Abstract
The feasibility for microplate-based screening of inhibitors of isozymes of cyclic nucleotide phosphodiesterase (PDE) was tested via the coupled action of a phosphatase on adenosine-5′-monophosphate and an improved malachite green assay of phosphate. Human full-length PDE4B2 and truncated mutant (152–528aa) were expressed in Escherichia coli via fusion to SUMO, which after purification through Ni-NTA column exhibited specific activities >0.017 U mg−1. In the presence of proteins <30 mg L−1, absorbance for 10 µΜ phosphate was measurable; a PDE isozyme of specific activity over 0.008 U mg−1 after reaction for 20 min thus suited for microplate-based screening of inhibitors. By using Biotek ELX 800 microplate reader, affinities of two forms of PEDE4B2 for cAMP, rolipram and papaverine varied over three magnitudes and were consistent with those by routine assay, respectively. Hence, the proposed method was promising for high-throughput-screening of inhibitors of phosphate-releasing enzymes bearing specific activities over 0.008 U mg−1.
Introduction
Cyclic nucleotide phosphodiesterase (PDE) isozymes are important targets to screen their inhibitors as potential drugsCitation1–3. High-throughput-screening (HTS) is always required for affordable cost and favorable efficiency to discover new inhibitors of any enzyme. For HTS of PDE inhibitors, a suitable method is hungered for high-throughput-assay (HTA) of PDE activities. The substrate 3′,5′-cyclic adenosine monophosphate (cAMP) or the product adenosine-5′-monophosphate (AMP) can be quantified to measure PDE activities. Chromatographic analyses of cAMP or AMP are clearly unsuitable for HTA of PDE activitiesCitation4. Fluorometric methods work for HTA of PDE activities, but suffer heavy cost and the sensitivity of fluorescence to organic solvents in stock solutions of candidate inhibitorsCitation5,Citation6. Classical enzyme-coupled spectrophotometric assay of AMP for HTA of PDE activities requires many auxiliary enzymes and co-substrates; the potential interference of candidate inhibitors with auxiliary enzymes, the heavy cost on auxiliary enzymes and co-substrates, and the unsatisfactory lower limits of quantification restrict their use for HTS of PDE inhibitorsCitation7,Citation8. Hence, new methods suitable for HTS of inhibitors of PDE isozymes are still needed.
Recently, a facile colorimetric method was developed to measure PDE activities via the coupled action of just one non-specific phosphatase to release phosphate from AMP and an improved malachite green assay of phosphateCitation9–11. This proposed method is clearly much cost-effective and may be applicable to HTS of inhibitors of PDE isozymes as well as other enzymes directly or indirectly releasing phosphate. For HTS of PDE inhibitors via the proposed method, both the release of phosphate from AMP and the quantification of phosphate by the improved malachite green assay should tolerate in samples co-existing proteins, PDE inhibitors and organic solvents from stock solutions of most inhibitors. The use of a non-specific phosphatase at high enough levels can effectively release phosphate from AMP; the improved malachite green assay of phosphate is resistant to common PDE inhibitors and dimethylsulfoxide (DMSO) as the organic solvent of common inhibitorsCitation9,Citation11. However, PDE isozymes had low specific activities and thus had to be used at high levels to produce sufficient quantities of phosphate for analysis, but the improved malachite green assay of phosphate tolerates limited levels of co-existing proteins. Removal of proteins in samples of phosphate before analysis hampers HTS. Hence, the proposed method is suitable for HTS of PDE inhibitors only when co-existing proteins cause no interference.
The use of a small quantity of a PDE isozyme can potentially avoid the interference of co-existing proteins with HTS of PDE inhibitors via the proposed method as long as the PDE isozyme has high enough a specific activity. Selective inhibitors of PDE isozyme 4 (PDE4) are potential anti-inflammation and anti-depression drugsCitation12,Citation13. Active full-length PDE4B2 was produced in Escherichia coli via fusion to maltose-binding protein, but the expressed PDE4 exhibited a low specific activity at low yieldCitation9–11. PDE4B has an inhibitory N-terminusCitation14,Citation15; the truncated mutant of PDE4B after the inhibitory N-terminus is deleted may have higher specific activity for HTS of inhibitors of PDE4 via the proposed method. Small-ubiquitin-related-modifier (SUMO) is a smaller tag for fused expression of eukaryotic proteins in Escherichia coli with higher efficiency and yieldCitation16,Citation17. Herein, with full-length PDE4B2 and its truncated mutant in fusion to SUMO as models of targets, we tested HTS of PDE inhibitors via the proposed method.
Materials and methods
Materials and reagents
Malachite green (free base), cAMP, calf intestinal alkaline phosphatase (CIAP), poly(vinylalcohol) (PVA) and p-toluenesulfonylfluoride (PMSF) were from Sigma-Aldrich (St. Louis, MO). Isopropanyl-β-D-thiogalactoside (IPTG), culture media and cell strains were from Sangon (Shanghai, China). Rolipram was from ICN Biochemicals. Ni-NTA sepharose gel was from Beijing Biotrand Biotechnology Co, Ltd (Beijing, China). Full-length human PDE4B2 was that we used previouslyCitation8. Other chemicals were analytical reagents.
Preparation of two fusion proteins
Full-length human PDE4B2 gene was truncated to retain its catalytic domain comprising amino acid residues from 152 to 528Citation14,Citation15. The full-length gene and the truncated mutant were cloned into pReceiver-B13 vector with 9His-SUMO at the N-terminus. With each vector, transformed cells of Escherichia coli BL21 (DE3) were amplified and induced by IPTG for 20 h at 16 °C. After induction, collected cells were lyzed by sonication in 20 mM Tris–HCl pH 8.0 containing 2.0 mM benzamidine, 400 mM NaCl, 1.0 mM β-mercaptoethanol, 1.0 mM PMSF and 1% Triton X-100 Citation9–11. Each fusion protein in lysates was purified via Ni-NTA affinity chromatography. In brief, Ni-NTA affinity column was equilibrated with 20 mM Tris–HCl at pH 8.0 containing 400 mM NaCl; unbound proteins were washed with the same buffer plus 20 mM imidazole; the bound protein was eluted out with the same buffer plus 100 mM imidazole. The preparation of each fusion protein contained few other proteins detectable by sodium–dodecylsulfate polyacrylamide gel electrophoresis.
HTA characterization of PDE4 and candidate inhibitors
To measure PDE activity, the reaction buffer was 20.0 mM Tris–HCl at pH 7.4 plus 0.10 mM EDTA and 10.0 mM MgCl2.Citation9–11 For routine characterization of PDE4 and inhibitors, all operations and data processing followed completely those described previously. One unit of PDE or CIAP hydrolyzed 1 μmol substrate per minute under stated conditions.
For HTA of PDE4 activity, 96-well microplates and Biotek ELX 800 microplate reader with a filter of 630 nm were employed. In detail, the total volume of PDE4 reaction mixture was 135 μL to contain all the required components including PDE, CIAP at activity no less than 25 U L−1 and an indicated inhibitor. During CIAP-coupled PDE4 reaction, the microplate was subjected to continuous vibration in an isolated room air-conditioned at 25°C. After reaction for <30 min, enzyme reactions were terminated by the addition of 25 μL solution of HClO4 to the final level of 0.5 M; after the addition of 30 μL solution of 1.0 mM malachite green and 0.15% PVA in 3 mM H2SO4 and continuous vibration for 1.0 min, 30 μL solution of 40.0 mM ammonium molybdate in 4.5 M sulfuric acid was added immediately and microplates were then subjected to continuous vibration again. Absorbance at 630 nm was measured with Biotek ELX 800 microplate reader from 10 to 30 min since the addition of molybdate, unless otherwise stated. For estimating Michaelis–Menten constant (Km), PDE activity under each condition was measured in six wells. For estimating inhibition constants of candidate inhibitors, final cAMP was fixed at 60 µM and the quantity of a purified PDE in each well was optimized to be <40 mg L−1 for the net increase in absorbance at 630 nm from 0.300 to 0.480; PDE activity under each condition was measured in triplicate at least. Effects of contaminants in PDE samples, proteins, DMSO and spontaneous hydrolysis of cAMP under those stated conditions were corrected with proper controls.
Other analyses and data processing
Proteins were quantified by the Bradford method with bovine serum albumin as the standardCitation18. Km was estimated via regression analysis according to Lineweaver–Burk plot. Inhibition percentages at 60 µM cAMP were plotted against logarithmic concentrations of a competitive inhibitor to estimate its inhibition constantCitation9,Citation11. The limit of quantification of phosphate was the lowest level of phosphate that was >5-fold of the standard error of estimate and had the coefficient of variation <20%Citation19.
Results and discussion
Routine characterization of two fusion proteins of PDE4B2
Two fusion proteins of PDE4B2 were purified by >8-fold through Ni-NTA column and the yield of activity of the full-length PDE4B2 from 1.0 mL regenerated Ni-NTA gel was nearly 3-folds of that from 1.0 mL fresh amylose resinCitation9. Of full-length PDE4B2 and the truncated mutant, their specific activities at 60 μM cAMP after once chromatography from Ni-NTA column were ∼0.017 and 0.40 U mg−1, while their highest specific activities were ∼0.05 and 1.0 U mg−1 after chromatography thrice, correspondingly. Moreover, the regeneration of Ni-NTA gel was easier than that of amylose resin. Hence, the expression of PDE isozymes via fusion to SUMO was favorable for screening their inhibitors.
Affinities of two forms of PDE4B2 for common ligands were compared. Of full-length PDE4B2 and the truncated mutant, Michaelis–Menten constants were 9.2 ± 0.6 μM (n = 3) and 54 ± 6 μM (n = 5). Rolipram is a selective while papaverine is a non-specific inhibitor of PDE4Citation9–14. On full-length PDE4B2 and the truncated mutant, inhibition constants of rolipram were 10 ± 2 nM (n = 3) and 0.33 ± 0.3 μM (n = 3), those of papaverine were ∼5.3 and 3.8 μM, correspondingly (). Affinities of two forms of PDE4B2 for cAMP, rolipram or papaverine were consistent with those reported, respectivelyCitation9–15, but the difference in their affinities for rolipram indicated that the truncated mutant was unsuitable for HTS of inhibitors of PDE4. Hence, for HTS of inhibitors of PDE4 via the proposed method or any other method, only full-length PDE4B2 of lower specific activity was suitable.
Table 1. Affinities of substrate and putative inhibitors of two forms of PDE4.
HTA of activities and HTS of inhibitors of PDE4B
In mixtures to quantify absorbance, there was ∼1.5-fold dilution of PDE reaction mixtures. To measure PDE activities for HTS of inhibitors, phosphate levels in PDE reaction mixtures were limited to 15.0 µM when 60.0 µM cAMP was used. In mixtures to quantify absorbance, the addition of a PDE at final 30 mg L−1 produced no precipitates in 40 min with phosphate at levels no more than 10.0 µM, but produced precipitates with phosphate at levels no less than 15.0 µM. Hence, proteins at high levels interfered with the improved malachite green assay of phosphate at levels >15.0 µM in PDE reaction mixtures.
For final phosphate from 3.0 to 10.0 µM in the absence of any protein, absorbance at 630 nm with the microplate reader was steady after color development for 5–30 min; in the presence of 30 mg L−1 of a purified PDE4B2, absorbance at 630 nm continued to increase slowly within 30 min, and reached the maxima before reaction for 40 min in total (). Usually, absorbance at 630 nm after reaction for 15 min was >98% of the maxima. As a result, with microplate, absorbance was measured after color development for 10–30 min. In this case, the response of absorbance at 630 nm to phosphate levels <10.0 µM in the presence of 10–30 mg L−1 proteins gave slopes and intercepts consistent with those in the absence of proteins, respectively (). The apparent absorptivity in this case was ∼70% of that determined with light path of 1.00 cmCitation9–11. The limit of quantification of phosphate with the microplate reader was ∼0.8 µM. Hence, in mixtures to quantify absorbance, proteins at 30 mg L−1 were tolerable by the proposed method at phosphate levels <10.0 µM, which corresponded to ∼45 mg L−1 proteins in PDE reaction mixtures.
Figure 1. Effects of reaction time to develop color on absorbance at 630 nm. 1, 3, 5 indicated final phosphate at 10.0, 6.3 and 3.3 µM, respectively, in the absence of proteins; 2, 4, 6 indicated final phosphate at 10.0, 6.3 and 3.3 µM, correspondingly, in the presence of the full-length PDE4B2 at final 30 mg L−1; 7 indicated the reagent blank without any phosphate or protein. All concentrations were those in mixtures to quantify absorbance. After the addition of molybdate, microplate was subjected to continuous vibration for 2 min before detection of absorbance at 630 nm with Biotek ELX 800 microplate reader.
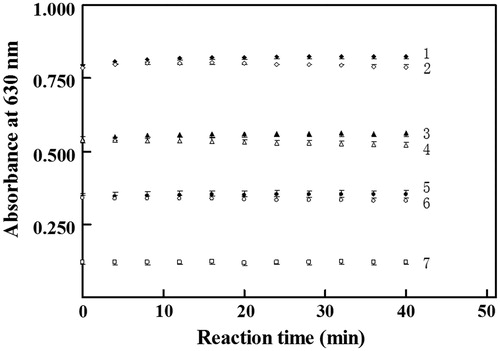
Figure 2. Linear responses of absorbance to phosphate levels. Time for color development with malachite green was from 10 to 25 min.
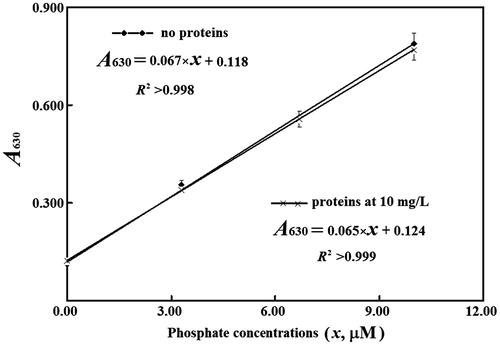
Based on those parameters, the lowest specific activity of a PDE isozyme suitable for HTS of its inhibitors via the proposed method was approximated. For HTS, a reaction period of 20 min is reasonable while the precision of 1% of inhibition percentages is sufficient. Random noise of absorbance is ∼0.003 with microplate readers and thus the threshold of the net increases in absorbance is 0.300 in the absence of any inhibitor. Such a threshold requires >6.5 µM phosphate in PDE reaction mixture. For reliable initial rates, when there was the production of 6.5 µM phosphate in PDE reaction mixture, cAMP should be over 45 µM with the truncated mutant, and over 25 µM with full-length PDE4B2, when their Michaelis–Menten constants were considered (). The use of higher levels of cAMP facilitated estimating inhibition percentages, but reduced the sensitivity to detect competitive inhibitors. Taken together, (a) in PDE reaction mixtures, cAMP was preset at 60 µM and phosphate levels were restricted to be <10.0 µM for confidence to tolerate co-existing proteins <45 mg L−1 and initial rate reactions of PDE; (b) for a minimum of 6.5 µM phosphate in PDE reaction mixtures after reaction for 20 min in the absence of any inhibitor, the specific activity of a PDE over 0.008 U mg−1 was required when proteins at 45 mg L−1 were used; (c) in PDE reaction mixtures, the final levels of inhibitors should be restricted to produce phosphate at levels >1.2 µM after reaction for 20 min so that reliable inhibition percentages were available and (d) full-length PDE4B2 after just once chromatography from Ni-NTA column should thus be suitable for HTS of its inhibitors when no other components in PDE reaction mixtures caused interference; specific activity of the truncated mutant met the criterion but the target itself was unsuitable for HTS of inhibitors of PDE4.
The proposed method tolerated >5% DMSO in final mixtures to develop color, which was an advantage over fluorometric methods for HTS of PDE inhibitorsCitation5,Citation6. However, PDE4B2 tolerated DMSO of 1% in reaction mixtures. The presence of papaverine and rolipram at final levels <80 μM did not alter absorbance at 630 nm for phosphate <10 μM. Linear response of the net increase in absorbance at 630 nm to quantities of either PDE4B2 was easily achieved; coefficients of variation of PDE activities with the net increases in absorbance at 630 nm over 0.060 were usually from 5% to 8%. Importantly, with two target models after once chromatography through Ni-NTA column, affinities of rolipram, cAMP and papaverine to full-length PDE4B2 or its truncated mutant via HTA mode varied over three magnitudes and were consistent with those via routine assay, respectively (). Hence, the proposed method was suitable for HTS of inhibitors of PDE isozymes bearing specific activities over 0.008 U mg−1 when candidate inhibitors caused negligible interference.
Conclusion
The proposed method tolerated co-existing proteins <30 mg L−1 in final mixtures to quantify absorbance, needed no peculiar reagents and was suitable for HTS of inhibitors of PDE isozymes bearing specific activities of no less than 0.008 U mg−1. Moreover, the improved malachite green assay of phosphate was applicable to HTS of inhibitors of other enzymes releasing phosphate at specific activities of no less than 0.008 U mg−1.
Declaration of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article. The work was supported by National Natural Science Foundation of China (nos. 81071427, 30472139), Natural Science Foundation Project of CQ (no. CSTC2012JJA0057) and the Education Ministry of China (no. 20125503110007).
Acknowledgements
We thank Dr Wei Li in College of Pharmaceutical Sciences for pReceiver-B13.
References
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 2006;109:366–98
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Ann Rev Biochem 2007;76:481–511
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol 2012;165:1288–305
- Liao F, Zhang XP, Zhou QX, et al. Assay of adenylyl cyclase activity by ion-exchange high-performance liquid chromatography. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2000;32:661–4
- Huang W, Zhang Y, Sportsman, JR. A fluorescence polarization assay for cyclic nucleotide phosphodiesterases. J Biomol Screen 2002;7:215–22
- Tian L, Wang RE, Fei Y, Chang YH. A homogeneous fluorescent assay for cAMP-phosphodiesterase enzyme activity. J Biomol Screen 2012;17:409–14
- Chock SP, Huang CY. An optimized continuous assay for cAMP phosphodiesteraseand calmodulin. Anal Biochem 1984;138:34–43
- Webb MR. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci USA 1992;89:4884–7
- Zhu S, Gan ZY, Li ZR, et al. The measurement of cyclic nucleotide phosphodiesterase 4 activities via the quantification of inorganic phosphate with malachite green. Anal Chim Acta 2009;636:105–10
- Zhu S, Yang GQ, Yang XL, et al. Soluble expression in E. coli of active human cyclic nucelotide phosphodiesterase isoform 4B2 in fusion with maltose-binding-protein. Biosci Biotechnol Biochem 2009;73:968–70
- Feng J, Chen Y, Pu J, et al. An improved malachite green assay of phosphate: mechanism and application. Anal Biochem 2011;409:144–9
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des 2009;15:1688–98
- Page CP, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol 2012;12:275–86
- Rocque WJ, Holmes WD, Patel IR, et al. Detailed characterization of a purified type 4 phosphodiesterase, HSPDE4B2B: differentiation of high- and low-affinity (R)-rolipram binding. Protein Expr Purif 1997;9:191–202
- Hoffmann R, Wilkinson IR, McCallum JF, et al. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: generation of a molecular model. Biochem J 1998;333:139–49
- Marblestone JG, Edavettal SC, Lim Y, et al. Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 2006;15:182–9
- Wang Z, Li H, Guan W, et al. Human SUMO fusion systems enhance protein expression and solubility. Protein Expr Purif 2010;73:203–8
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Liu H, Yang X, Liu L, et al. Spectrophotometric-dual-enzyme-simultaneous assay in one reaction solution: chemometrics and experimental models. Anal Chem 2013;85:2143–54
