Abstract
For the development of potent novel antileishmanial agents, 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4 dione) derivatives were synthesized from lawsone and evaluated for cytotoxicity on Leishmania donovani promastigotes as well as on leishmanial DNA topoisomerase-I. Enzyme inhibition studies were conducted with simultaneous and preincubation conditions. Total inhibition is compared to camptothecin (CPT), which was taken as positive control on both the systems of enzyme inhibition. The range of activity varied from 37.5 to 70 µM in simultaneous assay and 13–16 µM in preincubation assay. Furthermore, when evaluated against L. donovani promastigotes, the synthesized compounds exhibited the activity ranging from 2 to 14 µM. The results revealed that all the compounds exhibit promising antileishmanial activity.
Introduction
Leishmaniasis, a protozoan parasitic disease, is currently a global health problem occurring with visceral, cutaneous and mucocutaneous manifestations. Clinical implications of this disease range from the disfiguring skin lesions in the case of cutaneous leishmaniasis to the often fatal visceral leishmaniasis (VL)Citation1,Citation2. Visceral leishmaniasis (VL), also known as kala-azar, caused by Leishmania donovani, is often associated with a marked suppression of the cell-mediated immune response of the host, leading to severe morbidity, even mortality, if left untreatedCitation3. According to the World Health Organization [http://www.who.int/leishmaniasis/en/], leishmaniasis threatens about 350 million men, women and children in 88 countries around the world. As many as 12 million people are believed to be currently infected with about 1 to 2 million estimated new cases occurring every yearCitation4. It has to be noted that in spite of development of antimonial drugs, amphotericin B, pentamidine isethionate, sodium stibogluconate and miltefosine, there has been observed a rapid growth of resistance in leishmaniasisCitation5,Citation6.
Among the various biological catalysts which are established as cellular therapeutic targets, DNA topoisomerases are of particular importance. DNA topoisomerases are ubiquitous enzymes that play a pivotal role in modulating the dynamic nature of DNA secondary or higher order structures and thus support essential functions inside cells. These functions relate mainly to nucleic acid metabolism, namely replication, transcription, recombination and repairCitation7,Citation8. All known topoisomerases share two characteristics: (i) the ability to cleave and reseal the phosphodiester backbone of DNA in two successive trans-esterification reactions and (ii) once a topoisomerase is cleaved the DNA intermediate is formed, the enzyme allows the severed DNA end to come apart, opening a gate for the passage of another single or double stranded DNA segmentCitation9,Citation10.
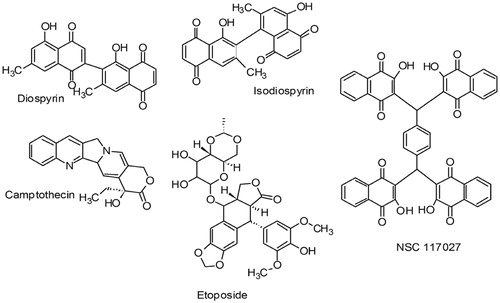
There are two facts which were accumulated together from literature to form the genesis of the present work. NSC 117027, a NCI repository compound has been shown to inhibit calf thymus DNA topoisomerase-I by Yves Pommier and co-workersCitation11. Few natural compounds containing naphthoquinone moiety like diospyrin and isodiospyrinCitation12–14 and non-naphthoquinone moiety like camptothecin (CPT)Citation15,Citation16 and etoposide have been proved to be potent antileishmanial agents by binding to DNA topoisomerases (). Unlike the prototypic human topoisomerase-I poison CPT; isodiospyrin does not induce human topoisomerase I–DNA covalent complexes. Isodiospyrin antagonizes CPT-induced, human topoisomerase I-mediated DNA cleavage. Diospyrin, on the other hand exerts its inhibitory effect by binding with the enzyme and stabilizing the topoisomerase I–DNA “cleavable complex”. Inspired from the above-mentioned facts, we attempted to develop naphthoquinone-based compounds with cytotoxic potential to L. donovani cells which can potentially target DNA topoisomerase-I. The compounds mentioned in the present work are synthesized from a natural compound lawsone or 2-hydroxy-1,4-naphthoquinone, which is a phytochemical constituent found in Lawsonia inermis. Since a long time, this plant is known to the people residing in Indian subcontinent due to its usage in making of skin and hair dye. All the compounds evaluated in the current work showed significant results in cell-based assay. As far as leishmanial DNA topoisomerase-I inhibition activity is concerned, both preincubation and simultaneous assays were conducted to understand the mechanism of action. The results demonstrated in the current work again proves that naphthoquinone scaffolds are potent against L. donovani cells and these compounds can be novel leads towards the development of better inhibitors of leishmaniasis.
Materials and methods
Chemistry
All chemicals used in this work were purchased from Sigma Aldrich Chemical Company (Bangalore, India). Solvents were used as obtained from the supplier (Merck Chemical Company, Bangalore, India) or redistilled as necessary. Thin layer chromatography was performed on readymade sheets from Merck Chemical Company. Melting points were measured on capillary melting point apparatus and were uncorrected. Mass spectra were recorded on a Agilent ESI-Q-TOF 6520 (New Delhi, India), with a resolution of 1 000 000 ppm. 1H NMR spectra and 13C NMR were recorded in DMSO with 400 MHz on a BRUKER Advance liquid state NMR (Bangalore, India) using Trimethylsilane as internal standard and the chemical shifts (δ) are reported in ppm. IR spectra were recorded on a SHIMADZU FT-IR 8400-S spectrophotometer (Mumbai, India).
Synthesis of compounds
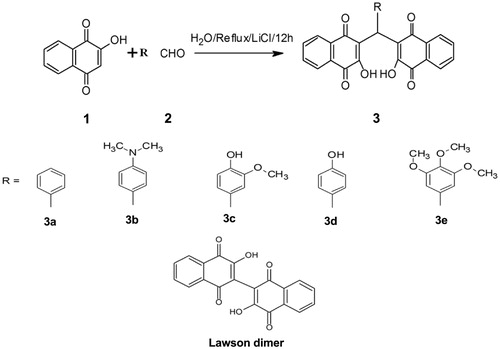
The compounds were synthesized in aqueous medium according to the procedure by Ayoob Bazgir and co-workersCitation17. Detailed experimental procedures and characterization data of synthesized compounds 3a–e () can be found in the supplementary information. Lawsone dimer has been procured as such from Sigma Aldrich Chemical Company.
Biological screening
Agarose (A6013), ficoll-400, polyvinyl-pyrollidone (PVP-360), M199, vitamins, amino acids CPT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenylterazolium bromide (MTT) and other chemicals were purchased from Sigma Aldrich (St. Louis, MO). pBlue script (SK) DNA was purchased from Novagen (Madison, WI, USA). Topoisomerase LDTOP1LS was obtained according to an already published procedureCitation18.
Liquid culture media
Liquid culture media was prepared using M199 powder (Sigma Aldrich, St. Louis, MO) 1.1% and HEPES buffer (pH 7.5) 20 mM. Buffered media was filter sterilized by passing through 0.22 μm pore-sized filter (Nalgene, Rochester, NY) and then heat inactivated fetal calf serum 10% (GIBCO-Invitrogen, Carlsbad, CA), Penicillin 100 U/ml and Streptomycin 100 μg/ml were added. This media was used for liquid culture of L. donovani promastigotes.
Drug solutions
All compounds, CPT, were dissolved in 100% DMSO at 20 mM concentration. All drug solutions were kept at room temperature. The final concentration of DMSO was kept at 4% and 0.5% for all in vitro and cellular experiments, respectively.
Parasite culture and maintenance
The L. donovani strain AG83 promastigotes were grown at 22 °C in Rays modified media and in M199 liquid media supplemented with 10% fetal calf serum as described previouslyCitation19.
Agarose gel electrophoresis
Agarose gel electrophoresis was carried out for the separation of DNA. Gel was prepared from 1.25 g of agarose, 125 ml of distilled water and 2.4 ml of TAE. Then agarose was boiled in hot water bath until the solution became clear and subsequently it was cooled to about 50–55° C. The melted agarose solution was poured into the casting tray and was cooled until it became solid and the gel was loaded in the electrophoresis chamber with 1 × TAE buffer at a constant voltage of 5–6 V/cm for 2–8 h. Different concentrations of compounds were added with enzyme and DNA control. DNA was stained by soaking in 0.5 µg/ml of ethidium bromide solution, visualized in U.V. transilluminator and photographed.
Plasmid relaxation assay
The type 1 DNA topoisomerase was assayed by decreased mobility of the relaxed isomers of supercoiled pBlue script (SK) DNA in agarose gel. Relaxation assay was carried out as described previously with LdTOPILS serially diluted in the relaxation buffer (25 mM Tris-HCl pH 7.5, 5% glycerol, 0.5 mM DTT, 10 mM MgCl2, 2.5 mM EDTA and 150 µg/ml BSA) supercoiled pBluescript (SK+) DNA and 50 mM KCl. The amount of supercoiled monomer DNA band fluorescence after ethidium bromide (EtBr) (0.5 µg/ml) staining was quantitated by using GEL Doc 2000 from Biorad (Hercules, CA, USA).
Antileishmanial activity in vitro
The effect of drug on the viability of L. donovani AG83 cells was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenylterazolium bromide (MTT) assayCitation20. The cells at the exponential phase were collected and transferred into 24-well plate (approximately 4 × 105 to 106 cells/well). The cells were then incubated for 48 h in the presence of various concentrations of compounds 3a–e and lawsone dimer individually. After incubation, the cells were centrifuged, and the supernatant was aspirated. The cell pellet was washed twice with PBS (1X) and was finally suspended in 100 µl of PBS (1×) in 96-well plates. Ten micro liters of MTT solution (10 µg/ml) were added to each sample of 96-well plates and samples were incubated for 4 h. After incubation, 100 µl of stop solution (stock: 4963 µl of isopropanol and 17 µl of 12 N HCl) was added and kept for 20 to 25 min at room temperature. The optical density was taken at A492 on an ELISA reader (Multiskan EX; Thermo Fisher Scientific, Waltham, MA).
Results
Initial screening of all compounds
The effect of these chemically synthesized derivatives (3a–e) and lawsone dimer on the unusual bi-subunit type IB topoisomerase of L. donovani (LdTOP1LS) were examined by plasmid relaxation assays as described in the section “Materials and methods”. The relaxation assays were carried out under standard assay conditions where the plasmid DNA and the enzyme were present at a molar ratio of 3:1. Under this reaction condition, in the absence of any inhibitor, LdTOP1LS relaxes supercoiled plasmid DNA completely after 30 min of incubationCitation21. Screening of all the above-mentioned compounds on relaxation activity of LdTOP1LS were carried out using 2% (v/v) of DMSO. All six compounds were initially screened at 200 µM concentrations. It was observed that four compounds, 3a, 3b, 3c and 3d were showing inhibition against DNA topoisomerase-I of L. donovani at a concentration of 200 µM. Compound 3e and lawsone dimer failed to show any inhibition. It implies that only compounds 3a–d could be potentially effective against DNA topoisomerase-I of L. donovani and were further investigated.
Simultaneous assay of four compounds with CPT as standard
In simultaneous relaxation assay, CPT concentrations were 50 and 100 µM, whereas concentration of compounds were 50, 100 and 200 µM. CPT showed 100% inhibition at a concentration of 100 µM. Compound 3a showed total inhibition at a concentration of 100 µM and the others, i.e. compound 3b, 3c and 3d, were showing total inhibition at a concentration of 200 µM (, lane 7, 11, 14, 17). The IC50 for the compounds 3a–d were calculated using the non-linear equation for sigmoidal response in Graph Pad Prism ver. 5.00 (La Jolla, CA) and are listed in ().
Figure 3. Inhibition of catalytic activity of LdTOP1LS by derivatives bis-naphthoquinone 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4 dione) analogs. (A) Relaxation of super coiled pBS (SK+) DNA with reconstituted LdTOP1LS at a molar ratio of 3:1. Lane 1, 90 fmol of pBS (SK+) DNA; lane 2, same as lane 1, but simultaneously incubated with 30 fmol of LdTOP1LS for 30 min at 37 °C; lane 3, same as lane 2, but in the presence of 2% (v/v) DMSO; lanes 4–5 same as lane 2, but in the presence of 50 and 100 µM of CPT, respectively; lanes 6–17 same as lane 2, but in the presence of 50, 100, 200 µM of compounds 3a, 3b, 3c and 3d, respectively. Positions of super coiled monomer (SM) and relaxed and nicked monomer (RL/NM) are indicated. (B) Preincubation of LdTOP1LS with respective inhibitors followed by the addition of DNA. Lane 1, 90 fmol of pBS (SK+) DNA; lane 2, same as lane 1, but the enzyme was pre-incubated with 2% (v/v) of DMSO; lanes 4–5, same as lane 2, but the enzyme was preincubated with 50 and 100 µM of CPT, respectively; lanes 6–17, same as lane 2, but the enzyme was preincubated with 25, 50, 100 µM of compounds 3a–e, respectively. Reactions were stopped by the addition of SDS to a final concentration of 0.5% and electrophoresed in 1% agarose gel.
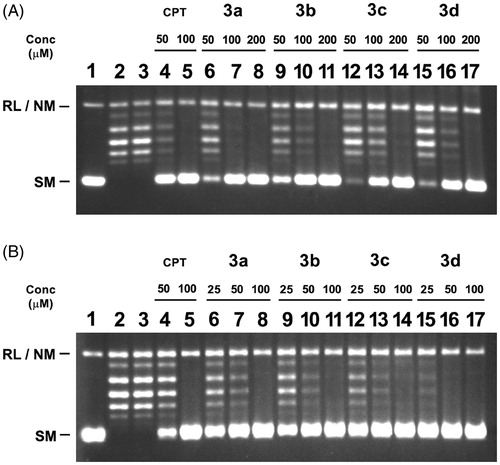
Table 1. Inhibitory concentration of the compounds on LdTOPILS.
Preincubation assay of four compounds with CPT as standard
To investigate whether these compounds interact with the enzyme (without DNA substrate), LdTOP1LS was preincubated with compounds 3a–d at different concentrations for 5 min at 37 °C before the addition of substrate DNA (). Inhibitory effects of these compounds in preincubation condition were compared with the inhibition by these compounds incubated simultaneously with the enzyme (LdTOP1LS) and supercoiled DNA in the relaxation reaction (). In the preincubation assay, CPT concentrations were 50 and 100 µM, whereas various concentrations of compounds were 25, 50 and 100 µM. CPT showed 100% inhibition at 100 µM concentration. The compounds showed total inhibition at 3a = 100; 3b = 100; 3c = 100 and 3d = 50 µM concentration, respectively (, lane 8, 11, 14, 16).
Cytotoxic effect against L. donovani promastigotes in vitro
The EC50 was calculated according to the already published procedureCitation21. The results shown are the means of three independent experiments with S.E. The fitted lines were generated with Graph Pad Prism using sigmoidal dose–response (variable slope model) equation. Cytotoxic assay of all the compounds tested revealed that the EC50 (the half maximal effective concentration) of compounds 3a, 3b, 3c, 3d, 3e and lawsone dimer obtained were 2, 6, 2, 9, 2.5 and 14 µM ( and ), respectively. It has to be noted that the compound 3e and lawsone dimer though did not inhibit DNA topoisomerase-I enzyme yet were active against L. donovani promastigotes. Both compound 3e and lawsone dimer exhibited a considerable amount of cytotoxicity for L. donovani promastigotes.
Figure 4. Quantitative representation for percentage of promastigotes survival of L. donovani in vitro in the presence of compounds 3a–e and lawsone dimer.
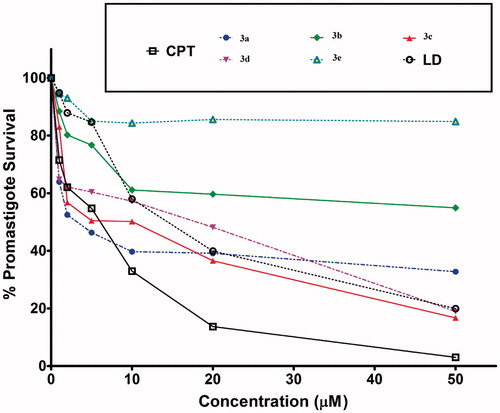
Table 2. EC50 values of the compounds of L. donovani promastigotes.
Discussion
The results shown here demonstrate the antileishmanial activity of 3,3′-(arylmethylene) bis(2-hydroxynaphthalene-1,4 dione) derivatives for the first time. Among the two methods used by us for the DNA topoisomerase-I enzyme assay, in simultaneous assay the compounds 3a–d showed IC50 in the range of 37.5–70 µM, whereas compound 3b, the N-dimethyl amino derivative, exhibited highest activity among all. Additionally, preincubation assay was also conducted for the dual purpose of knowing whether the compounds were able to inhibit the enzyme as such and to investigate whether the compounds work with the same mechanism as diospyrin, which is also a binapthoquinone and it inhibits topoisomerase-I and stabilizes the topoisomerase I–DNA “cleavable complex”Citation12. In our case, we notice that all the compounds except the lawsone dimer and the trimethoxy derivative (compound 3e) were active on both the simultaneous and preincubation assays. The preliminary results reported here suggest that the mechanism of the compounds may be similar to diospyrin. However, further studies regarding the mechanism of action of the compounds are currently being pursued and would be reported in future. The results also indicate that N-dimethyl amino and unsubtituted hydroxyl functional groups are important for inhibition whereas too much of substitution leads to a loss of activity against DNA topoisomerase-I. Though compound 3e and lawsone dimer did not inhibit the enzyme yet they exhibited considerable cytotoxic effects on leishmanial promastigotes. This could be a hint to the fact that compound 3e and lawsone dimer are interacting with some other biological target other than DNA topoisomerase-I. Due to the similarity between molecular skeleton of lawsone dimer and diospyrin, it was expected that the former would exhibit inhibition of the enzyme but on the contrary, lawsone dimer was proved completely inactive in enzyme assay and moderately active in cytotoxicity assay.
Conclusion
The study concludes with the fact that novel scaffolds have been successfully synthesized and evaluated against L. donovani promastigotes and DNA topoisomerase-I of the parasite. Both the simultaneous and preincubation assays have been conducted for a deeper insight into the mechanism of action. We noticed that more substitution in synthesized compounds lead towards decreased activity against DNA topoisomerase I of L. donovani. The mechanism of compound 3e and lawsone dimer remains unknown and are subject to further investigation. To further enhance the DNA topoisomerase I inhibiting capability of compounds, a profound analysis involving computational and medicinal chemistry approaches is necessary. The present investigation demonstrates that active compounds may act as lead molecules for further studies.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
GS and SVK thank the management and administration of Karunya University for their encouragement and support.
References
- Reithinger R, Dujardin J-C, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis 2007;7:581–96
- Zijlstra EE, Musa AM, Khalil EAG, et al. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 2003;3:87–98
- Haldar JP, Ghose S, Saha KC, Ghose AC. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun 1983;42:702–7
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect 2004;27:305–18
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev 2006;19:111–26
- Pérez-Victoria FJ, Sánchez-Cañete MP, Seifert K, et al. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist Update 2006;9:26–39
- Bjornsti MA, Wang JC. Expression of yeast DNA topoisomerase I can complement a conditional-lethal DNA topoisomerase I mutation in Escherichia coli. Proc Natl Acad Sci 1987;84:8971–5
- Stewart L, Ireton GC, Champoux JJ. Reconstitution of human topoisomerase I by fragment complementation. J Molec Biol 1997;269:355–72
- Wang JC. DNA topoisomerases: why so many? J Biol Chem 1991;266:6659–62
- Wang JC. DNA topoisomerases. Ann Rev Biochem 1996;65:635–92
- Mazumder A, Wang S, Neamati N, et al. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J Med Chem 1996;39:2472–81
- Ray S, Hazra B, Mittra B, et al. Diospyrin, A bisnaphthoquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Molec Pharmacol 1998;54:994–9
- Hazra B, Golenser J, Nechemiya O, et al. Inhibitory activity of diospyrin derivatives against Leishmania major parasites in vitro. Indian J Pharmacol 2002;34:422–7
- Ting C-Y, Hsu C-T, Hsu H-T, et al. Isodiospyrin as a novel human DNA topoisomerase I inhibitor. Biochem Pharmacol 2003;66:1981–91
- Sen N, Das BB, Ganguly A, et al. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J Biol Chem 2004;279:52366–75
- Sen N, Das BB, Ganguly A, et al. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 2004;11:924–36
- Tisseh ZN, Bazgir A. An efficient, clean synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives. Dyes Pigments 2009;83:258–61
- Das BB, Dasgupta SB, Ganguly A, et al. Leishmania donovani bisubunit topoisomerase I gene fusion leads to an active enzyme with conserved type IB enzyme function. FEBS J 2007;274:150–63
- Mittra B, Saha A, Chowdhury AR, et al. Luteolin, an abundant dietary component is a potent antileishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med (N Y) 2000;6:527–41
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 1983;65:55–63
- Chowdhury S, Mukherjee T, Sengupta S, et al. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Molec Pharmacol 2011;80:694–703