Abstract
Monoamine oxidase (MAO) inhibitors are generally used in the treatment of depressive disorders and some neurodegenerative illnesses, such as Parkinson’s disease and Alzheimer’s disease. The aim of this preliminary study was to investigate the MAO [MAO (E.C.1.4.3.4)] inhibiting effect of various apitherapeutic products, such as chestnut honey, pollen and propolis. Extracts’ MAO inhibition was measured using peroxidase-linked spectrophotometric assay in enzyme isolated from rat liver microsomes, and the values are expressed as the inhibition concentration (IC50) causing 50% inhibition of MAO. The antioxidant activity of the bee products was also determined in terms of total phenolic content (TPC) and ferric reducing/antioxidant power in aquatic extracts. All samples exhibited substantial inhibition of MAO, propolis having the highest. Inhibition was related to samples’ TPCs and antioxidant capacities. These results show that bee products possess a sedative effect and may be effective in protecting humans against depression and similar diseases.
Introduction
Monoamine oxidase inhibitors (MAOIs) are mainly used in psychiatry for the treatment of depressive and anxiety disorders and in neurology for the treatment of Parkinson’s disease and Alzheimer’s diseaseCitation1–3. Honey, pollen and propolis are all natural bee products, new therapeutic characteristics of which are emerging every day. To date, these products have only been known to be effective in physiological diseases, and their roles in psychological or neurodegenerative diseases are still unknown. Monoamine oxidases (MAOs) are a type of flavoprotein present in the outer mitochondrial membrane of neuronal and non-neuronal cellsCitation4,Citation5. Two isoforms have been identified, MAO-A and MAO-B. These enzymes are responsible for the oxidative deamination of endogenous and xenobiotic amines. They have different substrate preference, inhibitor specificity and tissue distributionsCitation6. Tyramine is a substrate for both MAO-A and MAO-B.
While the classic non-selective and irreversible MAOIs, such as phenelzine and tranylcypromine, are characterized by the risk of inducing a hypertensive crisis when dietary tyramine is ingested, the selective MAO-B inhibitor selegiline and the selective and reversible inhibitor of MAO-A (RIMA) moclobemide are free from this potential interactionCitation5,Citation6. Alzheimer’s disease has been linked with this mechanism, that is, increased MAO-B activity plus reduced free radical scavenging capacity. Parkinson’s and Alzheimer’s disease have been associated with oxidative stress and increasing MAO-B activityCitation5. MAO inhibition is accompanied by marked changes in the sensitivity of the organism to various dietary constituents (e.g. p-tyramine, tryptophan and other amines and amine precursors) as well as many drugs (e.g. sympathomimetics, opiates, reserpine and caffeine).
Although many studies have been conducted on bee products that have highly bioactive compounds, such as phenolic compounds, there are no studies of MAO inhibition. Previous studies to date have assessed the antioxidant, antimicrobial, anti-inflammatory, anti-carcinogenic and carbonic anhydrase (CA) inhibitory effects and the hepatoprotective role of the bee productsCitation2,Citation7–16. Similarly, MAO is inhibited by some plant extracts with a high content of phenolic compoundsCitation17–19 and some synthetic hydrazinesCitation20. There are information gaps on MAO inhibition by bee products, however, which this study is intended to help fill.
Materials and methods
Samples
Chestnut honey, pollen and propolis were collected from beekeepers in the Black Sea region of northern Turkey. Samples were stored in a refrigerator until use. Palynological identification of the pollen and honey samples was performed using microscopic analysis. Chestnut sativa was dominant (>45%) in the pollen in the samples.
Chemicals
All chemicals were reagent grade and used without further purification. Clorgyline (N-methyl-N-propargyl-3-(2,4-dichlorophenoxy) propylamine hydrochloride), Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), pargyline (N-methyl-N-propargylbenzylamine,%97), 4-aminoantipyrine (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one, ampyrone), p-triamine, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and horseradish peroxidase (Type VI) were purchased from Sigma-Aldrich (St. Louis, MO). TPTZ (2,4,6-tripyridyl-s-triazine) and Folin–Ciocalteau’s phenol reagent were obtained from Fluka Chemie GmbH (Switzerland).
Preparations of extract of honey, pollen and propolis samples
Aquatic honey, pollen and propolis extracts were prepared in different concentrations. Pollen and honey samples were dissolved easily and filtered with Whatman filter paper. The raw propolis samples were frozen at −20 °C for 24 h and ground to a fine powder. Five grams of powder was placed in a flask (250 mL) to which 100 mL double distilled water was added and shaken (Heidolph Promax 2020, Schwabach, Germany) for 72 h at 45 °C. The suspension was then filtered with Whatman filter paper. The filtrate was sonicated for 3 h using a sonicator apparatus (Elma® Transsonic Digital, Germany). Each sample was diluted to final concentrations and was kept at −20 °C until use.
Determination of total antioxidant capacity
Total antioxidant capacities of the bee products were measured using the total phenolic contents (TPC) and ferric reducing/antioxidant power (FRAP) methods. TPCs of the aquatic extracts were determined using Folin–Ciocalteu assayCitation21 with slight modificationsCitation22. Different concentrations of aqueous sample extracts were diluted. Gallic acid was used as the reference standard compound, and the results were expressed as milligrams gallic acid per gram sample. Subsequently, 680 µL distilled water, 20 µL aquatic extracts and 400 µL of 0.2 N Folin–Ciocalteu reagents were mixed in a tube and then vortexed. After 2 min, 400 µL Na2CO3 (7.5%) was added and incubated for 2 h at room temperature. Absorbance was measured at 760 nm at the end of the incubation period. The concentration of TPCs was calculated as milligrams of gallic acid equivalents of gram samples using a calibration curve. All measurements were performed in triplicate.
The reducing power ability of ferric tripyridyltriazine (Fe-III-TPTZ) complex from the sample aquatic extracts was measured using the methods described by Benzie and StrainCitation23 with some modifications. The test involved the reduction of ferric tripyridyltriazine (Fe-III-TPTZ) complex to a blue-colored Fe (II) TPTZ by samples’ antioxidant agents. Working FRAP reagent was prepared as required by mixing 25 mL of 300 mM acetate buffer, pH 3.6, with 2.5 mL of 10 mM TPTZ solution in 40 mM HCl and 2.5 mL of 20 mM FeCl3ċ6H2O solution. Subsequently, 3 mL freshly prepared FRAP reagent and 100 µL of the samples were mixed and incubated in 4 min at 37 °C. Absorbance was read at 595 nm against a reagent blank containing distilled water. For comparative purposes, Trolox® was also tested under the same conditions as a standard antioxidant compoundCitation23. FRAP values were expressed as millimoles of Trolox per gram of sample.
Isolation of mitochondria from rat liver
MAO was gradually purified from rat liver mitochondria by partial modification of the method described by Holt et al.Citation24. The animals were decapitated. The livers were immediately excised, placed in KCl (1.15%) and stored at −20 °C until use. After dissolution, the liver was decanted, washed in potassium phosphate buffer (0.2 M, pH 7.6) and homogenized 1:40 (w/v) in 0.3 M sucrose at 15 000/min using a homogenizator (Ika-Werke, Ultra Turrax® T25 Basic, Germany). Homogenate was centrifuged at 3000 rpm for 12 min. Obtained supernatant was re-centrifuged at 9450 rpm for 30 min and collapsed crude mitochondrial pellet. After the crude mitochondrial pellet was resuspended in 5 mL of 0.3 M sucrose, mitochondria were separated with density gradient centrifugation. The suspension was slowly layered onto 40 mL of 1.2 M sucrose and centrifuged at 21 753 rpm for 2 h and then decanted. Mitochondria obtained were suspended in 15 mL potassium phosphate buffer. Prepared mitochondria suspension was analyzed immediately because homogenates lose their activity in 24 h. In our preliminary experiments, homogenates exhibited a decline in activity in one day.
Measurement of inhibition
Activity measurements were performed using photometric assay. Enzyme inhibition was measured using the method described by Holt et al.Citation24 and Schmidt et al.Citation25 with minor modifications. Samples were serially diluted with distilled water, and 40 μL of each dilution was placed in 96-well microplates (PS Microplate, non-sterile, Greiner Bio-One, Germany) to give final concentrations from 5 to 0.00005 mg/mL (five dilutions for samples). Subsequently, 40 μL of water-diluted samples was placed in 96-well microplates to give final concentrations from 5 to 0.00065 mg/mL (at least five dilutions). Each test well contained 120 μL amino substrate solution (2.5 mM p-tyramine in potassium phosphate buffer), 40 μL chromogenic solution (1 mM vanillic acid, 0.5 mM 4-aminoantipyrine, 4 U/mL peroxidase in potassium phosphate buffer), 40 μL enzyme mixture obtained from rat liver homogenates and 40 μL of the bee sample extract. Chromogenic solutions were prepared daily and kept at 4 °C until use. Distilled water was used as a negative control. Background wells contained potassium phosphate buffer (0.2 M, pH 7.6) in place of the enzyme mixture. Reactions were observed at 490 nm using a microplate reader (Chromate 4300). Plates were incubated between readings at 37 °C. Absorbance readings were taken every 3 min over a period of 42 min.
Statistical analysis
The results were presented as mean values ± standard deviations of triplicate measurements. Data were tested using analysis of variance (ANOVA) with SPSS software (version 9.0 for Windows 98, SPSS Inc., Chicago, IL). The means statistically different from each other were compared using Duncan’s multiple comparison.
Result and discussion
This study investigated the inhibitory effect on MAO enzyme of aquatic extracts obtained from bee products (honey, pollen and propolis). Before inhibition was investigated, TPC levels and total antioxidant capacities of all three bee products were determined. The results obtained are shown in . TPC and FRAP activities in the aquatic extracts investigated were determined in the following descending order – propolis, pollen and honey. Propolis was the bee product with the highest antioxidant activity. Bee product phenolic compounds vary depending on the geographic characteristics and the plant flora of the region where they are collected. However, the TPC and FRAP values for the three bee products in this study did not exhibit similar results compared with other studies in the literature. Tezcan et al.Citation26 reported that the TPC of chestnut honey ranged between 0.95 mg/g and 1.13 mg/g. Similarly, another study reported aquatic pollen sample TPCsCitation15 of 56.60 mg/g. Studies showing the antioxidant property of propolis have reported that ethanolic propolis extracts have higher phenolic content levels and exhibit greater antioxidant activity in association with thisCitation16,Citation27. Recently, however, aquatic propolis extracts have been reported to contain significant amounts of phenolic content. Gülçin et al.Citation28 reported aquatic propolis extract TPC of 124 mg GAE/g. Sahin et al.Citation15 reported TPC levels of 13.45 mg/g in aquatic propolis samples. More phenolic material appears to pass into the aquatic environment with the extraction technique we used (89.51 mg GAE/g propolis).
Table 1. Total phenolic contents (TPC) and ferric reducing/antioxidant power (FRAP) of aqueous extracts.
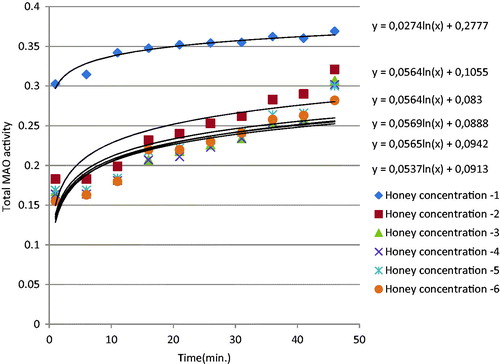
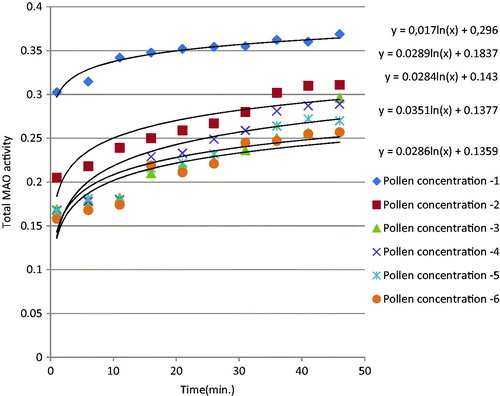
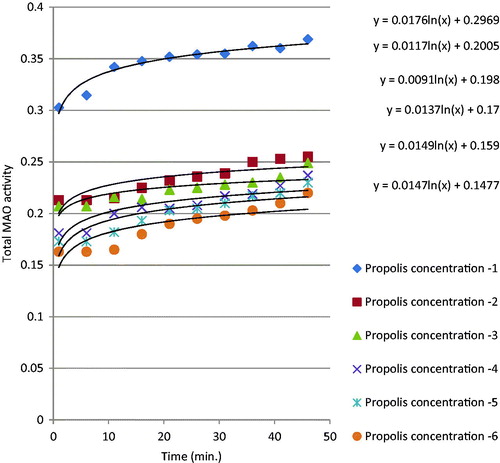
MAO activities detected by photometric assay were plotted over 42 min (). The total MAO activity curve was showed sigmoidal curve. This shows that the enzyme activity increases logarithmically. When honey, pollen and propolis were added to the enzyme environment, enzyme activity decreased significantly; in other words, all three natural products inhibited MAO (). show that inhibition rose significantly with increasing concentrations of honey, pollen and propolis. IC50 values were calculated in order to show total MAO enzyme inhibition values in a concrete form. IC50 value is defined as the level that inhibits 50% of the enzyme, and the values obtained are shown in . A low IC50 shows a high enzyme inhibition capacity. Propolis, pollen and honey, in decreasing order, all inhibited MAO enzyme activity (). We attribute propolis exhibiting higher MAO inhibition than pollen and honey to phenolic substances being present in greater amounts in its composition. In a previously studyCitation17 IC50 values of Clorgyline (selective MAO-A inhibitor) and Selegiline (selective MAO-B inhibitor) were found as 31 ± 10 nM and 111 ± 86 nM, respectively. Many researchers have reported a positive correlation between TPC and biological activities such as antioxidant, antimicrobial and anti-inflammatory effects in natural samples, as well as in bee productsCitation27,Citation29,Citation30. Similarly in this study, propolis had higher phenolic contents than the other bee products, and exhibited greater biological activity as a result.
Table 2. Inhibitor effects of aquatic bee products on total Mono amine oxidase (MAO).
Stafford et al.Citation17 studied MAO inhibition by various southern African traditional medicinal plants and reported that ethyl acetate extracts of Ruta graveolens exhibited the best MAO inhibitory activity (IC50: 5 ± 1 µg/mL). Alper et al.Citation31 suggested that the inhibition of MAO activity may attenuate the process of aging by reducing increased lipid peroxidation and concomitant oxidant stress. Some studies on MAO inhibition were conducted using alcoholic extracts, although aquatic extracts were also effective in our study. Propolis, pollen and honey have been used in different treatments in apitherapy. This study suggests that these apitherapeutic products may also have a role in the treatment of depressive disorders and some neurodegenerative illnesses. In the light of this study, more extensive and detailed studies on specific MAO-A and MAO-B inhibition activity are now needed. Extracts of Ginkgo biloba (EGb761®) have been reported to enhance dopaminergic neurotransmission in animal modelsCitation32. However, Jäger et al.Citation33 studied 17 different Danish folk medicine plants extracts and reported that some of the plant extracts (Borago officinalis L. and Arnica montana L.) exhibited significant inhibitory effects on MAO–A. It is very difficult to account for the inhibition mechanism, but both studies showed that the plants contain highly bioactive compounds, such as alkaloids, phenols, flavonoids, chalcones and coumarins, suggesting that these may responsible for the inhibitory effects on MAOCitation33.
Declaration of interest
This study was supported by Research Fund of Karadeniz Technical University (Project No: KTU-BAP 02-1159). The authors have declared no conflicts of interest with the presented data from this article.
References
- Laux G, Classen W, Sofic E, et al. Clinical, biochemical and psychometric findings with the new MAO-A-inhibitors moclobemide and brofaromine in patients with major depressive disorder. J Neural Transm 1990;32:189–95
- LeBlanc BW, Davis OK, Boue S, et al. Antioxidant activity of Sonoran Desert bee polen. Food Chem 2009;115:1299–305
- Li X-M, Juorio AV, Boulton AA. Some new mechanisms underlying the actions of deprenyl: possible relevance to neurodegeneration. Prog Brain Res 1995;106:99–112
- Greenawalt JW. Localization of monoamine oxidase in rat liver. Adv Biochem Psychopharmacol 1972;5:207–26
- Volz HP, Gleiter CH. Monoamine oxidase inhibitors. A perspective on their use in the elderly. Drugs Aging 1998;13:341–55
- Yamada M, Yasuhara H. Clinical pharmacology of MAO inhibitors: safety and future. Neurotoxicology 2004;25:215–21
- Al-Mamary MA, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res 2002;22:1041–7
- Özcan M, Ceylan A, Ünver A, Yetişir R. Antifungal effect of pollen and propolis extracts colleceted from different regions of Turkey. Uludağ Arıcılık Dergisi 2003;3:33–6
- Russo A, Cardile V, Sanchez F, et al. Chilean propolis: antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci 2004;76:545–58
- Almeida-Muradian LB, Pamplona LC, Coımbra S, Barth OM. Chemical composition and botanical evaluation of dried bee pollen pellets. J Food Compos Anal 2005;18:105-111
- Nasuti C, Gabbianelli R, Falcioni G, Cantalamessa F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr Res 2006;26:130–7
- Almaraz-Abarca N, Campos MG, Ávila-Reyes JA, et al. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J Food Compos Anal 2007;20:119–24
- Medeiros KCP, Figueiredo CAV, Figueredo TB, et al. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J Ethnopharmacol 2008;119:41–6
- Moreira L, Dias LG, Pereira JA, Estevinho L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem Toxicol 2008;46:3482–5
- (a) Kücük M, Kolayli S, Karaoglu Ş, et al. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem 2007;100:526–34. (b) Sahin H, Aliyazicioglu R, Yildiz O, et al. Honey, polen, and propolis extracts show potent inhibitory activity against the zinc metalloenzyme carbonic anhydrase. J Enzym Inhib Med Chem 2011;26:440–4
- Sahin H, Can Z, Yıldız O, et al. Inhibition of carbonic anhydrase isozymes I and II with natural products extracted from plants, mushrooms and honey. J Enzym Inhib Med Chem 2012;27:395–402
- Stafford GI, Pedersen PD, Jäger AK, Van Staden J. Monoamine oxidase inhibition by southern African traditional medicinal plants. S Afr J Bot 2007;73:384–90
- Kelekçi NK, Yabanoğlu S, Küpeli E, et al. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorgan Med Chem 2007;15:5775–86
- Mu LH, Wang B, Ren HY, et al. Synthesis and inhibitory effect of piperine derivates on monoamine oxidase. Bioorgan Med Chem Letter 2012;22:3343–8
- Dar A, Khan KM, Ateeq HS, et al. Inhibition of monoamine oxidase-A activity in rat brain by synthetic hydrazines: structure -- activity relationship (SAR). J Enzyme Inhib Med Chem 2005;20:269–74
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Viticult 1977;28:49–55
- Yıldız O, Alpaslan M. Properties of Rosehip Marmalades. Food Technol Biotechnol 2012;50:98–106
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 1996;239:70–6
- Holt A, Sharman DS, Baker GB, Palcic MM. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem 1997;244:384–92
- Schmidt K, Li Z, Schubert B, et al. Screening of entomopathogenic Deuteromycetes for activities on targets involved in degenerative diseases of the central nervous system. J Ethnopharmacol 2003;89:251–60
- Tezcan F, Kolaylı S, Sahin H, et al. Evaluation of organic acid, sacchraride composition and antioxidant properties of some authentic Turkish honeys. J Food Nutr Res 2011;50:33–40
- Aliyazıcioglu R, Şahin H, Erturk O, et al. Properties of phenolic composition and biological activity of propolis from Turkey. Int J Food Prop 2013;16:277–87
- Gülcin İ, Bursal E, Şehitoğlu MH, et al. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–38
- Ahn MR, Kumazawa S, Usui Y, et al. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem 2007;101:1383–92
- Gregoris E, Stevanato R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem Toxicol 2010;48:76–82
- Alper G, Özgönül M, Sağin F, et al. The effect of a monoamine oxidase (MAO)-B inhibitor; pargyline on oxidant stress/antioxidant status in aging rat tissues. Ege Tıp Dergisi 2007;46:27–32
- Fehske CJ, Leuner K, Muller WE. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol Res 2009;60:68–73
- Jäger AK, Gauguin B, Andersen J, et al. Screening of plants used in Danish folk medicine to treat depression and anxiety for affinity to the serotonin transporter and inhibition of MAO-A. J Ethnopharmacol 2013;145:822–5