Abstract
The cell-free culture filtrate of Bacillus cereus subsp. thuringiensis associated with an entomopathogenic nematode (EPN), Rhabditis (Oscheius) sp., exhibited strong antimicrobial activity. The ethyl acetate extract of the bacterial culture filtrate was purified by silica gel column chromatography to obtain two cyclic dipeptides (CDPs). The structure and absolute stereochemistry of this compound were determined based on extensive spectroscopic analyses (FABMS, 1H NMR, 13C NMR, 1H--1H COSY, 1H--13C HMBC) and Marfey’s method. The compounds were identified as cyclo(D-Pro-L-Met) and cyclo(D-Pro-D-Tyr). CDPs showed significantly higher activity than the standard fungicide bavistin against agriculturally important fungi, viz., Fusarium oxysporum, Rhizoctonia solani and Penicillium expansum. The highest activity of 2 µg/ml by cyclo(D-Pro-D-Tyr) was recorded against F. oxysporum, a plant pathogen responsible for causing fusarium wilt followed by R. solani, a pathogen that causes root rot and P. expansum. To our knowledge, this is the first report on the isolation of these compounds from Rhabditis EPN bacterial strain Bacillus cereus subsp. thuringiensis.
Introduction
Plant pathogenic fungi have evolved many ways to infect their hosts and can have devastating effects on commercial crop production. The fungus Fusarium oxysporum is found in both agricultural and non-cultivated soils throughout the world. The species consists of non-pathogenic and pathogenic isolates, both known as efficient colonizers of the root rhizosphere. The pathogenic isolates, grouped into formae specialis depending on their host rangeCitation1, cause wilt or rot disease in important agricultural and ornamental plant species, such as tomato, banana, cotton and tulip bulbs, thereby causing serious problems in commercial crop productionCitation2,Citation3. Recently, F. oxysporum has also been reported as an emerging human pathogen, causing opportunistic mycosesCitation4. Many strategies to control this fungal pathogen have been investigatedCitation5,Citation6. Currently, the most effective method in preventing crops from fusarium wilt is to mix the seed with chemical fungicides. The application of chemical fungicide induces other problems, such as harm to other living organisms and the reduction of useful soil microorganismsCitation7,Citation8. Therefore, public concern is focused on alternative methods of this fungal control, which can play a key role in natural product to reduce the concentration of chemical pesticidesCitation9. A promising strategy for the replacement of chemical pesticides has been the implementation of microbial natural product-based compounds.
The entomopathogenic nematode (EPN) Heterorhabditis and Steinernema together with their symbiotic bacteria Photorhabdus and Xenorhabdus, respectively, are obligate and lethal parasites of insectsCitation10. Infective juveniles (IJs), the only free-living stage, enter hosts through natural openings (mouth, anus and spiracles), or in some cases, through the cuticle. After entering the host’s hemocoel, nematodes release their bacterial symbiontsCitation11. The bacteria multiply rapidly and produce various metabolites that can overcome the insect immune system, kill the insect and inhibit the growth of various fungal and bacterial competitorsCitation12–14. By doing so, the bacterial symbionts are believed to prevent putrefaction of the insect cadaver and establish conditions that favor the development of both the nematode and bacterial symbionts. The antimicrobial nature of metabolites produced by Xenorhabdus spp. and Photorhabdus spp. is known, and several compounds with antibiotic activity have been isolated and identified. These include indolesCitation15, stilbenesCitation16, xenorhabdinsCitation17, xenocoumacinCitation18, nematophinCitation19, benzylineacetoneCitation20, xenortides and xenematideCitation21, and cyclolipopeptide (PAX)Citation22. These metabolites not only have diverse chemical structures, but also have a wide range of bioactivities of medicinal and agricultural interests such as antibiotic, antimycotic, insecticidal, nematicidal, antiulcer, antineoplastic and antiviral.
During our studies on EPN, we isolated a new EPN Rhabditis sp. from sweet potato weevil grubs collected from Central Tuber Crops Research Institute (CTCRI) farm, Thiruvananthapuram. To our knowledge, this is the first time Rhabditis sp. is being studied as an EPN. A specific bacterium isolated from third-stage IJs of the nematode or from the haemolymph of nematode infested Galleria mellonella larvae was found to be pathogenic to a number of insect pests (unpublished results). The antimicrobial substance producing bacterium was isolated from an entomopathogenic, Rhabditis (Oscheius) sp. resembling Rhabditis isolate Tumian 2007 at D2 and D3 expansion segments of 28S rDNACitation23. Molecular analyses revealed that the bacterium resembles Bacillus sp. (Accession No. CP001407). The bacterium has been deposited in IMTECH (Institute of Microbial Technology, Chandigarh, India) and the accession number is MTCC 5234. The cell-free culture filtrate of the bacteria was found to inhibit several pathogenic bacteria, fungi and a plant-parasitic nematode (Meloidogyne incognita)Citation24, suggesting that it could be a rich source of biologically active compounds. In this paper, we report the isolation, structure elucidation and antimicrobial activity of two diketopiperazines, especially against plant pathogenic fungi from the cell-free culture filtrate of the bacterium.
Materials and methods
Chemicals and media
All the chemicals used for extraction and column chromatography were of analytical grade and high performance liquid chromatography (HPLC) grade methanol was from Merck Limited, Mumbai, India. Silica gel (230–400 mesh) used for column chromatography and precoated silica gel 60 GF254 plates used for thin layer chromatography (TLC) were from Merck Limited, Darmstadt, Germany. Microbiological media were from Hi-Media Laboratories Limited, Mumbai, India. All other reagents were of analytical grade and the other chemicals used in this study were of the highest purity. The standard antibiotics ciprofloxacin, cefotaxime and amphotericin B were purchased from Sigma Aldrich (St. Louis, MO). The software used for the chemical structure drawing was Chemsketch Ultra, Advanced Chemistry Development Inc, Toranto, Canada.
Test microorganisms
Agriculturally important fungi: F. oxysporum MTCC 284, Rhizoctonia solani MTCC 4634, and Penicillium expansum MTCC 2006 and medically important fungi: Aspergillus flavus MTCC 183, Candida albicans MTCC 277; gram-positive bacteria: Bacillus subtilis MTCC 2756, Staphylococcus aureus MTCC 902; gram-negative bacteria: Escherichia coli MTCC 2622, Pseudomonas aeruginosa MTCC 2642. All the test microorganisms were purchased from Microbial Type Culture collection Centre, IMTECH, Chandigarh, India.
Isolation of Bacillus cereus subsp. thuringiensis
The bacterium was isolated from third-stage IJs of the nematode sample collected from sweet potato weevil grubs or from the haemolymph of nematode infested G. mellonella larvae. The strain was identified as Bacillus sp. (Accession No. CP001407) based on 16S rDNA and BLAST analysis. The strain was currently deposited in IMTECH (Institute of Microbial Technology, Chandigarh, India) and the accession number is MTCC 5234.
Fermentation and extraction
The bacterial fermentation was carried out using modified Tryptic soya broth (TSB) (tryptone 17 g/l, soytone 3.0 g/l, glucose 2.5 g/l, NaCl 5.0 g/l, yeast extract 10 g/l, water 1000 ml). A single colony of Bacillus sp. N strain from the agar plate was inoculated into the flask containing 100 ml sterile media. The flasks were incubated in a gyrorotatory shaker (150 rpm) at 30 °C in dark for 24 h. When the optical density of the culture at 600 nm was approximately 1.7, the bacterial cultures were transferred aseptically into 400 ml sterile medium and incubated in the gyrorotatory shaker at 30 °C in dark for 96 h. The culture media were then centrifuged (10 000 g, 20 min, 4 °C) followed by filtration through a 0.45 µm filter, to obtain cell-free culture filtrate. Thirty litres of cell-free culture filtrate were neutralized with concentrated hydrochloric acid and extracted with an equal volume of ethyl acetate thrice. The ethyl acetate layers were combined, dried over anhydrous sodium sulphate and concentrated at 30 °C using a rotary flash evaporator.
Purification of bioactive compounds
The oily yellow residue (9.3 g) obtained after drying was then loaded on a silica gel column (25 × 600 mm) previously equilibrated with hexane and eluted successively with 200 ml of 100% hexane, 200 ml of linear gradient hexane: dichloromethane (v/v, 75:25 to 25:75), 200 ml of 100% dichloromethane, 200 ml of linear gradient dichloromethane:ethyl acetate (v/v, 95:5 to 5:95), 200 ml of 100% ethyl acetate and finally with 200 ml of 100% methanol. Two fractions (100 ml each) were collected from each combination. Two fractions yielded white crystal compounds which were further purified by crystallization using hexane and benzene and the antibacterial activity of these fractions were determined by well diffusion assay against B. subtilis, which was selected as initial test microorganism.
TLC and HPLC
An aliquot of crystal compounds was loaded on the silica gel TLC plates (20–20 cm). The plates were developed using benzene: acetone (70:30) solvent for first and benzene:acetone (60:40) solvent for second compound. The spots were located by exposing the plate to iodine fumes. The purified crystal compounds were tested for their purity using HPLC, using LC-10AT liquid chromatograph (LC, Shimadzu, Singapore) equipped with a C-18 column (5 μm, 4.6 × 250 mm) and 100% methanol as a mobile phase with a flow rate of 1 ml/min. Ultraviolet (UV) detection was carried out with a diode array detector (Shimadzu).
Characterization of bioactive antifungal compound
UV spectrophotometer
UV-visible spectrum of the pure compounds was recorded on a Systronics Double Beam Spectrophotometer 2201, Ahmedabad, India at room temperature (scanning range 190–800 nm). About 1 mg of the isolated compound dissolved in 20 ml of chloroform was used to record the spectrum.
Nuclear magnetic resonance
The structure of the compounds was determined using nuclear magnetic resonance (NMR) spectroscopy (Bruker DRX 500 NMR instrument, Bruker, Rheinstetten, Germany) equipped with a 2.5-mm microprobe. NMR spectrometer using CDCl3 was deployed to measure 1H and 13C and 2D NMR. All spectra were recorded at 23 °C. One-dimensional 1H NMR experiments as well as two-dimensional 1H–1H correlation spectroscopy, 1H–13C heteronuclear multiple bond correlation and 1H–13C heteronuclear multiple quantum coherence (HMQC) experiments were performed according to Bruker standard pulse sequences. Proton chemical shifts were determined from one-dimensional 1H NMR and from HMQC experiments, and 13C chemical shifts were determined from HMQC and 1H–13C heteronuclear multiple bond correlation experiments. Chemical shifts are reported relative to the solvent peaks (CDCl3: 1H d 7.24 and Citation13C d 77.23).
High resolution mass spectrophotometer
High resolution mass spectrophotometer (HRMS) was performed on a Thermo Scientific Exactive Orbitrap LC-Mass Spectrometer (Massachusetts) with an electrospray ionization mode.
Optical rotations
Optical rotation of the compounds was measured using a Rudolph Research Autopol III polarimeter (New Jersey) at 25 °C in acetone.
Differential scanning calorimetry
The melting point of the pure compounds was measured with a differential scanning calorimeter in a Mettler Toledo DSC 822e instrument (Mettler-Toledo, Schcoerfenbach, Switzerland). Temperature ranges from 30 °C to 300 °C were employed.
Absolute configuration determination of compounds
A solution of two compounds (1.5 mg) in 6 M HCl (1 ml) was heated to 120 °C for 24 h. The solution was then evaporated to dryness and the residue redissolved in H2O (100 μl) and was then placed in a 1 ml reaction vial and treated with a 2% solution of FDAA (200 μl) in acetone followed by 1.0 M NaHCO3 (40 μl). The reaction mixture was heated at 47 °C for 1 h, cooled to room temperature, and then acidified with 2.0 M HCl (20 μl). In a similar fashion, standard d- and l-amino acids were derivatized separately. The derivatives of the hydrolysates and standard amino acids were subjected to HPLC analysis (Shimadzu LC-20AD, C18 column; 5 μm, 4.6 × 250 mm; 1.0 ml/min) at 30 °C using the following gradient program: solvent A, water +0.2% TFA; solvent B, MeCN; linear gradient 0 min 25% B, 40 min 60% B, 45 min 100% B; UV detection at 340 nmCitation25.
Antimicrobial activity
Antifungal activity
Minimum inhibitory concentration (MIC) was determined using potato dextrose agar media against the standard fungicide bavistin by the poisoned food techniqueCitation26 against A. flavus, F. oxysporum, R. solani and P. expansum. A stock solution of 2000 µg/ml of the test compound was prepared, which was further diluted with methanol to give the required concentrations 1000 to 1 µg/ml. One tube was used as solvent control. For C. albicans, the broth dilution methodCitation27 was adopted using potato dextrose broth against the standard fungicide amphotericin B. All experiments were in triplicate for each treatment against each fungus.
Antibacterial activity
The test compounds and antibiotics were screened for antimicrobial activity using the macro dilution method recommended by the National Committee for Clinical Laboratory Standards, USACitation28. To determine the MICs, the compounds were dissolved in methanol to give a stock concentration of 2000 μg/ml while the antibiotics were dissolved in sterile distilled water to give stock concentrations of 1000 μg/ml. All stock concentrations of compounds and antibiotics were filter-sterilized using 0.2 μm syringe filter. Two-fold serial dilutions of the antibiotics and compounds were made with nutrient broth to give concentrations ranging from 1 to 1000 μg/ml. Colony suspensions equivalent to a 0.5 McFarland standard were prepared and inoculated onto antibiotic-containing medium to yield a final inoculum of 104 CFU/ml. The tubes were incubated at 35 °C for 24 h. The MIC was defined as the lowest antibiotic concentration showing no growth. Triplicate sets of tubes were maintained for each concentration of test sample.
Agar disc-diffusion method
In vitro antibacterial and antifungal activity of the compounds was measured using agar disc-diffusion assay against the test bacteria and fungiCitation29. The sterile discs were impregnated with MIC concentration of test compounds. The ciprofloxacin (5 µg/disc) was used as positive reference standards for bacteria. Amphotericin B (Sigma) was used as reference standard for C. albicans. Bavistin was used as reference standard against other fungi. The antimicrobial activity was evaluated by measuring the zone of growth inhibition surrounding the discs. All the assays were carried out in triplicate.
Statistical analysis
All statistical analyses were performed with SPSS (Version 17.0; SPSS, Inc., Chicago, IL). Data for disc-diffusion assay was presented as means ± standard deviation. Statistical significance was defined as p < 0.05Citation30.
Results
Isolation and purification of bioactive compounds
The ethyl acetate extract of the cell-free culture filtrate of the bacteria showed antifungal activity against F. oxysporum. Silica gel column chromatography of this extract yielded two crystal compounds eluted at 20% ethyl acetate in DCM (DKP 1) and 55% ethyl acetate in DCM (DKP 2). These crystal compounds were further purified by crystallization using hexane and benzene. Initial bioactivity of these compounds was confirmed by testing against F. oxysporum.
Identification of bioactive compound
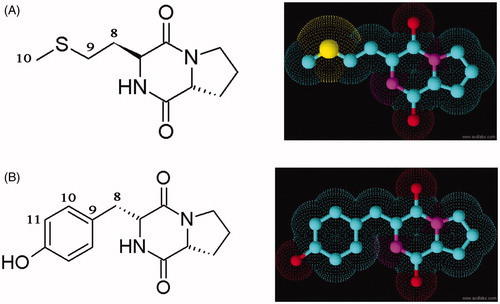
The pure compounds were subjected to various spectroscopic analyses, i.e. UV, NMR and HRMS. The structure of these two compounds corresponded to two different cyclic dipeptides (CDPs) (DKP 1--2). The cyclic dipeptide identified are Cyclo (D-Pro-L-Met) and Cyclo (D-Pro-D-Tyr) (). The 1H and Citation13C NMR spectra in all the DKPs showed the signal at δH 4.10 (t) with J value around 9 Hz with a corresponding δC of 59.0, along with multiplets around δH 2.3, 2.0 and 3.5 ppm, clearly indicated the presence of a proline residue. By comparison of NMRs of standard amino acid residue’s chemical shifts and multiplicities it was clear that DKP 2 contains tyrosine residues. Presence of an amide proton in all the DKPs along with mass spectral information and by comparison of standard DKPs it is decisive that they are all CDPs containing proline residue. Additional spectral information such as HMQC and 1H–1H COSY was also used. HMQC information was used to transfer assigned numbers from carbons in Citation13C NMR to the protons in 1H NMR. The key COSY cross-peaks between C9-C8, C1-C8 and δH 2.13 (s) in DKP 1 indicated the presence of methionine residue; COSY cross-peaks between C1–C8 and COSY cross-peaks between C1–C8, and δH 6.8 (d, 2H) and 7.0 (d, 2H) DKP 2 indicated the presence of Tyrosine residue. The retention times of the HPLC analysis of the 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (FDAA, Marfey’s reagent) derivatives of L-Pro, D-Pro, L-Tyr, D-Tyr, L-Met, D-Met are presented in the and Supplementary figures 1–3. FDAA derivatives of the acid hydrolysates of Pro and Met in the hydrolysate of DKP 1 were 18.281 and 22.211 min, respectively (). The FDAA derivatives of Pro and Tyr in the hydrolysate of DKP 2 were 18.326 and 20.727 min, respectively ().
Figure 2. HPLC profile of FDAA derivatives of the acid hydrolysates of DKPs: (A) cyclo(D-Pro-L-Met) and (B) cyclo-(D-Pro-D-Tyr).
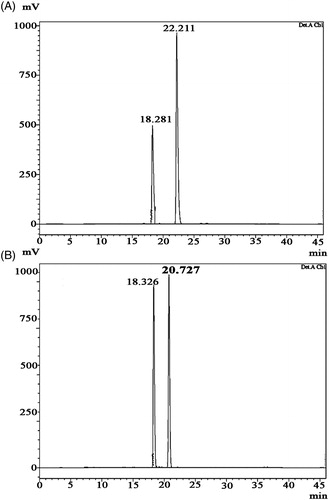
Table 1. HPLC reversed-phase retention times of the standard L and D-amino acid and CDPs derivatives.
DKP 1: Cyclo (D-Pro-L-Met), (3R,8aS)-3-[2(methylsulfanyl)ethyl]hexahydropyrrolo[1,2-a] pyrazine-1,4-dione: 13 mg; white amorphous powder; Rf 0.45 (35% Benzene in acetone); HPLC retention time: 2.711 min (); melting point: 155.03 °C (); = −85.3° (c = 0.10, EtOH); UV max: 210 nm (MeOH); NMR data (); HRMS [M + H]+ C10H17O2N2S calcd. for m/z 229.10053, found 229.10033 (Supplementary figure 4).
Figure 3. HPLC chromatogram of diketopiperazines on a reversed-phase C18 column (LC-20AD). Samples of 15 µl were injected to a column (250 mm × 4.6 mm × 5 mm), eluted with 100% methanol (A) cyclo(D-Pro-L-Met), retention time is 2.711 min. The calculated purity is 98% based on the peak area (B) cyclo-(D-Pro-D-Tyr), retention time is 2.820 min. The calculated purity is 92% based on the peak area.
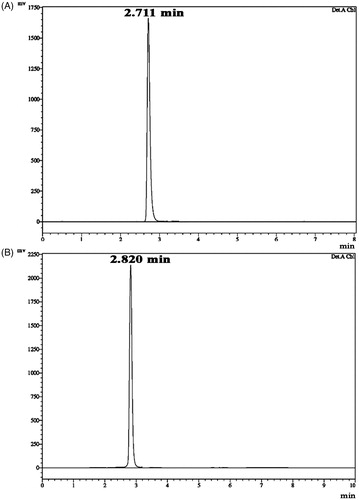
Figure 4. DSC curves of diketopiperazines. Temperatures corresponding to the onset of transition and midpoint of the transition region and enthalpy (ΔH) were recorded by means of the built-in software: (A) cyclo(D-Pro-L-Met) and (B) cyclo-(D-Pro-D-Tyr).
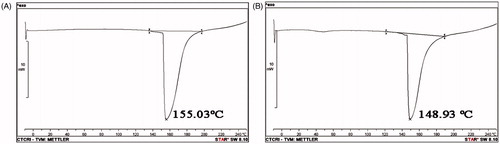
Table 2. NMR data for DKPs in CDCl3 (δ in ppm, J in Hz).
DKP 2: Cyclo (D-Pro-D-Tyr), (3R,8aR)-3-(4-hydroxybenzyl)hexahydropyrrolo[1,2-a]pyrazine-1,4-dione: 16 mg; white amorphous powder; Rf 0.32 (60% Benzene in acetone); HPLC retention time: 2.820 min (); melting point: 148.93 °C (); = +209.0° (c = 0.11, EtOH); UV max: 278 nm (MeOH); NMR data (); HRMS [M + H]+ C14H17O3N2 calcd. for m/z 261.12337, found 261.12333 (Supplementary figure 5).
Antimicrobial activity
The data of antimicrobial activity of DKPs are shown in . DKPs recorded considerably good activity against test organisms. DKPs showed significantly higher activity against agriculturally important fungi viz. F. oxysporum, R. solani and P. expansum. The highest activity of 2 µg/ml by DKP 2 was recorded against the plant pathogen F. oxysporum, which causes vascular wilt in plants. DKPs were also found to be very effective against R. solani and significant MIC was recorded by DKP 1 (4 µg/ml).The two DKPs were found to be having superior antifungal activity than the standard fungicide bavistin against F. oxysporum, R. solani and P. expansum. DKP 2 recorded lowest MIC against E. coli (16 µg/ml).
Table 3. MIC of diketopiperazines against test fungi.
Table 4. MIC and MBC of diketopiperazines against test bacteria.
Table 5. Antimicrobial activity of diketopiperazines.
Discussion
CDPs [also known as 2,5-dioxopiperazines; 2,5-diketopiperazines; cyclo(dipeptides); or dipeptide anhydrides] are relatively simple compounds and, therefore, are among the most common peptide derivatives found in nature. CDPs are more than simple curiositiesCitation31 and are well known for their economically beneficial biological activitiesCitation32. CDPs are relatively simple compounds, and therefore are among the most common peptide derivatives found in natureCitation32. They have been isolated from microorganisms, sponges and from a variety of tissues and body fluidsCitation33–35.
However, with a growing awareness of the diversity and biological roles played by many of the diketopiperazines found in nature, interest in these compounds has grownCitation36. Due to their relative simplicityCitation37 and stabilityCitation32, diketopiperazines provide excellent models for theoretical studies as well as the development of pharmaceutical compounds. The linear form of the dipeptide is often less stable in vivo than its cyclic counterpart, therefore making the latter variety far more promising in terms of drug candidacyCitation36. Both natural and synthetic diketopiperazines have a wide variety of biological activities, including antitumorCitation38, antiviralCitation39, antifungalCitation40 and antibacterialCitation41. Especially, 2,5-diketopiperazines recently have attracted attention due to their biological propertiesCitation42. The wide spectrum of their biological properties points to various therapeutic possibilities.
The cyclo(Pro-Tyr) was first isolated from Alternaria alternateCitation35,Citation43. Cyclo(Pro-Tyr) was also isolated from Streptomyces sp. TN256Citation44. Cain et al.Citation44 reported that cyclo(Pro-Tyr) exhibits no activity against strains of Micrococcus luteus, Mycobacterium smegmatis, Sacharomyces cerevisiae, Candida neoformans, Candida albicans and Aspergillus niger. Smaoui et al.Citation45 reported the antimicrobial activity of Cyclo(Pro-Tyr) against Micrococcus luteus LB 14110, S. enterica ATCC43972 and Fusarium sp. Cyclo(Pro-Tyr) demonstrated inhibitory effects against bioluminescence by V. harveyiCitation46.
Cyclo-(L-Pro-L-Met) was first reported from Antarctic sponge-associated bacterium, P. aeruginosaCitation47 and marine-derived actinomycete, Nocardiopsis sp. 03N67Citation48. Nothing is known about the antimicrobial activity of this compound in literatures. To our best knowledge this is the first report of the antimicrobial activity of cyclo(D-Pro-L-Met).
The diketopiperazines are heterocyclic compounds which exist in DD, DL, LD and LL forms. The stereochemistry of the compounds play an important role in biological activity. Li et al.Citation49 reported the production of two stereoisomeric CDPs, cyclo(L-Tyr-L-Pro) and cyclo(L-Tyr-D-Pro) from Lactobacillus reuteri, where cyclo(L-Tyr-L-Pro) is more effective against S. aureus which causes menstruation-associated toxic shock syndrome (TSS) in human.
We report herewith the antimicrobial effects of the CDPs DKPs 1–2 assayed against both medicinally and agriculturally important bacterium and fungi viz. B. subtilis, S. aureus, E. coli, P. aeruginosa, A. flavus, C. albicans, F. oxysporum, R. solani and P. expansum. The results showed that our DKPs exhibit potent inhibitory values in the range of µg/ml. The plant pathogen F. oxysporum, causing fusarium wilting, chlorosis, necrosis, premature leaf drop, browning of the vascular system, stunting and damping-off etc, and R. solani, associated with plant diseases such as collar rot, root rot, damping-off, wire stem etc, P. expansum, which causes post-harvest decay of stored apples and oranges, were strongly inhibited by three DKPs and the activity is superior than the standard antifungal agent bavistin.
Origin of DKPs has been questioned, once several DKPs have been found in fermentation broths and cultures of yeast, as well as in lichens and fungiCitation31. It is known that DKPs can be generated via non-enzymatic cyclization of linear dipeptides at extreme temperaturesCitation50. In our study, such a possibility of formation of DKPs during the fermentation process was ruled out by heat sterilization and incubation of the LB medium without Bacillus sp. N strain since we did not observe any detection of DKPs in the HPLC profiles of the corresponding ethyl acetate extracts.
Conclusion
In conclusion, cyclo(D-Pro-L-Met), cyclo (D-Pro-D-Tyr) and other diketopiperazines are the smallest cyclic peptides, commonly biosynthesized from amino acids by different organisms, including mammals, and are considered to be secondary functional metabolites or side products of terminal peptide cleavage and mainly act as cell signaling molecules. The antifungal activities of the compounds especially against plant pathogenic fungi F. oxysporum, R. solani and P. expansum have never been known. Here, we identified these compounds as a new antifungal agent against plant pathogenic fungi. Antifungal activity of compounds is an encouraging bioprobe to develop new antimicrobial therapeutics from such type of small molecules in near future.
Supplementary materials
Supplementary materials includes mass spectrum of three compounds, HPLC profile of FDAA derivatives of l and d standard amino acids and HRMS of DKPs.
Declaration of interest
The authors are grateful to Indian Council Medical Research (ICMR), Government of India for funding.
Acknowledgements
We thank the Director, CTCRI, for providing facilities for the work.
References
- Gordon TR, Martyn RD. The evolutionary biology of Fusarium oxysporum. Annu Rev Phytopathol 1997;35:111–28
- Brayford D. Fusarium oxysporum f. sp. radicis-lycopersici, IMI descriptions of fungi and bacteria no. 1270. Mycopathol 1996;133:61–3
- Ploetz RC. Population biology of Fusarium oxysporum f. sp. cubense. In: Ploetz RC, ed. Fusarium wilt of banana. St. Paul (MN): APS Press; 1990:63–76
- Albisetti M, Lauener RP, Gungor T, et al. Disseminated Fusarium oxysporum infection in hemophagocytic lymphohistiocytosis. Infection 2004;32:364–6
- Biondi N, Piccardi R, Margheri MC, et al. Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl Environ Microbiol 2004;70:3313–20
- Khan Z, Kim YH, Kim SG, Kim HW. Observation of the suppression of root-knot nematode (Meloigogyne arenaria) on tomato by incorporation of cyanobacteria power (Oscillatoria chlorine) into potting filed soil. Bioresour Technol 2007;98:69–73
- Khalifa EZ, El-Shenawy Z, Awad HM. Biological control of damping-off and root-rot of sugar beet. Egypt J Phytopathol 1995;23:39–51
- Lewis JA, Lumsden RD, Locke JC. Biocontrol of damping-off diseases caused by Rhizoctonia solani and Pythium ultimum with alginate prills of Gliocladium virens, Trichoderma hamatum and various food bases. Biocont Sci Technol 1996;6:163–73
- Sutton TB. Changing options for the control of deciduous fruit tree diseases. Annu Rev Phytopathol 1996;34:527–47
- Burnell AM, Stock SP. Heterorhabditis, Steinernema and their bacterial symbionts – lethal pathogens of insects. Nematol 2000;2:31–42
- Dowds BCA, Peters A. Virulence mechanisms. In: Gaugler R, ed. Entomopathogenic nematology. New York (NY): CABI; 2002:79–98
- Akhurst RJ. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Hettrorhabditidae and Steinernematidae. J Gen Microbiol 1982;128:3061–5
- Chen G, Dunphy GB, Webster JM. Antifungal activity of two Xenorhabdus species and Photorhabdus luminescens, bacteria associated with the nematodes Steinernema species and Heterorhabditis megidis. Biol Cont 1994;4:157–62
- Chen G, Maxwell P, Dunphy GB, Webster JM. Culture conditions for Xenorhabdus and Photorhabdus symbionts of entomopathogenic nematodes. Nematologica 1996;42:124–7
- Paul VJ, Frautschy S, Fenical W, Nealson KH. Antibiotics in microbial ecology, isolation and structure assignment of several new antibacterial compounds from the insect symbiotic bacteria Xenorhabdus spp. J Chem Ecol 1981;7:589–97
- Hu K, Li J, Webster JM. Nematicidal metabolites produced by Photorhabdus luminescens (Enterobacteriaceae), bacterial symbiont of entomopathogenic nematodes. Nematol 1999;1:457–69
- McInerney BV, Gregson RP, Lacey MJ, et al. Biologically active metabolites from Xenorhabdus spp. Part 1. Dithiolopyrrolone derivatives with antibiotic activity. J Nat Prods 1991;54:774–84
- McInerney BV, Taylor WC, Lacey MJ, et al. Biologically active metabolites from Xenorhabdus spp. Part 2. Benzopyran-1-one derivatives with gastroprotective activity. J Nat Prods 1991;54:785–95
- Li JX, Chen GH, Webster JM. Nematophin, a novel antimicrobial substance produced by Xenorhabdus nematophilus (Enterobactereaceae). Canadian J Microbiol 1997;43:770–3
- Ji DJ, Yi YK, Kang GH. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol Lett 2004;239:241–8
- Lang, G, Kalvelage T, Peters A, et al. Peptides from the entomopathogenic bacterium Xenorhabdus nematophilus. J Nat Prods 2008;71:1074–7
- Gualtieri M, Aumelasm A, Thaler JO. Identification of a new antimicrobial lysine-rich cyclolipopeptide family from Xenorhabdus nematophila. J Antibiot 2009;62:295–302
- Deepa I, Mohandas C, Makesh KT, et al. Identification of new entomopathogenic nematodes (EPNs) based on sequences of D2-D3 expansion fragments of the 28SrRNA. J Root Crops 2010;36:227–32
- Mohandas C, Sheeba M, Firoza AJ, Rajamma P. Bacteria associated with Rhabditis (Oscheius) spp. (Rhabditidae: Nematoda) for the biocontrol of insect pests. Proc Nat Seminar on Achievements and Opportunities in Post harvest Management and Value Addition in Root and Tuber Crops (NSRTC – 2);2007:195–8
- Marfey P. Determination of D-amino acids. II. Use of a bifunctional reagents, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Commun 1984;49:591–6
- Rollas S, Kalyoncuoglu N, Sur-Altiner D, Yegenglu Y. 5-(4-Aminophenyl)-4-substituted 2,4-dihydro-3H-1,2,4-triazole-3-thiones: synthesis, antibacterial and antifungal activities. Pharmazie 1993;48:308–9
- Clinical and Laboratory Standards Institute (CLSI). Reference methods for broth dilution antifungal susceptibility tests of yeasts. CLSI documents M27-S3. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA; 2008
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI documents M27-S3. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA; 2006
- Murray PR, Baron EJ, Pfaller MA, et al. Manual of clinical microbiology. Washington (DC): ASM; 1995
- Gomez KA, Gomez AA. Presentation of research results. Statistical Procedures for Agricultural Research. New York: A Wiley-Interscience Publication; 1984
- Krejcarek GE, Dominy BH, Lawton RG. The interaction of reactive functional groups along peptide chains. A model for alkaloid biosynthesis. Chem Commun 1968:1450–2
- Prasad C. Bioactive cyclic dipeptides. Peptides 1995;16:151–64
- Rudi A, Kashman Y, Benayahu Y, Schleyer M. Amino acid derivatives from the marine sponge Jaspis digonoxea. J Nat Prod 1994;57:829–32
- Strom K, Sjogren J, Broberg A, Schnurer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-pro) and 3-phenyllactic acid. Appl Environ Microbiol 2002;68:4322–7
- Rosa SD, Mitova M, Tommonaro G. Marine bacteria associated with sponge as source of cyclic peptides. Biomol Eng 2003;20:311–16
- Lucietto FR, Milne PJ, Kilian G, et al. The biological activity of the histidine-containing diketopiperazines cyclo(His-Ala) and cyclo(His-Gly). Peptides 2006;26:2706–14
- Anteunis MJO. The cyclic dipeptides: proper model compounds in peptide research. Bull Chem Soc Belgium 1978;87:627–50
- Nicholson B, Lloyd GK, Miller BR, et al. NPI-2358 is a tubulin-depolymerizing agent: in-vitro evidence for activity as a tumor vascular-disrupting agent. Anticancer Drugs 2006;7:25–31
- Sinha S, Srivastava R, De Clercq E, Singh RK. Synthesis and antiviral properties of arabino and ribonucleosides of 1,3-dideazaadenine, 4-nitro-1,3-dideazaadenine and diketopiperazine. Nucleos Nucleot Nucl Acids 2004;23:1815–24
- Houston DR, Synstad B, Eijsink VGH, et al. Structure-based exploration of cyclic dipeptide chitinase inhibitors. J Med Chem 2004;47:5713–20
- Kwon OS, Park SH, Yun B, et al. Cyclo(dehydroala-L-Leu), an α- glucosidase inhibitor from Penicillium sp. F70614. J Antibiot 2000;53:954–8
- McCleland K, Milne PJ, Lucietto FR, et al. An investigation into the biological activity of the selected histidine-containing diketopiperazines cyclo(His-Phe) and cyclo(His-Tyr). J Pharm Pharmacol 2004;56:1143–53
- Stierle AC, Cardellina JH, Strobel GA. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternate. Proc Natl Acad Sci USA 1988;85:8008–11
- Cain CC, Dongho L, Robert H, et al. Synergistic antimicrobial activity of metabolites produced by a nonobligate bacterial predator. Antimicrob Agents Chemother 2003;47:2113–17
- Smaoui SF, Mathieu F, Elleuch L, et al. Taxonomy, purification and chemical characterization of four bioactive compounds from new Streptomyces sp. TN256 strain. World J Microbiol Biotechnol 2012;28:793–804
- Teasdale ME, Donovan KA, Forschner-Dancause SR, Rowley DC. Gram-positive marine bacteria as a potential resource for the discovery of quorum sensing inhibitors. Marine Biotechnol 2011;13:722–32
- Jayatilake GS, Thornton MP, Leonard AC, et al. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J Nat Prod 1996;59:293–6
- Shin HJ, Mojid Mondol MA, Yu TK, et al. An angiogenesis inhibitor isolated from a marine-derived actinomycete, Nocardiopsis sp. 03N67. Phytochem Lett 2010;3:194–7
- Li J, Wangb W, Xua SX, et al. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc Natl Acad Sci USA 2011;108:3360–5
- Holden MTG, Chhabra SR, de Nys R, et al. Quorem-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram negative bacteria. Mol Microbiol 1999;33:1254–60