiviral sulfonamide derivatives. Mini Rev Med Chem 2004;4:189–200
- Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008–2012). Expert Opin Ther Pat 2012; 22:747–58
- Carta F, Supuran CT, Scozzafava A. Novel therapies for glaucoma: a patent review 2007–2011. Expert Opin Ther Pat 2012; 22:79–88
- Thiry A, Dogne JM, Supuran CT, Masereel B. Anticonvulsant sulfonamides/sulfamates/sulfamides with carbonic anhydrase inhibitory activity: drug design and mechanism of action. Curr Pharm Des 2008;14:661–71
- Temperini C, Cecchi A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. interaction of indapamide and related diuretics with 12 mammalian isozymes and X-ray crystallographic studies for the indapamide-isozyme II adduct. Bioorg Med Chem Lett 2008;18:2567–73
- Capkauskaite E, Zubriene A, Baranauskiene L, et al. Design of [(2-pyrimidinylthio)acetyl]benzenesulfonamides as inhibitors of human carbonic anhydrases. Eur J Med Chem 2012;51:259–70
- Sudzius J, Golovenko D, Matuliene J, et al. 4-[n-(substituted 4-pyrimidinyl)amino]benzenesulfonamides as inhibitors of carbonic anhydrase isozymes I, II, VII and XIII. Bioorg Med Chem 2010;18:7413–21
- Dudutiene V, Baranauskiene L, Matulis D. Benzimidazo[1,2-c][1,2,3]thiadiazole-7-sulfonamides as inhibitors of carbonic anhydrase. Bioorg Med Chem Lett 2007;17:3335–8
- Capkauskaite E, Baranauskiene L, Golovenko D, et al. Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem 2010;18:7357–64
- Fujikura T, Miigata K, Hashimoto S, et al. Studies on benzenesulfonamide derivatives with alpha- and beta-adrenergic antagonistic and antihypertensive activities. Chem Pharm Bull (Tokyo) 1982;30:4092–101
- Zanka A, Kubota A. Practical and efficient chlorination of deactivated anilines and anilides with ncs in 2-propanol. Synlett 1999;12:1984–6
- Nickson TE, Roche-Dolson CA. A convenient procedure for the chlorination of deactivated anilines. Synthesis 1985;6/7:669–70
- Phillips MA. The formation of 2-substituted benzimidazoles. J Chem Soc 1928;CCCXVII:2393–9
- Baranauskiene L, Hilvo M, Matuliene J, et al. Inhibition and binding studies of carbonic anhydrase isozymes I, II and IX with benzimidazo[1,2-c][1,2,3]thiadiazole-7-sulphonamides. J Enzyme Inhib Med Chem 2010;25:863–70
- Cimmperman P, Baranauskiene L, Jachimoviciute S, et al. A quantitative model of thermal stabilization and destabilization of proteins by ligands. Biophys J 2008;95:3222–31
- Jogaite V, Zubriene A, Michailoviene V, et al. Characterization of human carbonic anhydrase XII stability and inhibitor binding. Bioorg Med Chem 2012; doi: 10.1016/j.bmc.2012.10.016
- Kazlauskas E, Petrikaite V, Michailoviene V, et al. Thermodynamics of aryl-dihydroxyphenyl-thiadiazole binding to human Hsp90. PLoS ONE 2012;7:e36899
- Supuran CT. Diuretics: from classical carbonic anhydrase inhibitors to novel applications of the sulfonamides. Curr Pharm Des 2008;14:641–8
- Zubriene A, Kazlauskas E, Baranauskiene L, et al. Isothermal titration calorimetry and thermal shift assay in drug design. European pharmaceutical review 2011;16:56–9