Abstract
Human carbonic anhydrase XII (CA XII) is a single-pass transmembrane protein with an extracellular catalytic domain. This enzyme is being recognized as a potential biomarker for different tumours. The current study was aimed to generate monoclonal antibodies (MAbs) neutralizing the enzymatic activity of CA XII. Bioinformatics analysis of CA XII structure revealed surface-exposed sequences located in a proximity of its catalytic centre. Two MAbs against the selected antigenic peptide spanning 167–180 aa sequence of CA XII were generated. The MAbs were reactive with recombinant catalytic domain of CA XII expressed either in E. coli or mammalian cells. Inhibitory activity of the MAbs was demonstrated by a stopped flow CO2 hydration assay. The study provides new data on the surface-exposed linear CA XII epitope that may serve as a target for inhibitory antibodies with a potential immunotherapeutic application.
Introduction
Cancer is one of the leading causes of death in the world. According to the GLOBOCAN 2008 statistics, the most frequently diagnosed cancers are breast and lung cancers followed by stomach, liver, cervix, colorectal and prostate cancersCitation1. Cancer is a multifactorial disease with different phenotypic and genotypic characteristics, treatment regimes and recurrence risksCitation2,Citation3. Hypoxia is one of the features, which is shared by many cancer typesCitation4. Hypoxia state in cancer cells is different from that in normal tissues. Tumour cells develop faster than the vasculature leading to the absence of both oxygen and nutrientsCitation5. The lack of oxygen activates a heterodimeric transcription factor termed the hypoxia-inducible factor (HIF) that binds to a hypoxia response elements (HREs) of target genes: glucose transporters (GLUT1 and GLUT3), vascular endothelial growth factor (VEGF), angiogenin, platelet-derived growth factor-β, transforming growth factor-β, and insulin-like growth factor-II, various enzymes, proteins involved in tumour invasiveness, and other related proteinsCitation6,Citation7. During adaptation to hypoxia, cancer cells switch glucose metabolism from oxidative phosphorylation in mitochondria to lactic acid formation in the cytoplasmCitation8, which leads to an acidification of tumour microenvironmentCitation9. In the regulation of acid–base balance, an important role is played by the carbonic anhydrases (CAs) – zinc enzymes that exist in almost all organisms. They catalyze the reversible hydration of carbon dioxide to bicarbonate. Sixteen α-CA isoforms with different enzymatic activity, structure, cellular localization and tissue distribution are found in mammalsCitation10. They are also involved in CO2 and transport, respiration, bone resorption, ureagenesis, gluconeogenesis, lipogenesis, production of body fluidsCitation11. It is now known, that two hypoxia-inducible transmembrane carbonic anhydrases IX and XII (CA IX, CA XII) promote the development and survival of tumour cells through pH homeostasisCitation12.
Cancer-associated CA IX is present in few normal human tissues, but is ectopically expressed in some cancer cells, including colon, cervical, breast, renal and brain tumoursCitation13. Expression of CA IX is also associated with a poor prognosisCitation14. Therefore, CA IX is now recognized as cancer biomarker and also as a potential target for cancer therapyCitation15. In contrast, CA XII is present in a wide variety of normal tissues. However, expression of CA XII significantly increases during carcinogenesis, and it is known to be overexpressed in renal, brain, colon, breast, gastrointestinal, ovarian and pancreatic tumoursCitation16. Therefore, the diagnostic and prognostic significance of CA XII is now being intensively investigated. The association of CA XII expression with non-small cell lung cancers and correlation with better survival of patients has been demonstratedCitation16. In diffuse astrocytomas, CA XII expression correlated with poorer prognosisCitation17. Other studies indicate that CA XII, as well as CA IX, is not a specific biomarker of gastric tumoursCitation18. In human cervical tumours, CA XII expression was associated with lower risk of metastasis formationCitation19.
Based on structural features, cellular localization and the role of CA XII in tumour cell growth, CA XII is now considered as a promising target for specific cancer treatmentCitation20. Attempts are made to develop specific chemical inhibitors for CAs. Sulfonamides (acetazolamide, methazolamide, ethoxzolamide and dichlorophenamide) represent the main group of CA inhibitors. However, they strongly inhibit most CA isoforms, which leads to the necessity of development of new compounds that selectively inhibit tumour associated CA IX and CA XIICitation10. It was demonstrated that compounds based on coumarins (7-glycosylated 4-methyl coumarins) significantly inhibited the growth of primary tumour in mouse model by selectively inhibiting CA IX and CA XIICitation21.
Besides of small molecules, monoclonal antibodies (MAbs) are considered as highly specific inhibitors of CA IX and XIICitation22. MAbs are important diagnostic reagents and essential research tools for many immunoassay applicationsCitation23. In addition, MAb-based treatment of cancer is becoming one of the most successful therapeutic strategies. MAbs can not only inhibit the function of tumour antigen but also activate antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), leading to cancer cell deathCitation24. Considering the importance of CA IX in tumour development, chimeric MAb against CA IX has been generated and evaluated in a clinical trial (WX-G250, Rencarex; WILEX, Munich, Germany) for clear cell renal cell carcinoma treatmentCitation25. CA XII is still less studied and understood enzyme, involved in tumour progression. Recently, two research groups established different MAbs against CA XII – for immunohistochemistry and flow cytometry using random immunization method with A549 cell lysate or live cells, respectivelyCitation26,Citation27. It was demonstrated that the MAb 6A10 raised against A549 cells inhibits CA XII enzymatic activity and tumour growth in vitroCitation27.
In the current study, we applied bioinformatics analysis for tailored production of MAbs directed against a selected segment of the catalytic domain of CA XII and demonstrated the ability of newly developed MAbs to recognize full-length CA XII and inhibit its enzymatic activity.
Materials and methods
Bioinformatics analysis of CA XII protein and selection of a target peptide
Modelling of CA XII structure and selection of potential antigenic sequences located in a proximity of its catalytic centre was performed using Accelrys Discovery Studio Visualizer (version 3.1) computer program (Accelrys Software Inc., San Diego, CA). Alignment of selected sequences with murine proteins was performed by scanning against Uniprot database using BLAST programCitation28.
Synthetic peptide spanning selected CA XII sequence (aa 167–180) conjugated with keyhole limpet hemocyanin (KLH) was obtained from Mimotopes (Melbourne, Australia).
Production of recombinant CA XII
Recombinant catalytic domain of CA XII spanning amino acid (aa) residues from 30 to 291 was produced in E. coli cells and purified as described previouslyCitation29. In brief, E. coli Rosetta (DE3) strain cells (Novagen, Darmstadt, Germany) were transformed with recombinant expression vector pET21a-CA XII. Transformants were cultured in Luria Bertani (LB) medium, containing 100 µg/ml ampicillin, 34 µg/ml chloramphenicol and grown at 37 °C and 220 rpm for 16 h. The expression of CA XII was induced with 1 mM isopropyl b-d-thiogalactoside (IPTG) and 0.5 mM ZnSO4. The culture was grown for 4 h at 30 °C and 220 rpm. The cells were harvested, mixed with lysis buffer (20 mM Hepes, 0.1% Triton X-100, 0.15 M NaCl, (pH 8.5) and 1 mM PMSF) and disrupted by sonication. The soluble protein fraction was purified using a CA-affinity column containing p-aminomethylbenzene sulfonamide-agarose (Sigma-Aldrich Co, St. Louis, MO). Eluted CA XII protein was dialyzed against a storage buffer containing 10 mM Hepes (pH 7.5) and 50 mM NaCl.
Recombinant CA I, CA II, CA VII and CA XIII were expressed in E. coli and purified as described previouslyCitation30. Recombinant CA XII was expressed in mammalian cells and purified as described previouslyCitation31.
Immunization of mice
Three 6–8-week-old female BALB/c mice (obtained from a breeding colony at the Department of Immunology of the Center for Innovative Medicine, Vilnius, Lithuania) were immunized by a subcutaneous injection of 50 μg of synthetic peptide conjugated to KLH (Mimotopes). For an initial immunization, the antigen was emulsified in complete Freund adjuvant (Sigma-Aldrich Co) Subsequent immunizations at days 28 and 56 were performed without an adjuvant, with the antigen dissolved in PBS. Antisera were collected 2 weeks after each injection and tested by an indirect ELISA for the presence of antibodies specific to CA XII protein.
Generation of hybridomas
The mouse with the highest antibody titer against the recombinant CA XII was boosted subcutaneously with 50 μg peptide/KLH conjugate dissolved in PBS 3 days before the cell fusion. Hybridomas were generated as described by Kohler and MilsteinCitation32. Mouse splenocytes were fused with Sp2/0-Ag 14 mouse myeloma cells using polyethylene glycol 4000 (PEG; Carl Roth, Karlsruhe, Germany). Hybrid cells were selected in growth medium supplemented with HAT (hypoxanthine, aminopterin, thymidine) (50 x HAT media supplement, Sigma-Aldrich Co). Culture supernatants from wells with viable clones were screened by an indirect ELISA using recombinant CA XII protein. Stable hybridoma clones secreting specific antibodies to CA XII protein were obtained after two cloning cycles by a limiting dilution assay. Hybridoma cells were grown in complete Dulbecco’s modified Eagle’s medium (DMEM, Biochrom Merck Millipore, Darmstadt, Germany) supplemented with 15% fetal bovine serum (FBS, Biochrom Merck Millipore), 2 mM l-glutamine, 200 µg/ml gentamicine. All procedures involving experimental mice were performed under controlled laboratory conditions in strict accordance with the Lithuanian and European legislation.
Indirect ELISA
The specificities of mouse antisera and hybridoma supernatants were investigated by an indirect ELISA. Recombinant CA XII diluted in coating buffer (0.05 M sodium carbonate salt, pH 9.6) to 5 μg/mL was coated on plates (Nerbe plus GmbH, Winsen, Germany) at 50 μL aliquot per well and incubated at 4 °C overnight. The coated wells were blocked with 150 μL of 1% BSA for 1 h at room temperature (RT). Plates were washed 2 times with PBS-Tween buffer (0.1% Tween 20 in PBS). Antiserum samples or hybridoma growth medium were diluted in PBS-Tween buffer, added to the wells (50 μL/well) and incubated for 1 h at RT. The plates were rinsed 5 times with PBS-Tween buffer and then incubated with 50 μL horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad Laboratories, Hercules, CA) diluted 1:5000 in PBS-Tween buffer for 1 h at RT. The plates were washed 5 times with PBS-Tween buffer. Peroxidase activity was detected using 50 μL of ready-to-use TMB substrate (Sigma-Aldrich Co) per well. After 10 min of incubation at RT, the reaction was stopped by adding 25 μL aliquot per well of 10% H2SO4. The optical density (OD) was measured at 450 nm (reference filter 620 nm) using microplate reader (Tecan, Groedig, Austria).
The isotypes of the MAbs were determined by ELISA using the Monoclonal Antibody Isotyping kit (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s protocol.
Determination of the apparent dissociation constant (Kd)
The apparent dissociation constants (Kd) of the MAbs were determined by an indirect ELISA as described previouslyCitation33. Briefly, the MAbs were prepared in concentrations ranging from 1.9 × 10−13 M to 3.3 × 10−8 M and incubated in the microtiter plates coated with recombinant CA XII. The plates were then incubated with HRP-labelled anti-mouse IgG (Bio-Rad Laboratories) and developed with TMB substrate. The apparent Kd was calculated from a titration curve and defined as a molar concentration of the MAbs corresponding to the mid-point between maximum OD450 value and the background.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein samples were added to the Line Marker Reducing sample buffer (Thermo Fisher Scientific) and boiled for 5 min. Protein samples (1 µg per lane) were separated by electrophoresis on 12% polyacrylamide gel. The gels were visualized by staining with Coomassie brilliant blue (Sigma-Aldrich Co).
Immunoblotting
After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Carl Roth). The membranes were blocked with 5% milk powder in PBS for 1 h at RT. The membranes were incubated with undiluted hybridoma supernatants for 1 h at RT, followed by incubation with goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) (Bio-Rad Laboratories) diluted 1:4000 in PBS-Tween buffer. The enzymatic reaction was developed using tetramethylbenzidine (TMB) ready-to-use chromogenic substrate (Sigma-Aldrich Co).
Immunohistochemistry analysis
Human tumour samples were prospectively collected from 30 patients with different types of breast and lung cancers treated at the Institute of Oncology of Vilnius University. Informed consent was obtained and documented in writing before study entry. The study was approved by the Lithuanian Bioethics Committee (2013-02-12, No. 158200-13-564-180).
Immunohistochemical staining was performed on 30 formalin-fixed and paraffin-embedded samples of the breast and lung tumours at the National Center of Pathology (Vilnius, Lithuania). Tissue microarray (TMA) was constructed from 10% buffered formalin-fixed paraffin-embedded tissue blocks. One millimetre-diameter cores were punched from tumour areas randomly selected by pathologist (two cores per patient) using the tissue arraying instrument (3DHISTECH, TMA Master, Budapest, Hungary). Paraffin sections of the TMA were cut for IHC (approximately 2 μm thick) and were stained using an automated Dako Autostainer (Dako, Glostrup, Denmark). Immunohistochemistry was performed using the Dako REAL EnVision detection system with HRP (Dako) according to the manufacturer’s protocol. Retrieval was done by heating up to 97 °C for 1 h in a retrieval solution (EnVision™ FLEX target retrieval solution, High pH, Dako). Tissue sections were incubated in Envision FLEX Peroxidase-Blocking Reagent (Dako) for 5 min and then slides were incubated with the MAbs for 30 min. After rinsing, samples were incubated in poly-HRP-conjugated anti-rabbit/mouse IgG (EnVision™ FLEX/HRP, Dako) for 20 min. Slides were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution (EnVision™ FLEX DAB + Chromogen, Dako) two times for 5 min and counterstained with Hematoxylin for 10 min.
Determination of the inhibitory activity of the MAbs
The SX20 stopped-flow Spectrometer (Applied Photophysics, Surrey, United Kingdom) has been used for assaying the CA catalysed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.4) as buffer, and 20 mM Na2SO4 (for maintaining constant ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 sCitation34. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each MAb at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of both MAbs (10 µM) were prepared in distilled-deionised water and dilutions up to 0.001 nM were done thereafter with distilled-deionised water. Antibody and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow formation of the enzyme-inhibitor (E-I) complex (the proteins were incubated also for longer periods, of 1–24 h, but no differences of activity have been detected). The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlierCitation27,Citation35,Citation36, and represent the mean from at least three different determinations. Tested CA isoforms CA XII and CA II were recombinant ones obtained in house as reported earlierCitation27,Citation35,Citation37.
Results
Selection of CA XII target sequences
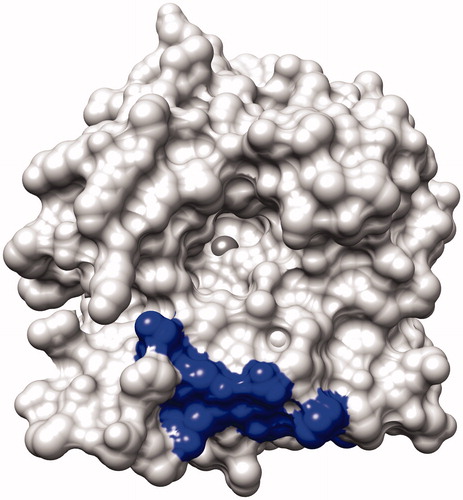
To generate inhibitory MAbs targeting the catalytic center of CA XII, bioinformatics approaches were applied. For this purpose, computer model of CA XII catalytic domain based on PDB entry 1JD0 was developed and surface-exposed sequences located in a close proximity of the catalytic center were selected. Five candidate peptides representing continuous sequences of CA XII catalytic domain were identified: #1 (aa 1–22), #2 (aa 70–77), #3 (aa 117–128), #4 (aa 233–242), #5 (aa 167–180). In the next step, the alignment of candidate sequences with murine proteins was performed in order to select antigenic peptides capable to induce immune response in mice. Peptides #1–4 shared 78–91% identity with different murine proteins such as murine CA XII (78% homology with peptide #1, 83% with peptide #2 and 91% with peptide #4) and murine KRAB box containing zinc finger protein (85% homology with peptide #3). In contrast, peptide #5 shared only 60% identity with murine protein (P2X purine receptor). Therefore, this peptide was selected for immunization experiments expecting to avoid immune tolerance in mice. The localization of the selected sequence #5 in CA XII model is presented in .
Generation of monoclonal antibodies against selected 167-180 aa sequence of CA XII
Synthetic peptide spanning sequence #5 (aa 167–180 of CA XII) conjugated to KLH carrier protein was used to immunize BALB/c mice in order to generate the MAbs. To evaluate the immunogenicity of the synthetic peptide, antiserum specimens were collected after each injection and tested for the presence of antibodies specific to recombinant CA XII protein by indirect ELISA. After three immunizations, the titers of CA XII-specific IgG antibodies in the sera of immunized mice ranged from 1:5000 to 1:20 000 (data not shown). One mouse with the highest antibody titer was sacrificed and spleen cells were fused with mouse myeloma cells following standard procedures. At day 12 after cell fusion, hybrid clones secreting CA XII-specific antibodies were screened by indirect ELISA on plates coated with recombinant CA XII protein. Two stable hybridoma cell lines producing CA XII-specific MAbs of IgG isotype were generated: clone #1D8 and clone #3C8 (both of subtype IgG1). The hybridomas were cultivated in culture and the supernatants were collected for further characterization of the MAbs.
Determination of MAb specificity and affinity
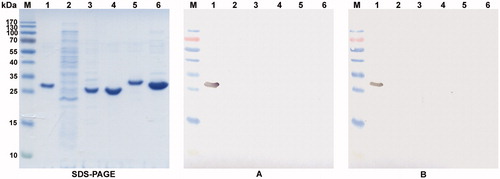
Both MAbs (clones #1D8 and #3C8) were reactive with recombinant CA XII expressed in E. coli cells by ELISA (data not shown). To test the reactivity of the MAbs with SDS-denaturated antigen, recombinant purified CA XII protein and crude lysate of E. coli transformed with pET21a-CA XII vector were denatured by boiling in SDS and 2-mercaptoethanol, and subjected to immunoblotting. The MAbs specifically recognized protein band of 31 kDa, which corresponded to recombinant CA XII protein (, lane 1) and did not cross-react with other proteins of E. coli lysate (, lane 2). The reactivity of the MAbs with other recombinant CAs, such as CA I, II, VII and XIII was tested both by ELISA and immunoblotting. For this purpose, recombinant purified CAs were used. The MAbs reacted exclusively with CA XII and did not react with CA I, II, VII and XIII (, lanes 3–6). This might be explained by differences in the respective aa sequences among different CA isoforms. The alignment of CA XII aa 167–180 sequence with the respective sequences of CA IV, VI and IX revealed low degree of similarity ranging from 38% between CA XII and CA VI to 50% between CA XII and CA IV, CA IX. The alignment with the respective sequences of CA I, II, VII, XIII and XIV revealed no similarity with CA XII.
Figure 2. The reactivity of the MAbs 1D8 and 3C8 with denaturated recombinant CA I, CA II, CA VII, CA XII and CA XIII proteins. Left panel – SDS-PAGE; right panel – immunoblot with the MAbs 1D8 (A) and 3C8 (B). Line M, pre-stained MW markers (Thermo Fisher Scientific); line 1, purified recombinant CA XII protein expressed in E. coli; line 2, lysate of E. coli Rosetta (DE3) cells; line 3--6, purified recombinant CA I, CA II, CA VII and CA XIII proteins, respectively.
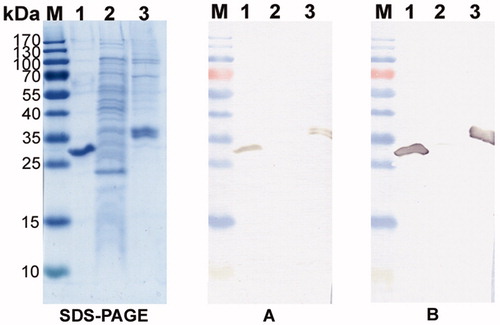
To evaluate the ability of the MAbs to recognize glycosylated form of CA XII, their reactivities with recombinant CA XII expressed in mammalian cells was tested. The MAbs were reactive with CA XII expressed in human cell line HEK293 as demonstrated both by ELISA and immunoblotting (, lane 3).
Figure 3. The reactivity of MAbs 1D8 and 3C8 with denaturated recombinant CA XII expressed in E. coli and HEK 293 cells. Left panel – SDS-PAGE; right panel – immunoblot with the MAbs 1D8 (A) and 3C8 (B). Line M, pre-stained MW markers (Thermo Fisher Scientific); line 1, purified recombinant CA XII protein expressed in E. coli; line 2, lysate of E. coli Rosetta (DE3) cells; line 3, purified recombinant CA XII expressed in HEK 293 cells.
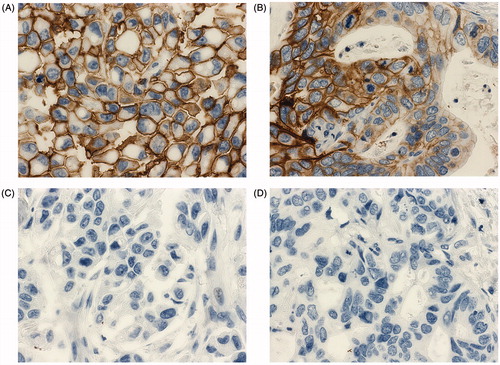
To test the ability of the MAbs to recognize cellular CA XII, they were employed in immunohistochemistry (IHC) assay using formalin-fixed and paraffin-embedded specimens of human breast and lung cancer tissues. The MAb 3D8 showed a strong specific immunostaining of tumour specimens () while an irrelevant MAb 1F8 of IgG1 subtype raised in-house against Porcine Parvovirus (PPV) used as a negative control did not show any unspecific staining of the same specimens (). In contrast to the MAb 3D8, the MAb 1D8 used at the same concentration was non-reactive with cellular CA XII in human tumour tissue specimens (data not shown). The expression of CA XII in the respective tumour tissue specimens was confirmed based on their immunostaining (data not shown) with CA XII-specific MAb 15A4Citation31.
Figure 4. Immunohistochemistry staining of human invasive ductal carcinoma of the breast (A, C) and human lung adenocarcinoma (B, D) specimens for CA XII expression using the MAb 3D8 (A, B) and an irrelevant MAb 1F8 (C, D). The hibridoma supernatant was diluted 1:10. Original magnification × 400.
To characterize the affinity of MAbs, their apparent Kd values were determined by an indirect ELISA. The Kd values for the MAbs 1D8 and 3C8, as calculated from three experiments, were 6.1 × 10−9 M and 1.19 × 10−9 M, respectively, which indicates high affinity binding.
Inhibitory activities of the MAbs
Inhibitory activities of the MAbs 1D8 and 3C8 were investigated by a stopped flow CO2 hydration assay. MAb concentrations for the inhibition test ranged from 0.001 nM to 10 µM. Prior to assay, CA XII was preincubated with each MAb for 15 min in order to allow formation of the enzyme−antibody complex. The optimum preincubation time was selected by incubating CA XII with the respective MAbs for longer periods of time (1−24 h). No differences in enzyme activity have been detected after 1−24 h of preincubation as compared to 15 min (data not shown). The inhibition constants for the MAbs 1D8 and 3C8 were calculated by non-linear least-squares methods from at least three different determinations. As a negative control, recombinant CA II was usedCitation27. Data on the inhibitory activities of MAbs 1D8 and 3C8 are summarized in . It was determined that the MAbs 1D8 and 3C8 are highly inhibitory against the target isoform – CA XII (IC50 values are 49.2 nM and 6.6 nM, respectively), whereas they are not at all inhibitory against the offtarget isoform – CA II (IC values>100 000 nM).
Table 1. Data on the inhibitory activities of the MAbs 1D8 and 3C8.
Discussion
CA XII is tumour-associated single-pass transmembrane protein with an extracellular catalytic domain. This enzyme is now being recognized as a potential biomarker for different tumours and a promising target for tumour-specific therapyCitation26. Along with CA IX, an established tumour marker, the significance of CA XII in tumour growth is now under investigation. Recent studies demonstrate that the combined silencing of CA IX and CA XII gives a dramatic decrease in the rate of growth of xenograft tumours in mice. Moreover, invalidation of CA IX leads to partial compensation by up-regulation of CA XII. For this reason, CA XII together with CA IX is considered as novel and potentially efficient drug targetCitation12. Several classes of chemical inhibitors of CAs are currently in use: sulphonamides, non-sulphonamides, organic inhibitors, anionic, inorganic inhibitorsCitation38,Citation39. However, it is a complicated task to design small-molecule inhibitors with high selectivity towards certain CA isoforms. To date, only a few small molecules have been characterized in CA-relevant cell and animal model systems. Ahlskog et al. have described the synthesis and properties of two novel acetazolamide derivatives, which preferentially target membrane-associated carbonic anhydrases on the surface of tumour cellsCitation40. Recently described cationic ruthenium(II) pentamethylcyclopentadienyl benzenesulfonamide sandwich complexes inhibited the growth of cancerous cells in vitro at low micromolar concentrations while expressing lower levels of toxicity towards the normal human cell lineCitation41. Morris et al. designed and synthesized a new class of carbohydrate-based small molecules that inhibit both CA IX and CA XII enzymes. These compounds were designed to selectively target cancer-relevant CA isozymes (with an extracellular active site) over other (intracellular) CA isozymesCitation42. Coumarins were recently discovered to act as inhibitors of CAs. A small series of 7-glycosylated 4-methyl coumarins were generally ineffective or weak inhibitors of CA I and II, but many of them were highly efficient as CA IX and XII inhibitors. One of these compounds significantly inhibited the growth of primary tumours formed by highly aggressive 4T1 cellsCitation21. These molecules constitute interesting candidates for the development of conceptually novel anticancer drugs. Along with small-molecule inhibitors, MAbs are considered as promising tools for cancer treatment. These two elements of different therapeutic strategies vary in several pharmacological properties – molecule size, route of administration, efficiency for tissue penetrationCitation43. The advantage of therapeutic MAbs is their capability to bind antigens with a highly specific selectivity and affinity, while small-molecule inhibitors are generally thought to be less specificCitation44,Citation45.
Two MAbs M75 and G250, directed at the PG-like domain of CA IX, are well known and have been described previously. MAb G250 was developed after immunization of mice with intact human renal cell carcinoma cells and the MAb M75 was derived after immunization with HeLa cellsCitation22,Citation46,Citation47. Both MAbs are used widely for immunohistochemistry and imaging of CA IX in hypoxic tumours. For anticancer immunotherapy, a chimeric version of G250 has been developed and phase III trials have now been initiated. Recent studies are focused on developing antibodies targeting CA IX catalytic domain. These antibodies have the advantage of specifically disrupting the catalytic activity of the enzyme, thus targeting its tumorigenic functions, including pH regulation. High throughput screening technologies, especially the use of phage display libraries, have been employed to efficiently identify CA IX-specific antibodiesCitation22.
Recently, novel MAb 6A10 that binds to the extracellular domain of CA XII and inhibits its catalytic activity at low nanomolar concentrations was described and patentedCitation27. The MAb 6A10 was generated by immunizing rats with A549 lung cancer cells and selecting positive hybridomas by flow cytometry using vital A549 cellsCitation27. Although no information on the epitope recognized by the MAb 6A10 is provided, most probably it is directed against a conformational epitope as the MAb was generated against cellular CA XII. In our study, we have used another approach to generate inhibitory murine MAbs against CA XII. We have applied computer modelling to identify potential target sequences of CA XII located near its catalytic center, and then selected antigenic sequences based on their alignment with murine proteins to avoid immune tolerance in mice. After immunization of mice with selected target peptide spanning aa 167–180 sequence of CA XII, two hybridoma cell lines producing high-affinity MAbs of IgG1 subtype were generated. The MAbs recognized recombinant extracellular domain of CA XII and did not cross-react with other CA isoforms, such as CA I, II, VII and XIII. Moreover, the MAbs raised against the peptide were reactive with recombinant CA XII expressed in HEK 293 cells, which differs from the E. coli-expressed protein according to its glycosylation pattern. The reactivity of the MAbs with glycosylated CA XII is in line with sequence analysis data showing that the aa 167–180 sequence of CA XII (VKYKGQEAFVPGF) does not contain potential glycosylation sites. The exclusive reactivity of the MAbs with CA XII might be explained by a low degree of similarity among the aa 167–180 sequence of CA XII and the respective aa sequences of other CA isoforms (0–50%). dIn addition, one of the MAbs (clone 3D8) was capable of recognizing cellular CA XII in formalin-fixed and paraffin-embedded specimens of human tumour tissues, which demonstrates the potential of this MAb as a specific diagnostic reagent. Inhibitory test by the stopped flow CO2 hydration assay has revealed that both MAbs 1D8 and 3C8 are highly inhibitory against CA XII. Although the MAbs 1D8 and 3C8 are directed to the same linear epitope of CA XII, their IC50 values are different (49.2 nM and 6.6 nM, respectively), which may be explained by their different affinities (Kd 6.1 × 10−9 M and 1.19 × 10−9 M, respectively). Inhibitory activity of the MAb 3C8 is comparable to that previously reported for the MAb 6A10 (3.1 nM), raised against cellular CA XIICitation27. In conclusion, our study provides new data on the surface-exposed linear CA XII epitope that may serve as a target for inhibitory antibodies with a potential immunotherapeutic application.
Declaration of interest
This work was supported by the Research Council of Lithuania (grant No. MIP-37/2012) and the EU FP7 project (Metoxia). The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90
- Essaghir A, Demoulin JB. A minimal connected network of transcription factors regulated in human tumours and its application to the quest for universal cancer biomarkers. PLoS One 2012;7:e39666
- Aktipis CA, Nesse RM. Evolutionary foundations for cancer biology. Evol Appl 2013;6:144–59
- Moehler TM, Ho AD, Goldschmidt H, Barlogie B. Angiogenesis in hematologic malignancies. Crit Rev Oncol Hematol 2003;45:227–44
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198–206
- Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun 2005;338:617–26
- Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001;93:266–76
- Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 2007;19:223–9
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–9
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Hilvo M, Baranauskiene L, Salzano AM, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–809
- Chiche J, Ilc K, Laferrière J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumour cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009;69:358–68
- Pastorekova S, Parkkila S, Zavada J. Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 2006;42:167–216
- Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumour oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 2001;61:6394–9
- Kallio H, Rodriguez Martinez A, Hilvo M, et al. Cancer-associated carbonic anhydrases ix and xii: effect of growth factors on gene expression in human cancer cell lines. J Cancer Mol 2010;5:73–8
- Ilie MI, Hofman V, Ortholan C, et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer 2011;128:1614–23
- Haapasalo J, Hilvo M, Nordfors K, et al. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol 2008;10:131–8
- Leppilampi M, Saarnio J, Karttunen TJ, et al. Carbonic anhydrase isozymes IX and XII in gastric tumours. World J Gastroenterol 2003;9:1398–403
- Kim JY, Shin HJ, Kim TH, et al. Tumor-associated carbonic anhydrases are linked to metastases in primary cervical cancer. J Cancer Res Clin Oncol 2006;132:302–8
- Chien MH, Ying TH, Hsieh YH, et al. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol 2012;48:417–23
- Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumours. J Med Chem 2011;54:8271–7
- McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012;3:84–97
- Saleem M, Kamal M. Monoclonal antibodies in clinical diagnosis: a brief review application. Afr J Biotechnol 2008;7:923–5
- Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Canc Immun 2012;12:14–21
- Tostain J, Li G, Gentil-Perret A, Gigante M. Carbonic anhydrase 9 in clear cell renal cell carcinoma: a marker for diagnosis, prognosis and treatment. Eur J Cancer 2010;46:3141–8
- Kobayashi M, Matsumoto T, Ryuge S, et al. CAXII Is a sero-diagnostic marker for lung cancer. PLoS One 2012;7:e33952
- Battke C, Kremmer E, Mysliwietz J, et al. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol Immunother 2011;60:649–58
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol 1990;215:403–10
- Jogaitė V, Zubrienė A, Michailovienė V, et al. Characterization of human carbonic anhydrase XII stability and inhibitor binding. Bioorg Med Chem 2013;21:1431–6
- Dudutienė V, Zubrienė A, Smirnov A, et al. 4-Substituted-2,3,5,6-tetrafluorobenzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, XII, and XIII. Bioorg Med Chem 2013;21:2093–106
- Dekaminaviciute D, Lasickiene R, Parkkila S, et al. Development and characterization of new monoclonal antibodies against human recombinant CA XII. Biomed Res Int 2013 [submitted]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495–7
- Zvirbliene A, Pleckaityte M, Lasickiene R, et al. Production and characterization of monoclonal antibodies against vaginolysin: mapping of a region critical for its cytolytic activity. Toxicon 2010;56:19–28
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumour-associated human carbonic anhydrase IX. Proc Natl Acad Sci U S A 2009;106:16233–8
- Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev 2005;24:487–99
- Lou Y, McDonald PC, Oloumi A, et al. Targeting tumour hypoxia: suppression of breast tumour growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76
- Krishnamurthy VM, Kaufman GK, Urbach AR, et al. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. Chem Rev 2008;108:946–1051
- Čapkauskaitė E, Zubrienė A, Baranauskienė L, et al. Design of [(2-pyrimidinylthio)acetyl]benzenesulfonamides as inhibitors of human carbonic anhydrases. Eur J Med Chem 2012;51:259–70
- Ahlskog JK, Dumelin CE, Trüssel S, et al. In vivo targeting of tumour-associated carbonic anhydrases using acetazolamide derivatives. Bioorg Med Chem Lett 2009;19:4851–6
- Loughrey BT, Williams ML, Healy PC, et al. Novel organometallic cationic ruthenium(II) pentamethylcyclopentadienyl benzenesulfonamide complexes targeted to inhibit carbonic anhydrase. J Biol Inorg Chem 2009;14:935–45
- Morris JC, Chiche J, Grellier C, et al. Targeting hypoxic tumour cell viability with carbohydrate-based carbonic anhydrase IX and XII inhibitors. J Med Chem 2011;54:6905–18
- Butowski N, Chang SM. Small molecule and monoclonal antibody therapies in neurooncology. Cancer Control 2005;12:116–24
- Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 2006;6:714–27
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006;6:343–57
- Oosterwijk-Wakka JC, Boerman OC, Mulders PF, Oosterwijk E. Application of monoclonal antibody g250 recognizing carbonic anhydrase ix in renal cell carcinoma. Int J Mol Sci 2013;14:11402–23
- Pastoreková S, Závadová Z, Kostál M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992;187:620–6