Abstract
Context: Lipoic acid (LA) and hyperbaric oxygenation therapy (HBOT) improve chronic wound healing.
Objective: We compared the effects of LA or its enantiomer R-(+)-lipoic acid (RLA) on wound healing.
Materials and methods: Groups LA + HBOT (L), RLA + HBOT (R) and placebo + HBOT (P). Lesion areas measured before treatment and on 20th and 40th day. The biopsies and plasma were harvested before treatment and on 7th and 14th (measurements of VEGF, vascular endothelial growth factor; EGF, epidermal growth factor, TNF-α and IL-6).
Results: Ulcers improved more on RLA. In both L and R groups, EGF and VEFG increased in time. RLA decreased IL-6 on T7 and T14, which did not happen with LA. TNF-α levels decreased on T14 in both LA and RLA.
Discussion: The improved wound healing is associated with increased EGF and VEGF and reduced plasma TNF-α and IL-6.
Conclusion: RLA may be more effective than LA in improving chronic wound healing in patients undergoing HBO therapy.
Introduction
Chronic leg wounds are generally caused by venous and/or arterial insufficiency, diabetes mellitus or pressure ulcers. Chronic wounds are not only a potentially disabling disorder that affects daily life of millions of Americans, but also cost the US health system over $25 billion dollars per yearCitation1. In addition to traditional treatment with wound dressing changes and topical use of antimicrobials, hyperbaric oxygenation therapy (HBOT) has been observed to promote wound healingCitation2–4 and documented by The Undersea and Hyperbaric Medicine Society to be an effective adjunctive therapyCitation5. However, it is known that exposure to oxygen at higher than atmospheric pressure leads to increased reactive oxygen species (ROS) formation, which is in direct proportion to the increased oxygen tensionCitation6. An overly produced ROS is detrimental because it significantly damages cell structures such as lipids, proteins and nucleic acids resulting in relevant alteration of health statusCitation7. It is a major concern to reduce or prevent oxidative damage in the treatment of chronic wounds since multiple HBOTs are usually required. Antioxidant supplementation has been advocated to be used during HBOT course to reduce potential oxidative damage. Lipoic acid (LA), a disulfide compound, is found naturally in mitochondria as the coenzyme for pyruvate dehydrogenase and a-ketoglutarate dehydrogenaseCitation8. Exogenous supplementation with LA has been reported to increase unbound LA levels, which act as a potent antioxidant to reduce oxidative stress both in vitro and in vivoCitation9. LA is both water and fat soluble, crosses biological membranes easily, thus reaching all the compartments of the cell and making it highly effective in reducing ROS including lipid peroxides in cellular membranes, as well as scavenging ROS at their mitochondrial sourceCitation10. The beneficial effect of LA supplementation in patients with chronic leg wounds undergoing HBOT has been reportedCitation11,Citation12. The beneficial effect of LA was believed due to its antioxidant activity either by directly interacting with ROS, thereby counteracting lipid and DNA oxidation induced by oxygen exposure, or by recycling vitamin E, thus enhancing the total antioxidant status of the plasma. The beneficial effect of LA may also due to its inhibitory effect on pro-inflammatory cytokineCitation8,Citation9. Enantiomer R-(+)-lipoic acid (RLA) is the form biosynthesized in humans which is essential for aerobic metabolism. RLA is the nutritionally and therapeutically preferred form due to its “vitamin-like” role in metabolismCitation13. RLA has been suggested to be not only as an in vivo ROS scavenger, but also an inducer of the oxidative stress responseCitation14.
Wound healing is a complex process that includes a series of overlapping phases including inflammation, epithelialization, angiogenesis and matrix depositionCitation15. An interaction may exist between oxidative stress and cytokine activity in wound healing processCitation16,Citation17. IL-6, a pleiotropic cytokine, has been hypothesized to play an essential role in modulating immune responses so as to promote collagen deposition and angiogenesisCitation18. TNF-α has been known to exert multiple regulatory effects on cell proliferation and differentiationCitation19. The vascular endothelial growth factor (VEGF) stimulates the formation of new blood vessels (angiogenesis). VEGF also acts as a mitogen for vascular endothelial cells, stimulating these cells to divide and multiply themselvesCitation15. Epidermal growth factor (EGF) is a growth factor that stimulates cell growth, proliferation and differentiation by binding to its receptor. Topical use of EGF has been suggested to accelerate the rate of healing of chronic wounds in humansCitation20,Citation21. Enantiomer RLA and S-(−)-LA (SLA) constitute the racemic mixture LA. Only the RLA exists in nature and is an essential cofactor of four mitochondrial enzyme complexesCitation22. RLA has been suggested to be nutritionally and therapeutically preferred form as antioxidantCitation13. Few studies compare individual enantiomers (RLA) with racemic LA. Several studies have demonstrated that LA either has lower activity than RLA or interferes with the specific effects of RLA by competitive inhibitionCitation23–25. We hypothesized that supplementation of RLA may be more effective than LA to HBOT in the treatment of chronic leg ulcer. Some literature reports indicate that RLA may act inducing genes expressions codifying proteases synthesis which are known to exert beneficial effects on cutaneous wounds healing. These effects are induced because of a direct action on growth factors and cytokines activityCitation8,Citation9. This randomized, placebo-controlled, double-blind study was carried out to compare the effects of RLA and LA on chronic wound healing in patients undergoing HBOT, by means of measuring improvement of ulcer sizes as well as changes of growth factor and inflammatory cytokines in biopsy specimens and blood plasma, which play the pivotal roles in the healing of chronic wound.
Materials and methods
This study was approved by the Institutional Review Board. A total of 27 patients (12 male and 15 female, mean ages of 66 (45–88) years old) were enrolled at the Vulnology and Diabetic Foot Unit, Peschiera, Italy. The inclusion criteria included: non-smokers (or those having quitted from smoking for more than one year), skin ulcer history more than 30 d, diabetic foot less than grade 4 according to WagnerCitation26, ankle blood pressure greater than 50 mmHg, a peri-lesional basal transcutaneous oximetry reading greater than 20 mmHg for diabetic ulcer or greater than 10 mmHg for vascular insufficiency ulcer. The patients characteristics and clinical data are listed in .
Table 1. Clinical data of groups R, L and P subjects undergoing HBO therapy.
The patients were randomly divided into three groups:
Group L: A total of 10 patients (four male and six female with a mean age of 59 (45–83) years old). There were total 13 ulcerated lesions: five caused by arterial impairment, four caused by venous insufficiency, three were diabetic/ischemic origin and one caused by trauma. The average ulcer size was 3.92 cm2 with a mean history of 217 d. The patients were treated with LA (600 Byodinoral-R, MDM, Milano, Italy), 600 mg orally 60 min before each session of HBOT.
Group R: A total of 10 patients (five male and five female with a mean age of 71.8 (58–82) years old). There were total 10 ulcerated lesions: five caused by arterial impairment, four were diabetic/ischemic origin and one with diabetic neuropathy. The average ulcer size was 7.45 cm2 with a mean history of 233 d. These patients were treated with RLA (Destior-R, MDM, Milano, Italy), 600 mg orally 60 min before each session of HBOT.
Group P: Seven patients (three male and four female with a mean age of 72.1 (49–88) years old). There were total seven ulcerated lesions: four were diabetic/ischemic origin, two caused by arterial impairment and one from venous insufficiency. The average ulcer size was 3.18 cm2 with a mean history 224 d. These patients were treated with placebo 60 min before each session of HBOT.
Hyperbaric oxygen therapy protocol
All patients were evaluated by past medical history and physical examination, chest X-ray, EKG and otoscopic examination to ascertain the safety for HBOT. HBOT was conducted at the Istituto Iperbarico SpA, the Diving and Hyperbaric Medicine Unit in Villafranca, Italy.
HBOT was administered once a day (110 min), 5 d a week for total 40 sessions. HBOT included three 24 min sessions with 100% O2 at 2.5 ATA and two interposed pauses of 5 min in air. The dive profile included two extra breathing periods of 100% O2 (always through an oral-facial mask): one during compression, from 1.6 ATA up to the final pressure of the treatment (2.5 ATA) and another one during decompression from the bottom depth (2.5 ATA) until reaching 1.3 ATA. The total times per dive was: 90 min of O2 with mask, 72 min at 2.5 ATA (usually took 12–15 min to reach this level) and 18 min for surfacing, including a further decompression stop of 3 min at 1.3 ATA.
Clinical evaluation
The skin lesions were clinically treated according to Schultz et al.Citation27 Clinical assessment of the wound conditions was conducted before treatment (T0), day 20 (T20) and day 40 (T40).
The samples of blood and tissue biopsy for cytokine and growth factors assay were taken before treatment (T0), day 7 (T7) and day14 (T14).
Preparation of blood and tissue samples
Venous blood was collected in heparinized tubes and centrifuged at 1000g for 15 min. The obtained plasma was stored at −80 °C until assay. A 5 mm diameter biopsy was taken from the center of the wound. The biopsy sample was immediately frozen at −20 °C. Frozen biopsies were homogenized in the lysis buffer (50 mM Tris–HCl, 1% Triton X-100, pH 7.4) and centrifuged at 12 000g for 5 min to remove particulate matter. The supernatant was assessed for protein content using the Bradford method (Sigma, St Louis, CA) and stored at −80 °C until assay.
Angiogenesis protein arrays
Protein arrays for human angiogenesis (EGF, VEGF) were used to analyze the biopsy lysates. IL-6 and TNF-α were assessed with ELISA kit (SearchLight, Pierce Biotechnology, Rockford, IL) in plasma, according to the manufacturer’s instructions. Results are expressed as ng/mg protein for biopsy and as ng/mL for plasma samples.
Statistical analysis
The Kruskal–Wallis and Mann–Whitney non-parametric statistical tests (the median [quartiles]) were employed to assess the significance of the differences in the concentration and activity of angiogenic/inflammatory factor levels between the time points and between the groups. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows version 11.0 (SPSS, Chicago, IL).
Results
Changes of ulcer sizes
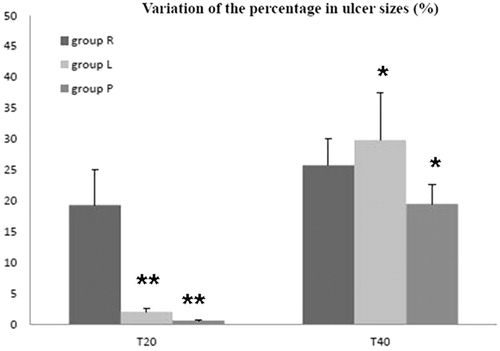
As shown in and , there was no significant reduction in ulcer lesion in placebo group after 20 d. Supplementation of RLA to HBOT significantly improved the wound healing. The reduction of ulcer lesion became significant on day 20, much earlier than with supplementation of LA to HBOT. In fact, in the latter group, the reduction of ulcer lesion became significant on day 40 only, similarly to what observed in group P.
Figure 1. The comparison of ulcer sizes at three time-points in each group including RLA + HBO treated (group R), LA + HBO treated (group L) and placebo + HBO treated (group P). The ulcer sizes were determined before (T0), after 20(T20) and 40(T40) days from starting the HBO therapy. The results were expressed as cm2 and data represent mean ± SD. As shown in this figure, the ulcer sizes became smaller in group R at both T20 and T40 significantly compared with its basal value T0. Simultaneously, only the results of T40 in groups L and P expressed improvement in ulcer size. *p < 0.05. Group R: n = 10; group L: n = 10; and group P: n = 6.
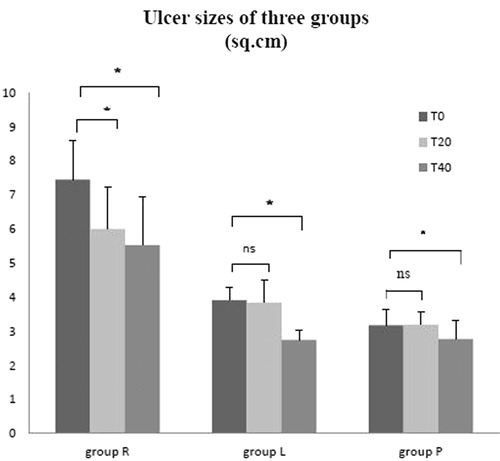
Figure 2. The comparison of ulcer sizes among the three groups at T20 and T40, respectively. The results were expressed as variation of the percentage in ulcer sizes and related to the basal value (T0), whereas the data represent mean ± SD. At T20, variation of the percentage of ulcer size in group R was significantly higher than that in groups L and P. Concurrently, the value in group L made no difference referred to group P. At T40, although there was no difference between groups R and L, both of them showed more effective in decreasing the areas of ulcer than group P. *p < 0.05 and **p < 0.01. Group R: n = 10; group L: n = 10; and group P: n = 6. T20 and T40 means after 20 and 40 d from starting the HBO therapy, respectively.
Changes of EGF, VEGF, IL-6 and TNF-α
The changes of EGF, VEGF, IL-6 and TNF-α during HBOT in the three groups were summarized in .
Table 2. Mean values (+/− SD) of IL-6, TNF-α, EGF and VEFG in experimental groups at different experimental times.
As showed, there was no significant change in EGF levels in placebo and HBOT patients. Supplementation of either LA or RLA to HBOT significantly increased EGF levels as detected on days 7 and 14.
As also showed, increased VEGF levels were noticed on supplementation of either LA or RLA on day 7, while the same did not happen in group P.
There was no significant changes in IL-6 values in group P patients, while supplementation of RLA to HBOT significantly decreased IL-6 values on days 7 and 14. The same was not observed for group L. The level of IL-6 measured on day 7 in group R resulted significantly lower with respect to group L. TNF-α values also did not change in group P, while decreased values were observed in groups R and L on day 14.
Discussion
The main findings in this study are that HBOT, supplemented with either LA or RLA had a better healing effect than HBOT alone in the treatment of chronic leg wound. Furthermore, RLA was more effective than racemic LA as supplemental agents to HBOT. This study also showed that the better wound healing effect was associated with increased EGF and VEGF beta production in the wound tissue and decreased TNF-α and IL-6 levels in the plasma.
Beneficial effects of HBOT on the stimulation of neovascularization have been reported in flaps, wounds, irradiated tissues and graftsCitation28. The beneficial effect of HBOT has been reviewed recentlyCitation29. However, potential oxidative damage to the body during HBOT has been a concern. Therefore, an antioxidant agent has been advised to be supplemented during HBOT. Alleva et al. evaluated the effects of LA in patients with chronic wounds undergoing HBOT. Their study demonstrated a synergistically beneficial effect of HBOT with LA supplementation in the treatment of chronic leg woundCitation12,Citation13. The data from this study are in agreement with their findings that HBOT in supplementation with LA significantly improved chronic wound healing. Our study further suggests that RLA may have better effect than LA in enhancing wound healing during HBOT.
LA and its reduced form, dihydrolipoic acid, are known for their biological antioxidant activity. LA acts as a scavenger of ROS and interacts with other antioxidants such as ascorbate, vitamin E and glutathione, contributing to their regenerationCitation11,Citation30,Citation31. Alleva et al.Citation12,Citation13 have reported its adjuvant effect in HBOT used for impaired wound healing treatment. Their data demonstrated that the supplementation of LA efficiently inhibited both DNA and lipid oxidation. Enantiomer RLA and SLA constitute the racemic mixture LA. Only RLA exists in nature and is an essential cofactor of four mitochondrial enzyme complexesCitation22. RLA has been suggested to be the eutomer – the nutritionally and therapeutically preferred formCitation13. Few studies compared individual enantiomers with racemic LA regarding chronic wound healing in vivo or in vitro. In this study, the improvement of ulcers size in groups L and P did not appreciated until T40, whereas the improvement in group R was achieved much earlier on T20. In our study, the beneficial effect of oral 600 mg RLA seems better than the same dosage of LA. There are several possible explanations for the better effect of RLA relative to LA. RLA is the naturally occurring enantiomer of LA in the body, a selective transport process of RLA may already exist in the cells. Uptake, distribution and action of RLA are more efficient. On contrary, as an unnatural mixture, LA may have to experience a complicated process to be active, including uptake, distribution and action. SLA was reported could interfere with the specific of RLA by competitive inhibitionCitation23, which would prevent LA from acting effectively. This study provides a new evidence that RLA is more effective than LA when supplemented to HBOT in chronic leg wound healing. Wound healing is a complex process. On the molecular level, the process of endothelial and vascular smooth muscle cells migration, proliferation and circulating angiogenic cells mobilization to the periphery is regulated by many factors including growth factors and cytokinesCitation30–32. When a cell is deficient in oxygen, it produces hypoxia-inducible factor (HIF), a transcription factor. HIF then stimulates the release of VEGF. Circulating VEGF then binds to VEGF receptors on endothelial cells, triggering a Tyrosine Kinase Pathway leading to angiogenesis. VEGF induces endothelial cell migration in wound healing through two primary mechanisms, chemotaxis and vasodilatation. In the initial phase of angiogenesis, endothelial cells migrate before mitotic divisionCitation33. It is unclear which molecules transduce the mitogenic signal, but VEGF induces endothelial cells grown on the surface of a collagen matrix to invade the underlying matrixCitation30, and stimulates their proliferative response. Furthermore, VEGF delays senescence and restores proliferative capacity to endothelial cells. It lengthens the life span of endothelial cells and prevents apoptosis by inducing the transient expression of two anti-apoptotic proteins in human endothelial cellsCitation29. These proteins may be responsible for VEGF’s prevention of apoptosis, induced by TNF-α in endothelial cells and by ionizing radiation in hematopoietic stem cellsCitation30. Controversy exists regarding the effect of HBOT on VEGF production. HBOT has been observed to up-regulate VEGF expression in an animal wound models and might be responsible for its beneficial effects in wound healingCitation33. HBOT preconditioning increased myocardial capillary density and the level of VEGF expression has also been observedCitation34. On the other hand, other wound models have demonstrated decreased VEGF expression and angiogenesis by short-term HBO treatmentCitation35. Alleva et al.Citation12,Citation13 have reported that HBOT increased local tissue VEGF gene expression and protein production in chronic leg wound patients. In this study, HBOT alone gradually and significantly increased VEGF production in the wound tissue, that is in agreement with their reports. The reason of different response of VEGF to HBOT in wound healing is unknown, as pointed out by Kalns et al.Citation35 that it is hard to interpret HBO’s role in angiogenic response currently. Our data suggest that increased VEGF production may be one factor contributing to the beneficial effect of HBOT in enhancing wound healing. Our data also suggest that VEGF production may not be just depend on IHF stimulation since HBOT significantly increased local tissue oxygen content. In this study, we noticed that when LA or RLA was supplemented to HBOT, VEGF production was significantly decreased in comparison to baseline and HBOT alone. In spite of decreased VEGF production, the wound achieved better healing when LA or RLA was added to the HBOT. Our results are in agreement with Alleva et al.’s study that HBOT alone increased VEGF gene expression and protein production in chronic wound patientsCitation9. LA supplementation inhibited VEGF production. The reason for this is not fully understood. As Alleva et al. suggested that as LA promotes wound healing, it would be expected even to increase angiogenesis by the expression of growth factors. However, these were actually down-regulated, suggesting that the alteration of the protease and inflammatory cytokine expression has a greater effect on the healing process.
Wound healing contains an ordered sequence of events including cell migration, proliferation and synthesis of extracellular matrix, angiogenesis and remodeling. EGF orchestrates the recruitment and growth of fibroblasts and epithelial cells in the evolution of granulation tissue. EGF enhances epidermal regeneration and tensile strength in experimental models of chronic woundsCitation30. An early study showed that topical application of EGF achieved a greater reduction in ulcer size and a larger number of healed ulcersCitation36. There is no report on the effect of HBOT on EGF in wound healing. Our study showed that HBOT alone has no significant effect on EGF production in chronic leg wound patients. However, EGF production was significantly increased when either LA or RLA was added to HBOT. This increased EGF production is associated with enhanced wound healing. Our study suggests that increased EGF production may be responsible to the beneficial effect of HBOT in combination with LA or RLA. TNF and IL-6 are key mediators of the inflammatory process, and also contribute to the reparative phase directly as well as indirectly (by inducing other cytokines and growth factors) affecting endothelial and fibroblast functionsCitation36. IL-6 was also shown to be essential for epithelialization and influence the formation of granulation tissue, as demonstrated in studies of wound healing in mice null for the IL-6 geneCitation30. As the repair process proceeds, fibroblasts display increased levels of molecular adhesion expression and do assume a myofibroblast phenotype, mediated in part by beta-TGF and PDGF-A and B, to facilitate wound contractionCitation33. Genes coding for growth factors, cytokines, cell-matrix adhesion molecules and proteases were expressed highly in the chronic wounds as mentioned by Alleva et al.Citation12,Citation13 Oxygenation induced overexpression of almost all of these genes at the first week of HBOT treatment. At prolonged oxygen exposure, some of them were repressed. High levels of chemokines and cytokines were observed in biopsies collected from chronic ulcers, and HBOT enhanced their expression. LA supplementation markedly repressed expression of inflammatory genes. Down regulation of IL-6 mRNA, observed at day 14 of LA supplementation, was associated with reduced plasma levels of the IL-6 protein. Our study is in agreement with their study showing that plasma levels of IL-6 increased following the HBOT therapy in patients receiving placebo, most likely due to inflammatory events, while LA inhibited IL-6 expression as well TNF-α production. In particular, the transition from chronic to acute or fully restored functional connective tissue is affected by these factors, and this represents a major interest in the treatment of chronic ulcerationCitation37–40.
Conclusions
Our results show that that LA and RLA in supplementation to the HBO promote the chronic wound healing. ALA R+ enantiomer seems more effective than the racemic compound. The improved wound healing is associated with increased local tissue EGF and VEGF production and reduced plasma TNF-α and IL-6. In addition to increased local oxygen availability, the modified growth factors and cytokines may be responsible for the beneficial effect of HBOT.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
We thank MDM, Milano, Italy for the generous gift of α-Lipoic acid capsules.
References
- Centers for Medicare and Medicaid Services. Guidance to surveyors for long term care facilities, 2012. Available from: http://www.cms.hhs.gov [last accessed 17 Jan 2013]
- Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010;33:998–1003
- Londahl M, Landin-Olsson M, Katzman P. Hyperbaric oxygen therapy improves health-related quality of life in patients with diabetes and chronic foot ulcer. Diabet Med 2011;28:186–90
- Hunter S, Langemo DK, Anderson J, et al. Hyperbaric oxygen therapy for chronic wounds. Adv Skin Wound Care 2010;23:116–9
- Gesell LB. Hyperbaric oxygen therapy indications. Committee Report. 12th ed. Durham, NC: Undersea and Hyperbaric Medical Society; 2008
- Narkowicz CH, Vial JH, McCartney P. Hyperbaric oxygen therapy increases free radical levels in the blood of humans. Free Radic Res Commun 1993;19:71–80
- Wells PG, McCallum GP, Chen CS, et al. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci 2009;108:4–18
- Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 1995;19:227–50
- Bustamante J, Lodge JK, Marcocci L, et al. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med 1998;24:1023–39
- Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 1997;29:315–31
- Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001;17:888–95
- Alleva R, Nasole E, Di Donato F, et al. α-Lipoic acid supplementation inhibits oxidative damage, accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy. Biochem Biophys Res Commun 2005;333:404–10
- Alleva R, Tomasetti M, Sartini D, et al. Alpha-lipoic acid modulates extracellular matrix and angiogenesis gene expression in non-healing wounds treated with hyperbaric oxygen therapy. Mol Med 2008;14:175–83
- Petersen SK, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB 2008;60:362–7
- Folkman J, Brem H. Angiogenesis and inflammation. In: Gallin J, Goldstein I, Snyderman R, eds. Inflammation: basic principles and clinical correlates. 2nd ed. New York: Raven Press; 1992:821–39
- Halliwel B, Gutteridge C, Cross CE. Free radicals, antioxidants and human disease: where are we now. J Lab Clin Med 1992;19:598–620
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18
- Tian YW, Stacey MC. Cytokines and growth factors in keratinocytes and sweat glands in chronic venous leg ulcers: an immunohistochemical study. Wound Rep Reg 2003;11:316–25
- Baker AE, Leaper DJ. Proteinases, their inhibitors and cytokine profiles in acute wound fluid. Wound Rep Reg 2000;8:392–8
- Brown GL, Curtsinger L, Jurkiewicz MJ, et al. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg 1991;88:189–94; discussion 195–6
- Falanga V, Eaglstein WH, Bucalo B, et al. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers. J Dermatol Surg Oncol 1992;18:604–6
- Raddatz G, Bisswanger H. Receptor site and stereospecifity of dihydrolipoamide dehydrogenase for R- and S-lipoamide: a molecular modeling study. J Biotechnol 1997;58:89–100
- Kilic F, Handelman GJ, Serbinova E, et al. Modelling cortical cataractogenesis: in vitro effect of a-lipoic acid on glucose-induced lens membrane damage, a model of diabetic cataractogenesis. Biochem Mol Biol Int 1995;37:361–70
- Streeper RS, Henriksen EJ, Jacob S, et al. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol 1997;273:E185–91
- Frölich L, Götz ME, Weinmüller M, et al. (r)-, but not (s)-alpha lipoic acid stimulates deficient brain pyruvate dehydrogenase complex in vascular dementia, but not in Alzheimer dementia. J Neural Transm 2004;111:295–310
- Wagner FW Jr. The diabetic foot. Orthopedics 1987;10:163–72
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:1–28
- Yogaratnam JZ, Laden G, Madden LA, et al. Hyperbaric oxygen: a new drug in myocardial revascularization and protection? Cardiovasc Revasc Med 2006;7:146–154
- Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 2011;127:131S–41S
- Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol 1997;38:79–101
- Sen CK, Roy S, Han D, Packer L. Regulation of cellular thiols in human lymphocytes by alpha-lipoic acid: a flow cytometric analysis. Free Radic Biol Med 1997;22:1241–57
- Pepper MS, Mandriota SJ, Jeltsch M, et al. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998;177:439–52
- Sheikh AY, Gibson JJ, Rollins MD, et al. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg 2000;135:1293–7
- Han C, Lin L, Zhang W, et al. Hyperbaric oxygen preconditioning alleviates myocardial ischemic injury in rats. Exp Biol Med (Maywood) 2008;233:1448–53
- Kalns JE, Dick EJ Jr, Scruggs JP, et al. Hyperbaric oxygen treatment prevents up-regulation of angiogenesis following partial-thickness skin grafts in the pig. Wound Repair Regen 2003;11:139–44
- Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 2000;115:12–8
- Grazul-Bilska AT, Johnson ML, Bilski JJ, et al. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787–800
- Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor: an endothelial cell mitogen related to PDGF. Science 1989;246:1309–12
- Folkman J, Brem H. Angiogenesis and inflammation. In: Gallin J, Goldstein I, Snyderman R, eds. Inflammation: basic principles and clinical correlates. 2nd ed. New York: Raven Press; 1992:821–39
- Pakele R, Watanabe T, Benedict CR. Induction of endothelial cell proliferation by angiogenic factors released by activated monocytes. Cardiovasc Radiat Med 2002;3:95–101
