Abstract
Synthesis and cytotoxic activities of 32 benzhydrylpiperazine derivatives with carboxamide and thioamide moieties were reported. In vitro cytotoxic activities of compounds were screened against hepatocellular (HUH-7), breast (MCF-7) and colorectal (HCT-116) cancer cell lines by sulphorhodamine B assay. In general, 4-chlorobenzhydrylpiperazine derivatives were more cytotoxic than other compounds. In addition, thioamide derivatives (6a–g) have higher growth inhibition than their carboxamide analogs.
Introduction
Cancer is the disease resulting from abnormal cells with abilities of uncontrolled dividing and invasion to other tissues through blood and lymph systems. Recently advanced treatment opportunities are unable to overcome the major problems of chemotherapy such as drug resistance and severe side effects due to the lack of specificity. Regarding issues lead the researchers to develop varying drug-like compounds targeting cancer.
Piperazine-1-carboxamides have diverse actions such as antagonism of CB1, human CCR2 chemokine, androgen and vanilloid receptors or inhibition of PDGFR phosphorylationCitation1–5.
Benzhydrylpiperazine scaffold is well known for its antihistaminic activityCitation6–11. Furthermore calcium channel blockingCitation12–19, dopaminergicCitation20–23, antimicrobialCitation24–41 and antiviralCitation42,Citation43 activities are often mentioned in literature.
Anticancer activity of benzhydrylpiperazines has recently advancedCitation44–51. Kumar et al. have performed cytotoxicity assays to several 1-benzhydrylpiperazine derivatives substituted with variable sulfonyl chlorides, acid chlorides and isothiocyanates. These derivatives have potent cytotoxicity over breast cancer (MCF-7), hepatocellular (HepG-2), cervix (HeLa) and colon carcinoma (HT-29) cell linesCitation44. Yarim et al., also performed cytotoxicity screenings for some 4-chlorobenzhydrylpiperazines substituted with variable benzoyl chloride derivatives and reported their high activities against liver (HUH-7, FOCUS, MAHLAVU, HepG-2, Hep-3B), breast (MCF-7, BT20, T47D, CAMA-1), colon (HCT-116), gastric (KATO-3) and endometrial (MFE-296) cancer cell linesCitation45. In addition, our work group has recently reported a study in which sulfonamide and benzamide derivatives of benzhydrylpiperazines were discussed for their cytotoxicities against HUH-7, MCF-7 and HCT-116 cancer cell linesCitation52.
In this study, we reported the synthesis, purification and characterization of some novel compounds bearing benzhydrylpiperazine backbone. Those compounds were tested for their cytotoxic activities against hepatocellular (HUH-7), breast (MCF-7) and colorectal (HCT-116) cancer cell lines with sulphorhodamine B (SRB) assay. We aimed to develop a structure activity relationship for benzhydrylpiperazine derivatives in accordance with their cytotoxic activity results.
Materials and methods
Chemistry
All chemicals and reagents used in the current study were of analytical grade. The reactions were monitored by thin layer chromatography (TLC) on Merck pre-coated silica GF254 plates (Merck KGaA, Darmstadt, Germany). Melting points (°C) of the compounds were determined by using a Mettler Toledo FP62 capillary melting point apparatus (Mettler-Toledo, Greifensee, Switzerland) and are uncorrected. Ultraviolet spectra were recorded with Agilent 8453 UV-Visible Spectrophotometer (Agilent Technologies, Santa Clara, CA). Infrared spectra were recorded on a Perkin-Elmer Spectrum One series FT-IR apparatus (Version 5.0.1, Perkin Elmer, Norwalk, CT), using potassium bromide pellets, the frequencies were expressed in cm−1. The 1H- and 13C-NMR spectra were recorded with a Varian Mercury-400 FT-NMR spectrometer (Varian Inc., Palo Alto, CA), using tetramethylsilane (TMS) as the internal reference, with dimethylsulfoxide (DMSO-d6) as solvent, the chemical shifts were reported in parts per million (ppm). Coupling constants were recorded in Hertz (Hz). The mass spectra were recorded with a Waters 2695 Alliance Micromass ZQ LC/MS instrument (Waters Corp., Milford, MA). Elemental analyses were performed on LECO 932 CHNS (LECO-932, St. Joseph, MI) instrument and were within ±0.4% of the theoretical values.
General procedure for preparation of benzhydrole derivatives
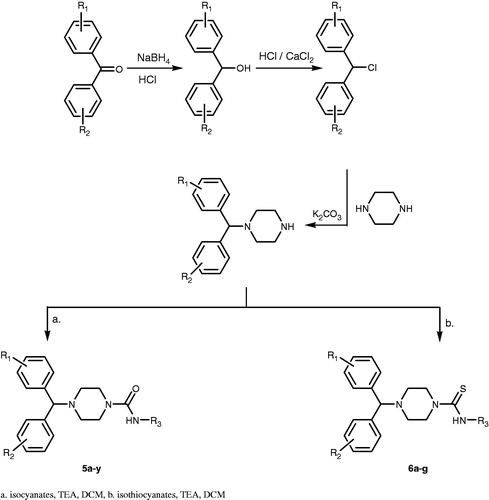
Ten millimoles (2.2 g) of benzophenone was dissolved in 10 ml of ethanol. In a separate flask, 11 mmol (0.4 g) of sodium borohydride (NaBH4) was dissolved in 2 ml of ethanol. Sodium borohydride solution was slowly added to benzophenone solution with a Pasteur pipette. Reaction mixture was allowed to continue stirring for a further 30 min. For the work up of reaction, 2 ml of concentrated HCl was added to a 20 ml ice-water solution. Reaction mixture was poured into this ice cold solution slowly with stirring. White solid product was collected with vacuum filtration and washed twice with distilled water. 4-Chlorobenzophenone and 4,4′-difluorobenzophenone were also reacted with sodium borohydride to give 4-chlorobenzhydrole and 4,4′-difluorobenzhydrole, respectively, according to above procedure.
General procedure for preparation of benzhydryl chloride derivatives
Ten millimoles (1.84 g) of benzhydrole was added to 15 ml of concentrated HCl. 10 mmol (1.1 g) of anhydrous calcium chloride was added to the mixture to be refluxed at 85 °C for 4 h with stirring. After the reaction is completed, the flask was cooled to room temperature and extracted twice with 20 ml of ethyl acetate. Organic layers were combined together, washed with brine and water, then dried over anhydrous sodium sulfate. Followed by the concentration under vacuo, the product was collected as brown liquid. 4-Chlorobenzhydryl chloride and 4,4′-difluorobenzhydryl chloride were also synthesized from 4-chlorobenzhydrole and 4,4′-difluorobenzhydrole according to above procedure.
General procedure for preparation of benzhydrylpiperazine derivatives
Nine millimoles (0.78 g) of piperazine was dissolved in dimethylformamide. Anhydrous potassium carbonate was added to the solution and stirred for 10 min. Followed by the addition of 9 mmol (1.82 g) of benzhydryl chloride, reaction mixture was heated at 80 °C for 8 h. After completion, dimethylformamide was removed under vacuo, then the residue was taken in water and extracted with ethyl acetate. Organic layer was washed with water and dried over anhydrous sodium sulfate. The solvent was evaporated and white solid product was obtained. 1-[(4-Chlorophenyl)(phenyl)methyl]piperazine and 4,4′-benzhydrylpiperazine were also synthesized from 4-chlorobenzhydryl chloride and 4,4′-difluorobenzhydryl chloride consecutively according to above procedure.
General procedure for preparation of N-alkyl-4-[benzhydryl/4-chlorobenzhydryl/4,4′-difluorobenzhydryl]piperazine-1-carboxamides
Two millimoles (0.515 g) of 1-benzhydrylpiperazine or 1.7 mmol (0.515 g) of 1-(4,4′-difluorobenzhydryl)piperazine or 0.872 mmol (0.2632 g) of 1-(4-chlorobenzhydryl)piperazine was dissolved in 20 mL of dry dichloromethane. Reaction flask was taken into ice bath and triethylamine (1:3 moles) was added to the solution. Ice bath was removed after 10 min and appropriate isocyanate derivative (1:1 mole) was added. Reaction was mixed overnight at room temperature. After the reaction is completed, solution was extracted with water and ammonium chloride solution (10%), respectively. Dichloromethane layer was washed with water again and dried with anhydrous sodium sulfate. Solvent was evaporated under vacuo and solid product was recrystallized with ethanol/water.
N-sec-Butyl-4-(diphenylmethyl)piperazine-1-carboxamide (5a, CAS No: 1071382-92-7)
White, opaque, needle-shaped crystals, 68% (0.240 g), m.p. 198.4 °C. UV (MeOH, λmax, nm); 205 (log ε: 5.17), 224 (log ε: 4.69). FT-IR (KBr, cm−1); 3342 (N–H), 3022 (C–H, aromatic), 2959 (C–H, aliphatic), 1619 (C=O, amide), 1540 (C=C, aromatic), 1246 (C–N). 1H-NMR (DMSO, ppm); 0.78 (t, 3H, –CH2–CH3, J = 7.6 Hz); 0.98 (d, 3H, –CH–CH3, J = 6.8 Hz); 1.35 (m, 2H, –CH2–CH3); 2.23 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.28 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.53 (m, 1H, –NH–CH–); 4.29 (s, 1H, (Ar)2CH–); 6.02 (d, 1H, –CONH–, J = 7.6 Hz); 7.20 (m, 2H, diphenyl H4, H4′); 7.30 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 7.6 Hz); 7.43 (t, 4H, diphenyl H2, H6, H2′, H6′, J = 7.2 Hz). 13C-NMR (DMSO, ppm); 11.43 (C21); 21.45 (C22); 29.90 (C20); 44.25 (C14,16); 47.82 (C19); 52.06 (C15,17); 75.59 (C7); 127.56 (C4,11); 128.29 (C2,6,9,13); 129.20 (C3,5,10,12); 143.30 (C1,8); 157.76 (C18). MS (m/z); 352.8 (M+); 253.7 ((C6H5)2CHN(C2H4)2NH⌉+); 167.5 ((C6H5)2CH⌉+). Elemental analysis of C22H29N3O (MW: 351.49 g/mol); C 75.18, H 8.32, N 11.96 (Calcd.); C 75.12, H 8.27, N 11.85 (Found).
N-tert-Butyl-4-(diphenylmethyl)piperazine-1-carboxamide (5b)
White, opaque, needle-shaped crystals, 62% (0.436 g), m.p. 192.4 °C. UV (MeOH, λmax, nm); 206 (log ε: 5.13), 227 (log ε: 4.62). FT-IR (KBr, cm−1); 3322 (N–H), 3023 (C–H, aromatic), 2970 (C–H, aliphatic), 1621 (C=O, amide), 1536 (C=C, aromatic), 1260 (C–N). 1H-NMR (DMSO, ppm); 1.22 (s, 9H, –C(CH3)3); 2.23 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.25 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 4.29 (s, 1H, (Ar)2CH–); 5.68 (s, 1H, CONH); 7.19 (m, 2H, diphenyl H4, H4′); 7.30 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 7.6 Hz); 7.43 (t, 4H, diphenyl H2, H6, H2′, H6′, J = 7.2 Hz). Elemental analysis of C22H29N3O (MW: 351.49 g/mol); C 75.18, H 8.32, N 11.96 (Calcd.); C 74.60, H 8.21, N 11.84 (Found).
N-Isopropyl-4-(diphenylmethyl)piperazine-1-carboxamide (5c)
White, opaque, clustered crystals, 94% (0.318 g), m.p. 220.4 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.21), 227 (log ε: 4.81). FT-IR (KBr, cm−1); 3367 (N–H), 3060 (C–H, aromatic), 2964 (C–H, aliphatic), 1611 (C=O, amide), 1538 (C=C, aromatic), 1254 (C–N). 1H-NMR (DMSO, ppm); 0.98 (d, 6H, –CH(CH3)2, J = 6.8 Hz); 2.19 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.25 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 3.68 (m, 1H, –CH(CH3)2); 4.25 (s, 1H, (Ar)2CH–); 6.05 (d, 1H, CONH, J = 7.6 Hz); 7.15 (m, 2H, diphenyl H4, H4′); 7.26 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 7.2 Hz); 7.39 (t, 4H, diphenyl H2, H6, H2′, H6′, J = 6.8 Hz). Elemental analysis of C21H27N3O (MW: 337.46 g/mol); C 74.74, H 8.06, N 12.45 (Calcd.); C 74.89, H 7.73, N 12.30 (Found).
N-Ethyl-4-(diphenylmethyl)piperazine-1-carboxamide (5d)
White, shiny, flat crystals, 84% (0.294 g), m.p. 208.9 °C. UV (MeOH, λmax, nm); 203 (log ε: 5.11), 221 (log ε: 4.58). FT-IR (KBr, cm−1); 3365 (N–H), 3024 (C–H, aromatic), 2978 (C–H, aliphatic), 1622 (C=O, amide), 1545 (C=C, aromatic), 1259 (C–N).1H-NMR (DMSO, ppm); 0.98 (t, 3H, –CH3, J = 7.6 Hz); 2.23 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.01 (m, 2H, –CH2–); 3.28 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 4.28 (s, 1H, (Ar)2CH–); 6.41 (t, 1H, CONH, J = 5.2 Hz); 7.20 (m, 2H, diphenyl H4, H4′); 7.29 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 8 Hz); 7.43 (t, 4H, diphenyl H2, H6, H2′, H6′, J = 7.2 Hz). Elemental analysis of C22H29N3O (MW: 351.49 g/mol); C 74.27, H 7.79, N 12.99 (Calcd.); C 73.77, H 7.46, N 12.93 (Found).
N-(2,6-Dichlorophenyl)-4-(diphenylmethyl)piperazine-1-carboxamide (5e)
White, opaque, powdered crystals, 88% (0.386 g), m.p. 234.6 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.32), 226 (log ε: 4.72). FT-IR (KBr, cm−1); 3237 (N–H,), 3025 (C–H, aromatic), 2967 (C–H, aliphatic), 1638 (C=O, amide), 1528 (C=C, aromatic), 1255 (C–N). 1H-NMR (DMSO, ppm); 2.29 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.44 (t, 4H, piperazine H2, H6, J = 4 Hz); 4.33 (s, 1H, (Ar)2CH–); 7.15–7.3 (m, 10H, diphenyl); 7.40–7.47 (m, 3H, 2,6-dichlorophenyl); 8.34 (s, 1H, CONH). Elemental analysis of C24H23Cl2N3O (MW: 440.36 g/mol); C 65.46, H 5.26, N 9.54 (Calcd.); C 65.37, H 5.36, N 9.62 (Found).
N-(2-Benzylphenyl)-4-(diphenylmethyl)piperazine-1-carboxamide (5f)
White, opaque, feather-like crystals, 89% (0.412 g), m.p. 192.1 °C. UV (MeOH, λmax, nm); 203 (log ε: 5.12), 224 (log ε: 4.26). FT-IR (KBr, cm−1); 3251 (N–H), 3060 (C–H, aromatic), 2954 (C–H, aliphatic), 1637 (C=O, amide), 1524 (C=C, aromatic), 1253 (C–N). 1H-NMR (DMSO, ppm); 2.24 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.37 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.91 (s, 2H, –CH2–); 4.29 (s, 1H, (Ar)2CH–); 7.05–7.25 (m, 10H, diphenyl); 7.30 (m, 5H, phenyl); 7.44 (m, 4H, N-phenyl); 7.97 (s, 1H, CONH). Elemental analysis of C31H31N3O (MW: 461.60 g/mol); C 80.66, H 6.77, N 9.10 (Calcd.); C 80.90, H 6.48, N 9.13 (Found).
Ethyl 2-[4-(diphenylmethyl)piperazino]carbamoyl]acetate (5g, CAS No: 1350123-57-7)
White, opaque, powdered crystals, 69% (0.263 g), m.p. 150 °C. UV (MeOH, λmax, nm); 202 (log ε: 4.87), 223 (log ε: 4.35). FT-IR (KBr, cm−1); 3360 (N–H), 3026 (C–H, aromatic), 2986 (C–H, aliphatic), 1755 (C=O, ester), 1636 (C=O, amide), 1531 (C=C, aromatic), 1192 (C–O), 1147 (C–N). 1H-NMR (DMSO, ppm); 1.17 (t, 3H, –CH2–CH3, J = 6.8 Hz); 2.25 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.31 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.68 (d, 2H, –NH–CH2–, J = 5.6 Hz); 4.05 (q, 2H, –O–CH2–); 4.30 (s, 1H, (Ar)2CH–); 6.93 (t, 1H, CONH, J = 6 Hz); 7.19 (t, 2H, diphenyl H4, H4′, J = 7.2 Hz); 7.29 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 7.2 Hz); 7.44 (d, 4H, diphenyl H2, H6, H2′, H6′, J = 7.6 Hz). Elemental analysis of C22H27N3O3 (MW: 381.47 g/mol); C 69.27, H 7.13, N 11.02 (Calcd.); C 69.24, H 6.96, N 10.96 (Found).
N-Allyl-4-(diphenylmethyl)piperazine-1-carboxamide (5h, CAS No: 1349487-56-4)
White, shiny, flat crystals, 96% (0.323 g), m.p. 213.6 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.32), 226 (log ε: 4.75). FT-IR (KBr, cm−1); 3343 (N–H), 3027 (C–H, aromatic), 2954 (C–H, aliphatic), 1625 (C=O, amide), 1546 (C=C, aromatic), 1255 (C–N). 1H-NMR (DMSO, ppm); 2.23 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.30 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.63 (t, 2H, –CH2–, J = 5.2 Hz); 4.29 (s, 1H, (Ar)2CH-); 5.0 (dd, 2H, –CH=CH2, J1=17.2 Hz, J2 = 8 Hz, J3 = 1.6 Hz); 5.78 (m, 1H, –CH=CH2); 6.61 (t, 1H, CONH, J = 5.2 Hz); 7.17 (t, 2H, diphenyl H4, H4′, J = 7.6 Hz); 7.29 (t, 4H, diphenyl H3, H5, H3′, H5′, J = 7.6 Hz); 7.43 (d, 4H, diphenyl H2, H6, H2′, H6′, J = 8.8 Hz). Elemental analysis of C21H25N3O (MW: 335.44 g/mol); C 75.19, H 7.51, N 12.53 (Calcd.); C 75.09, H 7.25, N 12.46 (Found).
N-sec-Butyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5i)
White, opaque, powdered crystals, 54% (0.208 g), m.p. 157.7 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.24), 225 (log ε: 4.71). FT-IR (KBr, cm−1); 3310 (N–H), 3076 (C–H, aromatic), 2965 (C–H, aliphatic), 1615 (C=O, amide), 1548 (C=C, aromatic), 1247 (C–N), 1223 (C–F). 1H-NMR (DMSO, ppm); 0.8 (t, 3H, –CH2CH3, J = 7.2 Hz); 0.98 (d, 3H, –CH–CH3, J = 6.8 Hz); 2.24 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 2.5 (m, 2H, –CH–CH2–CH3); 3.28 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.54 (m, 1H, –NH–CH–); 4.38 (s, 1H, (Ar)2CH–); 6.04 (d, 1H, CONH, J = 7.6 Hz); 7.10–7.16 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.41–7.45 (m, 4H, diphenyl H3, H5, H3′, H5′). MS (m/z); 388.95 (M+); 290.00 ((4-F-C6H5)2CH[N(C2H4)2N]H⌉+); 203.5 (100%, (4-F-C6H5)2CH⌉+). Elemental analysis of C22H27F2N3O (MW: 387.46 g/mol); C 68.20, H 7.02, N 10.84 (Calcd.); C 67.44, H 7.01, N 10.89 (Found).
N-tert-Butyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5j)
White, opaque, feather-like crystals, 82% (0.317 g), m.p. 162.4 °C. UV (MeOH, λmax, nm); 208 (log ε: 5.32), 227 (log ε: 4.78). FT-IR (KBr, cm−1); 3332 (N–H), 3046 (C–H, aromatic), 2968 (C–H, aliphatic), 1623 (C=O, amide), 1537 (C=C, aromatic), 1259 (C–N), 1219 (C–F). 1H-NMR (DMSO, ppm); 1.22 (s, 9H, C(CH3)3); 2.20 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.24 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.38 (s, 1H, (Ar)2CH–); 5.68 (s, 1H, CONH); 7.10–7.16 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.41–7.45 (m, 4H, diphenyl H3, H5, H3′, H5′). MS (m/z); 388.88 (100%, M+); 203.51 ((4-F-C6H5)2CH⌉+). Elemental analysis of C22H27F2N3O (MW: 387.46 g/mol); C 68.20, H 7.02, N 10.84 (Calcd.); C 67.96, H 7.32, N 10.87 (Found).
N-Butyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5k)
White, opaque, flat crystals, 45% (0.174 g), m.p. 132.9 °C. UV (MeOH, λmax, nm); 209 (log ε: 5.43), 226 (log ε: 4.83). FT-IR (KBr, cm−1); 3402 (N–H), 3073 (C–H, aromatic), 2962 (C–H, aliphatic), 1629 (C=O, amide), 1531 (C=C, aromatic), 1251 (C–N), 1217 (C–F). 1H-NMR (DMSO, ppm); 0.85 (t, 3H, –CH3, J = 7.2 Hz) 1.20-1.27 (m, 2H, –CH2–CH3); 1.31–1.37 (m, 4H, –CH2CH2CH3–); 2.21 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 2.95–3.06 (q, 2H, –NH–CH2–); 3.27 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 4.38 (s, 1H, (Ar)2CH–); 6.38 (t, 1H, CONH); 7.1–7.15 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.41–7.45 (m, 4H, diphenyl H3, H5, H3′, H5′). MS (m/z); 388.93 (100%, M+); 203.55 ((4-F-C6H5)2CH⌉+). Elemental analysis of C22H27F2N3O (MW: 387.46 g/mol); C 68.20, H 7.02, N 10.84 (Calcd.); C 67.92, H 6.82, N 10.85 (Found).
N-Ethyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5l)
White, opaque, cotton-like crystals, 83% (0.297 g), m.p. 175 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.39), 225 (log ε: 4.81). FT-IR (KBr, cm−1); 3349 (N–H), 3060 (C–H, aromatic), 2972 (C–H, aliphatic), 1617 (C=O, amide), 1544 (C=C, aromatic), 1253 (C–N), 1216 (C–F). 1H-NMR (DMSO, ppm); 0.98 (t, 3H, –CH3, J = 6.8 Hz); 2.21 (t, 4H, piperazine H3, H5, J = 4 Hz); 3.05–2.98 (m, 2H, –CH2–); 3.28 (t, 4H, piperazine H2, H6, J = 4 Hz); 4.38 (s, 1H, (Ar)2CH–); 6.42 (t, 1H, CONH, J = 5.2 Hz); 7.11–7.15 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.42–7.45 (m, 4H, diphenyl H3, H5, H3′, H5′). 13C-NMR (DMSO, ppm); 16.26 (C20); 35.44 (C19); 44.03 (C14,16); 51.84 (C15,17); 73.48 (C7); 115.91–116.12 (C3,5,10,12); 130.03–130.12 (C2,6,9,13); 139.20, 139.17 (C1,8); 157.96–160.52 (C4,11); 162.95 (C18). MS (m/z); 360.85 (M+); 203.53 (100%, (4-F-C6H5)2CH⌉+). Elemental analysis of C20H23F2N3O (MW: 359.41 g/mol); C 66.84, H 6.45, N 11.69 (Calcd.); C 66.44, H 6.28, N 11.68 (Found).
N-Isopropyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5m)
White, opaque, powdered crystals, 92% (0.345 g), m.p. 169.9 °C. UV (MeOH, λmax, nm); 205 (log ε: 5.25), 223 (log ε: 4.47). FT-IR (KBr, cm−1); 3331 (N–H), 3074 (C–H, aromatic), 2976 (C–H, aliphatic), 1615 (C=O, amide), 1547 (C=C, aromatic), 1252 (C–N), 1215 (C–F). 1H-NMR (DMSO, ppm); 1.01 (d, 6H, –CH(CH3)2, J = 6.8 Hz); 2.21 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.28 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 3.68–3.76 (m, 1H, –CH(CH3)2); 4.38 (s, 1H, (Ar)2CH–); 6.10 (d, 1H, CONH, J = 7.6 Hz); 7.11–7.15 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.42–7.45 (m, 4H, diphenyl H3, H5, H3′, H5′). MS (m/z); 374.87 (M+); 289.72 ((4-F-C6H5)2CHN(C2H4)2NH⌉+); 203.54 (100%, (4-F-C6H5)2CH⌉+). Elemental analysis of C21H25F2N3O (MW: 373.44 g/mol); C 67.54, H 6.75, N 11.25 (Calcd.); C 67.87, H 6.64, N 11.20 (Found).
Ethyl 2-[bis(4-fluorophenyl)methyl]piperazino]carbamoylacetate (5n)
White, opaque, powdered crystals, 20% (0.08 g), m.p. 152.3 °C. UV (MeOH, λmax, nm); 203 (log ε: 4.89), 221 (log ε: 4.29). FT-IR (KBr, cm−1); 3359 (N–H), 3070 (C–H, aromatic), 2978 (C–H, aliphatic), 1748 (C=O, ester), 1640 (C=O, amide), 1602 (C=C, aromatic), 1224 (C–O), 1198 (C–N), 1153 (C–F). 1H-NMR (DMSO, ppm); 1.17 (t, 3H, –CH3, J = 7.2 Hz); 2.23 (t, 4H, piperazine H3, H5, J = 5.2 Hz); 3.11 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.68 (d, 2H, –NH–CH2–, J = 6 Hz); 4.03–4.08 (q, 2H, –O–CH2–); 4.39 (s, 1H, (Ar)2CH–); 6.93 (t, 1H, CONH, J = 6 Hz); 7.11–7.16 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.42–7.46 (m, 4H, diphenyl H3, H5, H3′, H5′). Elemental analysis of C22H25F2N3O3 (MW: 417.45 g/mol); C 63.30, H 6.04, N 10.07 (Calcd.); C 63.46, H 6.05, N 10.02 (Found).
N-(4-Bromophenyl)-4-[bis(4-fluorophenyl)methyl]piperazine-1-carboxamide (5o)
White, opaque, powdered crystals, 67% (0.325 g), m.p. 210.9 °C. UV (MeOH, λmax, nm); 202 (log ε: 4.31), 237 (log ε: 4.15) , 246 (log ε: 4.12). FT-IR (KBr, cm−1); 3290 (N–H), 3044 (C–H, aromatic), 2999 (C–H, aliphatic), 1646 (C=O, amide), 1506 (C=C, aromatic), 1246 (C–N), 1224 (C–F). 1H-NMR (DMSO, ppm); 2.29 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.45 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.44 (s, 1H, (Ar)2CH–); 7.12–7.47 (m, 12H, aromatic H’s); 8.61 (s, 1H, CONH). Elemental analysis of C24H23BrClN3O (MW: 484.82 g/mol); C 59.27, H 4.56, N 8.64 (Calcd.); C 59.02, H 4.38, N 8.73 (Found).
N-sec-Butyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5p)
White, shiny, clustered crystals, 62% (0.240 g), m.p. above 300 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.32), 226 (log ε: 4.51). FT-IR (KBr, cm−1); 3393 (N–H), 3027 (C–H, aromatic), 2970 (C–H, aliphatic), 1618 (C=O, amide), 1533 (C=C, aromatic), 1246 (C–N), 1091 (C–Cl). 1H-NMR (DMSO, ppm); 0.78 (t, 3H, –CH2–CH3, J = 7.6 Hz); 1.00 (d, 3H, –CH–CH3, J = 6.8 Hz); 1.32–1.40 (m, 2H, –CH–CH2–CH3); 2.22 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.28 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 3.51–3.55 (m, 1H, –NHCH); 4.35 (s, 1H, (Ar)2CH–); 6.03 (d, 1H, CONH, J = 8 Hz); 7.18–7.46 (m, 9H, diphenyl). Elemental analysis of C22H28ClN3O (MW: 385.93 g/mol); C 68.47, H 7.31, N 10.89 (Calcd.); C 68.65, H 7.20, N 10.93 (Found).
N-tert-Butyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5q)
White, shiny, flat crystals, 36% (0.137 g), m.p .190.3 °C. UV (MeOH, λmax, nm); 207 (log ε: 5.29), 225 (log ε: 4.52). FT-IR (KBr, cm−1); 3371 (N–H), 3027 (C–H, aromatic), 2968 (C–H, aliphatic), 1629 (C=O, amide), 1538 (C=C, aromatic), 1257 (C–N), 1092 (C–Cl). 1H-NMR (DMSO, ppm); 1.19 (s, 9H, –C(CH3)3); 2.19 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.21 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.31 (s, 1H, (Ar)2CH–); 5.65 (s, 1H, CONH); 7.17–7.42 (m, 9H, diphenyl). Elemental analysis of C22H28ClN3O (MW: 385.93 g/mol); C 68.47, H 7.31, N 10.89 (Calcd.); C 68.67, H 7.23, N 10.93 (Found).
N-Ethyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5r)
White, shiny, clustered crystals, 17% (0.06 g), m.p. 288.6 °C. UV (MeOH, λmax, nm); 205 (log ε: 5.24), 224 (log ε: 4.46). FT-IR (KBr, cm−1); 3363 (N–H), 3020 (C–H, aromatic), 2970 (C–H, aliphatic), 1620 (C=O, amide), 1539 (C=C, aromatic), 1254 (C–N), 1090 (C–Cl). 1H-NMR (DMSO, ppm); 0.98 (t, 3H, –CH3, J = 7.2 Hz); 2.22 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.00–3.03 (m, 2H, –CH2–); 3.27 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 4.34 (s, 1H, (Ar)2CH–); 6.41 (t, 1H, CONH, J = 5.6 Hz); 7.18–7.46 (m, 9H, diphenyl). Elemental analysis of C20H24ClN3O (MW: 357.88 g/mol); C 67.12, H 6.76, N 11.74 (Calcd.); C 67.22, H 6.69, N 11.79 (Found).
N-Isopropyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5s)
White, shiny, flat crystals, 34% (0.128 g), m.p. 198.6 °C. UV (MeOH, λmax, nm); 205 (log ε: 5.15), 223 (log ε: 4.45). FT-IR (KBr, cm−1); 3390 (N–H), 3020 (C–H, aromatic), 2969 (C–H, aliphatic), 1617 (C=O, amide), 1532 (C=C, aromatic), 1252 (C–N), 1092 (C–Cl). 1H-NMR (DMSO, ppm); 1.01 (d, 6H, –CH(CH3)2, J = 6.8 Hz); 2.22 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 3.27 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 3.68–3.75 (m, 1H, –CH(CH3)2); 4.34 (s, 1H, (Ar)2CH–); 6.08 (d, 1H, CONH, J = 7.6 Hz); 7.18–7.46 (m, 9H, diphenyl). Elemental analysis of C21H26ClN3O (MW: 371,9 g/mol); C 67.82, H 7.05, N 11.30 (Calcd.); C 67.88, H 7.11, N 11.35 (Found).
N-Allyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5t)
White, opaque, powdered crystals, 27% (0.1 g), m.p. 172.7 °C. UV (MeOH, λmax, nm); 204 (log ε: 5.11), 225 (log ε: 4.38). FT-IR (KBr, cm−1); 3356 (N–H), 3027 (C–H, aromatic), 2981 (C–H, aliphatic), 1622 (C=O, amide), 1543 (C=C, aromatic), 1252 (C–N), 1094 (C–Cl). 1H-NMR (DMSO, ppm); 2.23 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.30 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 3.63 (t, 2H, NH–CH2–CH=, J = 4.8 Hz); 4.34 (s, 1H, (Ar)2CH–); 4.97–5.08 (dd, 2H, –CH=CH2, J1 = 16 Hz, J2 = 10 Hz, J3 = 1.6 Hz); 5.75–5.82 (m, 1H, –CH=CH2); 6.62 (t, 1H, CONH); 7.18–7.46 (m, 9H, diphenyl). Elemental analysis of C21H24ClN3O (MW: 371.9 g/mol); C 68.19, H 6.54, N 11.36 (Calcd.); C 68.52, H 6.43, N 11.43 (Found).
N-(2,6-Dichlorophenyl)-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5u)
White, shiny, powdered crystals, 38% (0.178 g), m.p. 224.6 °C. UV (MeOH, λmax, nm); 205 (log ε: 4.47), 245 (log ε: 4.12). FT-IR (KBr, cm−1); 3316 (N–H), 3020 (C–H, aromatic), 2963 (C–H, aliphatic), 1645 (C=O, amide), 1519 (C=C, aromatic), 1254 (C–N), 1089 (C–Cl). 1H-NMR (DMSO, ppm); 2.31 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.46 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 4.41 (s, 1H, (Ar)2CH–); 7.21–7.49 (m, 12H, aromatic H’s); 8.37 (s, 1H, CONH). Elemental analysis of C24H22Cl3N3O (MW: 474.81 g/mol); C 60.71, H 4.67, N 8.85 (Calcd.); C 60.70, H 4.77, N 9.18 (Found).
N-(2-Phenylethyl)-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5v)
White, opaque, feather-like crystals, 49% (0.212 g), m.p. 147.8 °C. UV (MeOH, λmax, nm); 205 (log ε: 4.52), 245 (log ε: 4.07). FT-IR (KBr, cm−1); 3307 (N–H), 3022 (C–H, aromatic), 2955 (C–H, aliphatic), 1617 (C=O, amide), 1543 (C=C, aromatic), 1256 (C–N), 1091 (C–Cl). 1H-NMR (DMSO, ppm); 2.22 (t, 4H, piperazine H3, H5, J = 4.4 Hz); 2.69 (t, 2H, –CH2–C6H5, J = 6.8 Hz); 3.19 (q, 2H, –NHCH2); 3.28 (t, 4H, piperazine H2, H6, J = 5.2 Hz); 4.34 (s, 1H, (Ar)2CH–); 6.55 (t, 1H, CONH, J = 5.6 Hz); 7.15–7.46 (m, 14H, aromatic H’s). Elemental analysis of C26H28ClN3O (MW: 433.97 g/mol); C 71.96, H 6.50, N 9.68 (Calcd.); C 72.04, H 6.72, N 9.70 (Found).
N-(4-Bromophenyl)-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5w)
White, opaque, feather-like crystals, 37% (0.180 g), m.p. 195.5 °C. UV (MeOH, λmax, nm); 203 (log ε: 4.33), 236 (log ε: 4.11). FT-IR (KBr, cm−1); 3316 (N–H), 3028 (C–H, aromatic), 2966 (C–H, aliphatic), 1634 (C=O, amide), 1537 (C=C, aromatic), 1243 (C–N), 1089 (C–Cl). 1H-NMR (DMSO, ppm); 2.30 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.45 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.39 (s, 1H, (Ar)2CH–); 7.21–7.47 (m, 13H, aromatic H’s); 8.6 (s, 1H, CONH). Elemental analysis of C24H23BrClN3O (MW: 484.82 g/mol); C 59.46, H 4.78, N 8.67 (Calcd.); C 59.43, H 4.97, N 8.84 (Found).
N-(2-Benzylphenyl)-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5x)
White, shiny, needle-shaped crystals, 44% (0.219 g), m.p. 174.6 °C. UV (MeOH, λmax, nm); 205 (log ε: 4.57), 224 (log ε: 4.21). FT-IR (KBr, cm−1); 3332 (N–H), 3026 (C–H, aromatic), 2967 (C–H, aliphatic), 1626 (C=O, amide), 1523 (C=C, aromatic), 1251 (C–N), 1089 (C–Cl). 1H-NMR (DMSO, ppm); 2.23 (bs, 4H, piperazine H3, H5); 3.36 (bs, 4H, piperazine H2, H6); 3.91 (s, 2H, –CH2–); 4.34 (s, 1H, (Ar)2CH–); 7.05–7.47 (m, 18H, aromatic H’s); 7.96 (s, 1H, CONH). 13C-NMR (DMSO, ppm); 37.74 (C25); 44.46 (C14,16); 51.91 (C15,17); 74.48 (C7); 125.46 (C20); 126.52 (C22); 126.97 (C29); 127.23 (C21); 127.79 (C27,31); 128.31 (C11); 128.91 (C9,13); 129.23 (C23); 129.33 (C28,30); 129.42 (C10,12); 130.11 (C3,5); 130.64 (C2,6); 132.09 (C4); 136.87 (C19); 138.23 (C1); 141.05 (C26); 142.26 (C8); 142.64 (C24); 156.06 (C18). MS (m/z); 496.9 (M+, 100%); 498.9 (M + 2, 33%); 287.8 ((4-Cl-C6H5)(C6H5)CH-N(C2H4)2N⌉+); 201.6 ((4-Cl-C6H5)(C6H5)CH⌉+). Elemental analysis of C31H30ClN3O (MW: 496.04 g/mol); C 75.06, H 6.10, N 8.47 (Calcd.); C 75.13, H 6.28, N 8.54 (Found).
N-(4-Cyanophenyl)-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carboxamide (5y)
White, shiny, powdered crystals, 26% (0.114 g), m.p. 196.8 °C. UV (MeOH, λmax, nm); 202 (log ε: 4.82), 270 (log ε: 4.59). FT-IR (KBr, cm−1); 3278 (N–H), 3027 (C–H, aromatic), 2951 (C–H, aliphatic), 2221 (C≡N), 1653 (C=O, amide), 1513 (C=C, aromatic), 1245 (C–N), 1089 (C–Cl). 1H-NMR (DMSO, ppm); 2.32 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.48 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.41 (s, 1H, (Ar)2CH–); 7.21–7.47 (m, 9H, diphenyl); 7.61–7.67 (m, 4H, 4-cyanophenyl); 8.97 (s, 1H, CONH). Elemental analysis of C25H23ClN4O (MW: 430.93 g/mol); C 69.68, H 5.38, N 13.00 (Calcd.); C 69.74, H 5.50, N 13.06 (Found).
General procedure for preparation of N-Alkyl-4-[4-chlorobenzhydryl/4,4′-difluorobenzhydryl]piperazine-1-carbothioamides
A total of 1.7 mmol (0.515 g) 1-[Bis(4-fluorophenyl)methyl]piperazine or 0.872 mmol (1 mol, 0.2632 g) 1-[(4-chlorophenyl)(phenyl)methyl]piperazine was dissolved in 20 ml dry dichloromethane. Reaction flask was taken into ice bath and triethylamine (1:3 moles) was added to the solution. After 10 min, ice bath was removed and suitable isothiocyanate derivative (1:1 mole) was added. Reaction was stirred overnight at room temperature. After the reaction was completed, solution was extracted in order with water and ammonium chloride solution (10%). Dichloromethane layer was washed with water again and dried with anhydrous sodium sulfate. Solvent was evaporated under vacuo and solid product was recrystallized with ethanol/water.
N-tert-Butyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carbothioamide hydrochloride (6a)
Yellowish white, opaque, powdered crystals, 14% (0.06 g), m.p. 176.8 °C. UV (MeOH, λmax, nm); 205 (log ε: 4.23), 223 (log ε: 4.11). FT-IR (KBr, cm−1); 3258 (N–H), 3057 (C–H, aromatic), 2972 (C–H, aliphatic), 1606 (C=C, aromatic), 1288 (C–N), 1236 (C=S, thioamide), 1189 (C–F). 1H-NMR (DMSO, ppm); 1.45 (s, 9H, –C(CH3)3); 2.96–3.15 (m, 4H, piperazine H3, H5); 3.65 (t, 4H, piperazine H2, H6); 4.59 (d, 1H, (Ar)2CH–, J = 14.4 Hz); 5.75 (d, 1H, CSNH, J = 8.8 Hz); 7.17–7.33 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.95 (bs, 4H, diphenyl H3, H5, H3′, H5′); 12.55 (bs, 1H, N–H salt). MS (m/z); 404.90 (100%, M+ – Cl); 205.3 ((4-F-C6H5)2CH⌉+). Elemental analysis of C22H28ClF2N3S (MW: 439.99 g/mol); C 60.05, H 6.41, N 9.55, S 7.29 (Calcd.); C 59.55, H 6.45, N 9.47, S 6.64 (Found).
N-Cyclohexyl-4-[bis(4-fluorophenyl)methyl]piperazine-1-carbothioamide (6b)
White, shiny, needle-shaped crystals 50%, (0.214 g), m.p. 198.2 °C. UV (MeOH, λmax, nm); 202 (log ε: 4.10), 224 (log ε: 4.02), 248 (log ε: 3.88). FT-IR (KBr, cm−1); 3328 (N–H), 3060 (C–H, aromatic), 2996 (C–H, aliphatic), 1603 (C=C, aromatic), 1299 (C–N), 1221 (C=S, thioamide), 1104 (C–F). 1H-NMR (DMSO, ppm); 1.13–1.21 (m, 5H, cyclohexyl); 1.53–1.79 (m, 6H, cyclohexyl); 2.22 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.71 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 4.12 (s, 1H, CSNH); 4.40 (s, 1H, (Ar)2CH–); 7.09–7.14 (m, 4H, diphenyl H2, H6, H2′, H6′); 7.39–7.43 (m, 4H, diphenyl H3, H5, H3′, H5′). 13C-NMR (DMSO, ppm); 24.99 (C21,23); 25.18 (C22); 31.97 (C20,24); 47.06 (C14,16); 50.85 (C15,17); 54.28 (C19); 72.32 (C7); 115.14 (C10,12); 115.35 (C3,5); 129.35 (C9,13); 129.43 (C2,6); 138.12 (C8); 138.15 (C1); 159.79 (C11); 162.21 (C4); 180.14 (C18). MS (m/z); 430.95 (100%, M+); 203.65 ((4-F-C6H5)2CH⌉+). Elemental analysis of C24H29F2N3S (MW: 429.57 g/mol); C 67.10, H 6.80, N 9.78, S 7.46 (Calcd.); C 66.94, H 6.94, N 9.89, S 7.42 (Found).
N-Ethyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carbothioamide (6c)
White, opaque, powdered crystals, 15% (0.056 g), m.p. 150.6 °C. UV (MeOH, λmax, nm); 204 (log ε: 4.25), 225 (log ε: 4.13). FT-IR (KBr, cm−1); 3294 (N–H), 3020 (C–H, aromatic), 2966 (C–H, aliphatic), 1531 (C=C, aromatic), 1255 (C–N), 1229 (C=S, thioamide), 1289 (C–N), 1091 (C–Cl). 1H-NMR (DMSO, ppm); 1.06 (t, 3H, –CH3, J = 6.8 Hz); 2.27 (t, 4H, piperazine H3, H5, J = 5.2 Hz); 3.52–3.45 (m, 2H, –CH2–); 3.75 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.39 (s, 1H, (Ar)2CH–); 7.19–7.46 (m, 9H, diphenyl); 7.61 (t, 1H, CSNH). Elemental analysis of C20H24ClN3S (MW: 373.94 g/mol); C 64.24, H 6.47, N 11.24, S 8.57 (Calcd.); C 64.44, H 6.19, N 11.35, S 8.67 (Found).
N-Isopropyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carbothioamide (6d)
White, shiny, needle-shaped crystals, 39% (0.15 g), m.p. 252.4 °C. UV (MeOH, λmax, nm); 203 (log ε: 4.31), 223 (log ε: 4.15). FT-IR (KBr, cm−1); 3371 (N–H), 3059 (C–H, aromatic), 2967 (C–H, aliphatic), 1539 (C=C, aromatic), 1270 (C–N), 1232 (C=S, thioamide), 1232 (C–N), 1001 (C–Cl). 1H-NMR (DMSO, ppm); 1.09 (d, 6H, –(CH3)2, J = 6.8 Hz); 2.27 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.76 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.39 (s, 1H, (Ar)2CH–); 4.44–4.53 (m, 1H, –CH(CH3)2); 7.19–7.46 (m, 10H, diphenyl H’s + NH). 13C-NMR (DMSO, ppm); 38.79–40.05 (C20,21); 46.93–47.01 (C14,15,16,17); 50.93 (C19); 73.36 (C7); 127.05 (C11); 127.57 (C9,13); 128.42 (C10,12); 128.52 (C3,5); 129.35 (C2,6); 131.32 (C4); 141.28 (C8); 141.64 (C1); 180.17 (C18). MS (m/z); 388.8 (M+, 100%); 390.8 (M + 2, 33%); 201.5 (4-Cl-C6H5)(C6H5)CH+). Elemental analysis of C21H26ClN3S (MW: 387.97 g/mol); C 65.01, H 6.75, N 10.83, S 8.26 (Calcd.); C 64.88, H 6.88, N 10.87, S 8.29 (Found).
N-Allyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carbothioamide (6e)
White, opaque, powdered crystals, 10% (0.040 g), m.p. 139.4 °C. UV (MeOH, λmax, nm); 204 (log ε: 4.27), 225 (log ε: 4.13). FT-IR (KBr, cm−1); 3296 (N–H), 3023 (C–H, aromatic), 2960 (C–H, aliphatic), 1528 (C=C, aromatic), 1252 (C–N), 1224 (C=S, thioamide), 1223 (C–N), 1090 (C–Cl). 1H-NMR (DMSO, ppm); 2.28 (t, 4H, piperazine H3, H5, J = 5.2 Hz); 3.79 (t, 4H, piperazine H2, H6, J = 4 Hz); 4.15 (t, 2H, –CH2–CH=CH2, J = 5.6 Hz); 4.39 (s, 1H, (Ar)2CH–); 5.01–5.11 (dd, 2H, –CH=CH2, J1 = 17.2 Hz, J2 = 8.6 Hz, J3 = 1.6 Hz); 5.80–5.90 (m, 1H, –CH=CH2); 7.19–7.46 (m, 9H, diphenyl); 7.80 (t, 1H, CSNH, J = 5.6 Hz). Elemental analysis of C21H24ClN3S (MW: 385.95 g/mol); C 65.35, H 6.27, N 10.89, S 8.31 (Calcd.); C 65.71, H 6.44, N 11.01, S 8.28 (Found).
N-Benzyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carbothioamide (6f)
White, opaque, featherlike crystals, 23% (0.1 g), m.p. 157.2 °C. UV (MeOH, λmax, nm); 203 (log ε: 4.51), 226 (log ε: 4.33). FT-IR (KBr, cm−1); 3236 (N–H), 3020 (C–H, aromatic), 2813 (C–H, aliphatic), 1539 (C=C, aromatic), 1246 (C–N), 1211 (C=S, thioamide), 1246 (C–N), 1001 (C–Cl). 1H–NMR (DMSO, ppm); 2.31 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.83 (t, 4H, piperazine H2, H6, J = 4.4 Hz); 4.41 (s, 1H, (Ar)2CH–); 4.79 (d, 2H, –CH2–, J = 5.2 Hz); 7.19–7.47 (m, 14H, aromatic H’s); 8.19 (t, 1H, CSNH, J = 5.6 Hz). Elemental analysis of C25H26ClN3S (MW: 436.01 g/mol); C 68.87, H 6.01, N 9.64, S 7.35 (Calcd.); C 69.02, H 5.98, N 9.80, S 7.46 (Found).
N-Butyl-4-[(4-chlorophenyl)(phenyl)methyl]piperazine-1-carbothioamide hydrochloride (6g)
White, opaque, feather-like crystals, 20% (0.080 g), m.p. 125.5 °C. UV (MeOH, λmax, nm); 204 (log ε: 4.43), 227 (log ε: 4.35). FT-IR (KBr, cm−1); 3261 (N–H), 3028 (C–H, aromatic), 2958 (C–H, aliphatic), 1541 (C=C, aromatic), 1298 (C–N), 1201 (C=S, thioamide), 1201 (C–N), 1001 (C–Cl). 1H-NMR (DMSO, ppm); 0.87 (t, 3H, –CH2CH2CH3, J = 7.2 Hz); 1.20–1.29 (m, 2H, –CH2CH2CH3); 1.44–1.52 (m, 2H, –CH2CH2CH3); 2.27 (t, 4H, piperazine H3, H5, J = 4.8 Hz); 3.42–3.47 (q, 2H, –NHCH2–); 3.75 (t, 4H, piperazine H2, H6, J = 4.8 Hz); 4.39 (s, 1H, (Ar)2CH–); 7.19–7.46 (m, 9H, diphenyl); 7.58 (t, 1H, CSNH, J = 5.6 Hz). Elemental analysis of C22H28ClN3S (MW: 401.17 g/mol); C 65.73, H 7.02, N 10.45, S 7.98 (Calcd.); C 66.06, H 7.07, N 10.56, S 8.05 (Found).
Cytotoxicity studies
The cytotoxic activity of the synthesized compounds was investigated initially on liver (HUH-7), breast (MCF-7) and colon (HCT-116) cancer cell lines, by means of SRB assays in triplicate. Serial dilutions from 100 μM to 2.5 μM were used, 5-fluorouracil (5-FU) was the reference compound and camptothecin (CPT) was the positive control for the cytotoxic effect.
Cell culture
The human cancer cell lines were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin. Each cell line was maintained in an incubator at 37 °C supplied with 5% CO2 and 95% air.
NCI-60 SRB assay
Cancer cells (range of 2000 cell/well to 5000 cell/well) were inoculated into 96-well plates in 200 μl of media and incubated in 37 °C incubators containing 5% CO2 and 95% air. After a 24 h incubation period, one plate for each cell line was fixed with 100 μl of 10% ice-cold trichloroacetic acid (TCA). This plate represents the behavior of the cells just prior to the drug treatment and is accepted as the time-zero plate. The compounds to be tested were solubilized in DMSO to a final concentration of 40 mM and stored at +4 °C. While treating the cells with the compounds, the corresponding volume of the compound was applied to the cell to achieve the desired drug concentration and diluted through serial dilution. After the drug treatment, the cells were incubated in 37 °C incubators containing 5% CO2 and 95% air for 72 h. Following the termination of the incubation period after the drug treatment, the cells were fixed with 100 μl of 10% ice-cold TCA and incubated in the dark at +4 °C for 1 h. Then the TCA was washed away with ddH2O five times and the plates were left to air dry. For the final step, the plates were stained with 100 μl of 0.4% SRB solution in 1% acetic acid solution. Following staining, the plates were incubated in dark for 10 min at room temperature. The unbound dye was washed away using 1% acetic acid and the plates were left to air dry. To measure the absorbance results, the bound stain was then solubilized using 200 μl of 10 mM Tris-Base. The OD values were obtained at 515 nm.
Results and discussion
Chemistry
The synthesis of the benzhydrylpiperazine derivatives (5a–y) and (6a–g) is outlined in . Reduction with sodium borohydride of benzophenone, 4-chlorobenzophenone and 4,4′-difluorobenzophenone afforded benzhydrole derivatives which were chlorinated with HCl and anhydrous calcium chloride. Resulting benzhydryl chloride derivatives were used for N-alkylation of piperazine to give 1-benzhydrylpiperazine, 4-chlorobenzhydrylpiperazine and 4,4′-difluorobenzhydrylpiperazine. The final step was nucleophilic addition to isocyanates or isothiocyanates in order to obtain benzhydrylpiperazine derivatives (5a–y) and (6a–g).
Synthesized compounds were identified with IR, UV and 1H-NMR spectra. In addition, some compounds were selected for LC-MS and 13C-NMR spectral evaluation. In UV spectra of carboxamide derivatives there are two significant bands at 205 and 224 nm, which represent π → π* and n → π* transitions. In UV spectra of thioamide derivatives there are three significant bands nearly at 202, 224 and 248 nm, which represent π → π* and n → π* transitions. In IR spectrum of carboxamide derivatives, characteristic N–H stretching band is observed nearly at 3332 cm−1. Other stretching bands are observed approximately at 3020 cm−1 (C–H; aromatic), 2965 cm−1 (C–H; aliphatic), 1625 cm−1 (C=O; amide), 1520 cm−1 (C=C; aromatic) and 1250 cm−1 (C–N). In IR spectrum of thioamide derivatives, characteristic N–H stretching band is observed nearly at 3330 cm−1. Other stretching bands are observed approximately at 3060 cm−1 (C–H; aromatic), 2995 cm−1 (C–H; aliphatic), 1600 cm−1 (C=C; aromatic), 1300 cm−1 (C–N) and 1220 cm−1 (C=S) and 1100 cm−1 (C–F). In H1-NMR spectra of carboxamide derivatives the protons of piperazine are seen approximately at 2.23 and 3.36 ppm as broad singlets. Diphenylmethyl C–H gives a singlet nearly at 4 ppm. Aromatic rings give multiplets at 7–7.5 ppm. Amide N–H gives a singlet nearly at 8 ppm. In H1-NMR spectra of thioamide derivatives, the protons of piperazine are seen at 2.5 (t, 4H, J = 5.2 Hz) ppm and 3.5 (t, 4H, H1, J = 4 Hz) ppm approximately. Diphenylmethyl C–H gives a singlet nearly at 4.5 ppm. Protons of aromatic rings give multiplets at 7–7.5 ppm. Thioamide N–H is observed approximately at 7.5 ppm. The 13C-NMR spectrum of 5x shows characteristic peaks of the carboxamide derivatives approximately at 45 and 50 ppm for piperazine ring, 75 ppm for diphenylmethyl carbon and 150 ppm for carbonyl group. The 13C-NMR spectrum of 6b shows characteristic peaks of the thioamide derivatives nearly at 45 and 50 ppm for piperazine ring, 70 ppm for diphenylmethyl carbon and 180 ppm for thiocarbonyl group.
Structures of the prepared benzhydrylpiperazine derivatives are illustrated in .
Table 1. Structural and physical information of compounds 5a–y and 6a–g.
Cytotoxicity
The cytotoxic activity of the synthesized compounds 5a–y and 6a–g was investigated on liver (HUH-7), breast (MCF-7) and colon (HCT-116) cancer cell lines, by means of SRB assays in triplicate. As shown in , all tested compounds were screened with mean 50% growth inhibition concentration (GI50) in micromolar concentration range.
Table 2. Cytotoxic activity data for compounds 5a–y and 6a–g.
Most of the nonsubstituted benzhydrylpiperazine derivatives are inactive or they have low activities against all cancer cell lines. It should also be noted that, in general, 4-chlorobenzhydrylpiperazine derivatives have higher activities becoming superior over their 4,4′-difluoro and nonsubstituted counterparts. Moreover, thioamide derivatives are more potent than carboxamide derivatives against all cancer cell lines. Corresponding compound groups representing these findings are detailed in .
Table 3. GI50 (µM) values of some carboxamide and thioamide derivatives for detailed discussion of SAR.
Compounds 5c, 5m, 5s and 6d have the same substituents on NH group (R3 = isopropyl). Compound 5c has no cytotoxicity against any of these cancer cell lines. However, 5m has slight cytotoxicity, 5s has good cytotoxicity and 6d has the highest cytotoxicity against all three cancer cell lines.
Compounds 5e and 5u have the same substituents on NH group (R3 = 2,6-dichlorophenyl). 5e has no cytotoxicity against any of the cancer cell lines. Interestingly, 5u has increased cytotoxicity against all the cancer cell lines.
Compounds 5f and 5x have the same substituents on NH group (R3 = 2-benzylphenyl). 5f has no cytotoxicity against none of these cancer cell lines. However, 5x has good cytotoxicity against all the cancer cell lines.
Compounds 5h, 5t and 6e have the same substituents on NH group (R3 = allyl). 5h has no cytotoxicity against HUH-7 and HCT-116 cell lines nevertheless it has good cytotoxicity against MCF-7 cell line. However, 5t has elevated cytotoxicity and 6e has the highest cytotoxicity against all three cancer cell lines.
In general, nonsubstituted benzhydryl derivatives are inactive or have low inhibition whereas 4-chlorobenzhydryl derivatives are more active than other compounds against HUH-7 cell line.
The most active compounds against HUH-7 cell line are 5y (GI50 = 1.29 μM) and 6a (GI50 = 5.97 μM). Additionally, most of the compounds have higher cytotoxicity against HUH-7 than reference compound 5-fluorouracil.
Among the carboxamide derivatives, compounds bearing electron withdrawing substituents on phenyl ring such as 5o (GI50 = 9.46 μM), 5u (GI50 = 6.44 μM), 5w (GI50 = 8.54 μM) and 5y (GI50 = 1.29 μM) are highly active against HUH-7 cell line. In addition, alkyl substituted derivatives, except thioamide derivatives, have no (5a–d, 5h, 5n) or low inhibition (5i–m, 5p–t).
Thioamide derivatives are generally cytotoxic against HUH-7 cell line. It can be noted that thioamides show higher activity than their carboxamide derivatives, which can be exemplified by compounds 5j (GI50 = 29.96 μM) compared with 6a (GI50 = 5.97 μM), 5r (GI50 = 20.92 μM) compared with 6c (GI50 = 10.81 μM), 5s (GI50 = 15.36 μM) compared with 6d (GI50 = 6.20 μM) and 5t (GI50 = 16.29 μM) compared with 6e (GI50 = 9.95 μM).
The most active compounds against MCF-7 cell line are 5u (GI50 = 6.14 μM) and 6e (GI50 = 4.94 μM). Furthermore, we observed that compound 6e was less toxic in MCF-12A (GI50 = 8.5 μM), which is a normal-like breast epithelial cell line ().
Table 4. MCF-7 (breast cancer cell line) and MCF-12A (normal-like breast epithelial cell line) cytotoxicity comparison of compound 6e (µM).
Against MCF-7 cell line, nonsubstituted benzhydryl carboxamide derivatives (except 5d and 5h) and 5i, 5j, 5l, 6b, 6c show no inhibition. Alkyl-substituted carboxamide derivatives have low activity values such as 5d (GI50 = 25.7 μM), 5k (GI50 =19.03 μM), 5m (GI50 = 45.23 μM), 5n (GI50 = 36.14 μM), 5r (GI50 = 60.24 μM). However, compounds such as 5u (GI50 = 6.14 μM) and 5y (GI50 = 6.34 μM) that contain phenyl ring with electron withdrawing substituents are highly cytotoxic.
Against HCT-116 cell line, 5b (GI50 = 1.01 μM) and 5y (GI50 = 1.81 μM) are the most active derivatives. In addition, most of the compounds have higher cytotoxicity against HCT-116 than reference compound 5-fluorouracil.
With the exception of 5b, nonsubstituted benzhydryl carboxamide derivatives present no inhibition against HCT-116 cell line. 4-Chlorobenzhydryl carboxamide derivatives are higher in activity than 4,4′-difluorobenzhydryl carboxamide derivatives demonstrated with compounds 5i (GI50 = 24.48 μM) and 5p (GI50 = 9.33 μM) or compounds 5j (GI50 = 28.4 μM) and 5q (GI50 = 9.33 μM). Thioamides generally show good activity values considering HCT-116 cell line.
Conclusion
In this study, 32 benzhydrylpiperazine derivatives with carboxamide and thioamide moieties were prepared. In vitro cytotoxic activities were screened against hepatocellular (HUH-7), breast (MCF-7) and colorectal (HCT-116) cancer cell lines by SRB assay. Most of the compounds presented higher cytotoxicity against HUH-7 and HCT-116 cancer cell lines in comparison with reference compound 5-fluorouracil. Interestingly, 4-chlorobenzhydrylpiperazine derivatives were more active than benzhydrylpiperazine and 4,4′-difluorobenzhydrylpiperazine derivatives. In addition, thioamide derivatives were observed to have markedly elevated cytotoxicity values opposed to their carboxamide analogs. Future synthesis of similar derivatives will take place to create a larger set of compounds, in order to produce a rational quantitative structure-activity relationship (QSAR) mapping. Since 4-chlorobenzhydrylpiperazine derivatives are chiral compounds, further exploration of chiral separation methods will be performed. The primary ambition regarding future research is to evaluate the mechanism of cytotoxicity.
Declaration of interest
The authors have declared no conflicts of interest.
References
- Gao L, Li M, Meng T, et al. Asymmetric synthesis and biological evaluation of N-cyclohexyl-4-[1-(2,4-dichlorophenyl)-1-(p-tolyl)methyl]piperazine-1-carboxamide as hCB1 receptor antagonists. Eur J Med Chem 2011;46:5310–16
- Cumming JG, Bower JF, Waterson D, et al. The design and synthesis of novel, potent and orally bioavailable N-aryl piperazine-1-carboxamide CCR2 antagonists with very high hERG selectivity. Bioorg Med Chem Lett 2012;22:3895–9
- Kinoyama I, Taniguchi N, Toyoshima A, et al. (+)-(2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-dimethyl-N-[6-(trifluoromethyl)pyridin-3-yl]piperazine-1-carboxamide (YM580) as an orally potent and peripherally selective nonsteroidal androgen receptor antagonist. J Med Chem 2006;49:716–26
- Sun Q, Tafesse L, Islam K, et al. 4-(2-Pyridyl)piperazine-1-carboxamides: potent vanilloid receptor 1 antagonists. Bioorg Med Chem Lett 2003;13:3611–16
- Matsuno K, Nakajima T, Ichimura M, et al. Potent and selective inhibitors of PDGF receptor phosphorylation. 2. Synthesis, structure activity relationship, improvement of aqueous solubility, and biological effects of 4-[4-(N-substituted(thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. J Med Chem 2002;45:4513–23
- Albro LP, Baltzly R, Phillips AP. Unsymmetrically disubstituted piperazines II. Histamine antagonists. J Org Chem 1949;14:771–4
- Baltzly R, DuBreuil S, Ide WS, Lorz E. Unsymmetrically disubstituted piperazines III. N-methyl-N′-benzhydrylpiperazines as histamine antagonists. J Org Chem 1949;14:775–82
- Iemura R, Kawashima T, Fukuda T, et al. Synthesis of 2-(4-substituted-1-piperazinyl)benzimidazoles as H1-antihistaminic agents. J Med Chem 1986;29:1178–83
- Beck K, Hamlin K, Weston A. Histamine antagonists. IV. C-methyl derivatives of 1,4-disubstituted piperazines. J Am Chem Soc 1952;74:605–8
- Vandenberk J, Kennis L, Van der Aa M, et al. Piperazine derivatives. Janssen Pharmaceutica, US 4250176; February 1981
- Wang L, Wang T, Yang B, et al. Design, synthesis, and anti-allergic activities of novel (R)(-)-1-[(4-chlorophenyl)phenylmethyl]piperazine derivatives. Med Chem Res 2010;21:124–32
- Zamponi G, Feng Z, Zhang L, et al. Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg Med Chem Lett 2009;19:6467–72
- Alps B. Drugs acting on calcium channels – potential treatment for ischemic stroke. Brit J Clin Pharmaco 1992;34:199–206
- Miyake N, Fujita R, Ishikawa M, et al. Reversal of multidrug resistance in human leukemia K562 by tamolarizine, a novel calcium antagonist. Jpn J Pharmacol 2000;82:265–8
- Kimura M, Masuda T, Yamada K, et al. Novel diphenylalkyl piperazine derivatives with dual calcium antagonistic and antioxidative activities. Bioorg Med Chem Lett 2002;12:1947–50
- Pajouhesh H, Feng Z, Ding Y, et al. Structure-activity relationships of diphenylpiperazine N-type calcium channel inhibitors. Bioorg Med Chem Lett 2010;20:1378–83
- Kam Y, Rhee H, Rhim H, et al. Synthesis and T-type calcium channel blocking activity of novel diphenylpiperazine compounds, and evaluation of in vivo analgesic activity. Bioorg Med Chem 2010;18:5938–44
- Kurokawa M, Sato F, Hatano N, et al. A new class of calcium antagonists. Synthesis and biological activity of 11-[(ω-aminoalkanoyl)amino]-6,6a,7,8,9,10,10a,11-octahydrodibenzo[b,e]thiepin derivatives. J Med Chem 1991;34:593–9
- Doddareddy M, Choo H, Cho Y, et al. 3D pharmacophore based virtual screening of T-type calcium channel blockers. Bioorg Med Chem 2007;15:1091–105
- Sasse B, Mach U, Leppaenen J, et al. Hybrid approach for the design of highly affine and selective dopamine D-3 receptor ligands using privileged scaffolds of biogenic amine GPCR ligands. Bioorg Med Chem 2007;15:7258–73
- Jung J, Jung S, Koh H. Asymmetric synthesis of chiral piperazinylpropylisoxazoline ligands for dopamine receptors. Eur J Med Chem 2007;42:1044–8
- Kimura M, Masuda T, Yamada K, et al. Synthesis of novel diphenyl piperazine derivatives and their activities as inhibitors of dopamine uptake in the central nervous system. Bioorg Med Chem 2003;11:1621–30
- Abou-Gharbia M, Patel UR, Moyer JA, Muth EA. Psychotropic agents: synthesis and antipsychotic activity of substituted β-carbinoles. J Med Chem 1987;30:1100–5
- Okachi R, Niino H, Kitaura K, et al. Synthesis and antibacterial activity of 2,2′-dithiobis(benzamide) derivatives against Mycobacterium species. J Med Chem 1985;28:1772–9
- Punkvang A, Saparpakorn P, Hannongbua S, et al. Insight into crucial inhibitor-enzyme interaction of arylamides as novel direct inhibitors of the enoyl ACP reductase (InhA) from Mycobacterium tuberculosis: computer-aided molecular design. Monatsh Chem 2010;141:1029–41
- Dinakaran M, Senthilkumar P, Yogeeswari P, et al. Antimycobacterial activities of novel 2-(sub)-3-fluoro/nitro-5,12-dihydro-5-oxo-benzothiazolo[3,2-a]quinoline-6-carboxylic acid. Bioorg Med Chem 2008;16:3408–18
- Senthilkumar P, Dinakaran M, Banerjee D, et al. Synthesis and antimycobacterial evaluation of newer 1-cyclopropyl-1,4-dihydro-6-fluoro-7-(substituted secondary amino)-8-methoxy-5-(sub)-4-oxoquinoline-3-carboxylic acids. Bioorg Med Chem 2008;16:2558–69
- Upadhayaya R, Vandavasi J, Kardile R, et al. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur J Med Chem 2010;45:1854–67
- Kumar A, Siddiqi MI. Receptor based 3D-QSAR to identify putative binders of Mycobacterium tuberculosis Enoyl acyl carrier protein reductase. J Mol Model 2010;16:877–93
- Lu XY, Chen YD, You QD. 3D-QSAR studies of arylcarboxamides with inhibitory activity on InhA using pharmacophore-based alignment. Chem Biol Drug Des 2010;75:195–203
- Sriram D, Senthilkumar P, Dinakaran M, et al. Antimycobacterial activities of novel 1-(cyclopropyl/tert-butyl/4-fluorophenyl)-1,4-dihydro-6-nitro-4-oxo-7-(substituted secondary amino)-1,8-naphthyridine-3-carboxylic acid. J Med Chem 2007;50:6232–9
- Senthilkumar P, Dinakaran M, Yogeeswari P, et al. Synthesis and antimycobacterial activities of novel 6-nitroquinolone-3-carboxylic acids. Eur J Med Chem 2009;44:345–58
- Kumar A, Siddiqi M. CoMFA based de novo design of pyrrolidine carboxamides as inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. J Mol Model 2008;14:923–35
- Lu X, Chen Y, Jiang Y, You Q. Discovery of potential new InhA direct inhibitors based on pharmacophore and 3D-QSAR analysis followed by in silico screening. Eur J Med Chem 2009;44:3718–30
- Muddassar M, Jang J, Gon H, et al. Identification of novel antitubercular compounds through hybrid virtual screening approach. Bioorg Med Chem 2010;18:6914–21
- Chandra J, Sadashiva C, Kavitha C, Rangappa K. Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis(4-fluorophenyl)methyl]piperazine. Bioorg Med Chem. 2006;14:6621–7
- Verderame M. 1,4-Disubstituted piperazines. 3. Piperazinylbenzothiazoles. J Med Chem 1972;15:693–4
- Aytemir M, Ozcelik B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur J Med Chem 2010;45:4089–95
- Patel I, Parmar S. Synthesis and studies of novel optically active Schiff’s base derivatives and their antimicrobial activities. E-J Chem 2010;7:617–23
- Srinivasan S, Gupta S, Marwah R, et al. Synthesis, characterization and in vitro biological studies of novel N-aryl piperazinyl fluoroquinolones. Res J Pharm Biol Chem Sci 2010;1:208–18
- Shivakumara K, Prakasha K, Gowda D. Synthesis and antimicrobial activity of amino acids conjugated diphenylmethylpiperazine derivatives. E-J Chem 2009;6:473–9
- Chern J, Shia K, Hsu T, et al. Design, synthesis, and structure-activity relationships of pyrazolo[3,4-d]pyrimidinies: a novel class of potent enterovirus inhibitors. Bioorg Med Chem Lett 2004;14:2519–25
- Curreli F, Zhang H, Zhang X, et al. Virtual screening based identification of novel small-molecule inhibitors targeted to the HIV-1 capsid. Bioorg Med Chem 2011;19:77–90
- Kumar C, Prasad S, Vinaya K, et al. Synthesis and in vitro antiproliferative activity of novel 1-benzhydryl-piperazine derivatives against human cancer cell lines. Eur J Med Chem 2009;44:1223–9
- Yarim M, Koksal M, Durmaz I, Atalay R. Cancer cell cytotoxicities of 1-(4-Substitutedbenzoyl)-4-(4-chlorobenzhydryl)piperazine derivatives. Int J Mol Sci 2012;13:8071–85
- Huang W, Liu M, Li Y, et al. Design, synthesis and antitumor activity of novel chromone and aurone derivatives. Bioorg Med Chem 2007;15:5191–7
- Huang W, Ding Y, Miao Y, et al. Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur J Med Chem 2009;44:3687–96
- Gan L, Fang B, Zhou C. Synthesis of azole-containing piperazine derivatives and evaluation of their antibacterial, antifungal and cytotoxic activities. B Kor Chem Soc 2010;31:3684–92
- Kumar C, Swamy S, Thimmegowda N, et al. Synthesis and evaluation of 1-benzhydryl-sulfonyl-piperazine derivatives as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Med Chem Res 2007;16:179–87
- Wang W, Xu X, Chen Y, et al. Apoptosis of human Burkitt’s lymphoma cells induced by 2-N,N-diethylaminocarbonyloxymethyl-1-diphenyl-methyl-4-(3,4,5-trimethoxybenzoyl)piperazine hydrochloride (PMS-1077). Arch Pharm Res 2009;32:1727–36
- Demma M, Maxwell E, Ramos R, et al. SCH529074, a small molecule activator of mutant p53, which binds p53 DNA binding domain (DBD), restores growth-suppressive function to mutant p53 and interrupts HDM2-mediated ubiquitination of wild type p53. J Biol Chem 2010;285:198–212
- Gurdal EE, Yarim M, Durmaz I, Cetin-Atalay R. Cytotoxic activities of some novel benzhydrylpiperazine derivatives. Arzneimittel-Forsch 2013; Accepted Manuscript