Abstract
In biological systems, the Keap1/Nrf2/antioxidant response element pathway determines the ability of mammalian cells to adapt and survive conditions of oxidative, electrophilic and inflammatory stress by regulating the production of cytoprotective enzymes NAD(P)H:quinone oxidoreductase 1 (NQO1, EC 1.6.99.2) being one of them. Novel biologically active benzenesulfonamides 2, 3, 5–7, penta-2,4-dienamide 4 and chromene-2-carboxamide 8 structurally augmented with an electron-deficient Michael acceptor enone or cyanoenone functionalities were prepared. A new biological activity was conferred to these molecules, that of induction of NQO1. The potency of induction was increased by incorporation of a nitrile group adjacent to the enone and the dinitrophenyl derivative 3 was the most promising inducer. Also, molecular docking of the new compounds in the Nrf2-binding site of Keap1 was performed to assess their ability to inhibit Keap1 which biologically leads to a consequent Nrf2 accumulation and enhanced gene expression of NQO1. Docking results showed considerable interactions between the new molecules and essential binding site amino acids.
Introduction
Sulfonamides represent a group of compounds exerting a wide range of pharmacological activities such as antibacterialCitation1,Citation2, anti-inflammatoryCitation3, anticancerCitation4,Citation5 and antiviralCitation6 through various mechanisms of actions. On the other hand, enone and cyanoenones are electron-deficient functional groups that when present in a molecule can act as Michael acceptors thus enhancing the molecule’s ability to function as an antioxidantCitation7,Citation8. In biological systems, the Kelch-like ECH-associated protein 1 (Keap1)-nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response elements (AREs) pathway determines the ability of mammalian cells and organisms to adapt and survive conditions of oxidative, electrophilic and inflammatory stress by regulating the expression of a network of more than 100 cytoprotective genesCitation9,Citation10. NAD(P)H:quinone oxidoreductase 1 (NQO1, EC 1.6.99.2) is one such cytoprotective enzyme which is upregulated (induced) in response to various types of endogenous and exogenous electrophilesCitation9–11. Under basal conditions, Nrf2 is continuously targeted for ubiquitination and proteasomal degradation by the Cullin-3 (Cul3)-based ubiquitin ligase substrate adaptor protein Keap1Citation12,Citation13. Reactive cysteine residues within Keap1 serve as sensors for electrophiles and oxidantsCitation14,Citation15. Keap1 reacts rapidly with cyano enones, and certain monocyclic, tricyclic and pentacyclic cyano enones represent the most potent NQO1 inducers known to date in cells and in animalsCitation16–18. Consequent to its modification by electrophiles, the substrate adaptor activity of Keap1 is impaired, Nrf2 is stabilized, and the expression of its target genes and related proteins, exemplified by NQO1, is enhanced. Such Nrf2-dependent induction has been shown to be protective in numerous animal models of chronic disease, including cancer, cardiovascular disease and neurodegenerative conditionsCitation19. In continuation of our work on sulfonamidesCitation20–23, we synthesized several new derivatives bearing a (cyano)enone Michael acceptor moiety. We found that introduction of this functionality confers an additional biological activity of sulfonamides – induction of the cytoprotective enzyme NQO1.
Materials and methods
Chemistry
Melting points (°C, uncorrected) were determined in open capillaries on a Gallenkemp melting point apparatus (Sanyo Gallenkemp, Southborough, UK). Pre-coated silica gel plates (silica gel 0.25 mm, 60 G F 254; Merck, Darmstadt, Germany) were used for thin layer chromatography, dichloromethane/methanol (9.5:0.5 mL) mixture was used as a developing solvent system. IR spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). NMR spectra in (DMSO-d6) were recorded on Bruker Ac-500 ultra shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 500 MHz, using TMS as internal standard. Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All compounds were within ±0.4% of the theoretical values.
Synthesis of benzenesulfonamide derivatives 2–8
2-Cyano-N-(4-sulfamoylphenyl)acetamide (1)
Compound 1 was prepared according to a previously reported methodCitation19.
General procedure for synthesis of 2-cyano-3-(4-substituted)-N-(4-sulfamoylphenyl)-acrylamides (2–8)
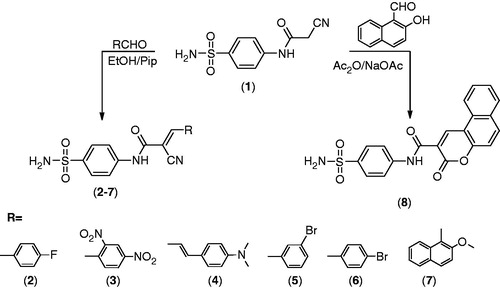
A mixture of 1 (2.11 g, 0.01 mol) and aromatic aldehydes (0.012 mol) in absolute ethanol (20 mL) containing piperidine (0.5 mL) was refluxed for 4 h. The obtained solid was filtered while hot and crystallized from dioxane to give 2–8, respectively.
2-Cyano-3-(4-fluorophenyl)-N-(4-sulfamoylphenyl)acrylamide (2)
Yield % 83; m.p. 265.9 °C; IR (KBr, cm−1): 3337, 3310, 3290 (NH, NH2), 3065 (CH arom.), 2966, 2876 (CH aliph.), 2205 (C≡N), 1685 (C=O), 1396, 1184 (SO2): 1H-NMR in (DMSO-d6) δ: 7.0–8.1 [m, 10H, Ar-H + SO2NH2], 8.3 [s,1H, CH], 10.9 [s, 1H, NH, D2O exchangeable]. 13C-NMR in (DMSO-d6) δ: 105.6, 114.3 (2), 114.9, 122.6 (2) 126.7 (2), 127.8 (2), 129.9, 130.8, 133.7, 152.6, 163.1, 164.9. 19F-NMR (DMSO-d6) δ: −130.25 [s, 1F, C6H4–F]. Mass m/z (rel.int.): M+ + 2 347 (3.1), M+ + 1 346 (1.9), M+ 345 (2.6 %), 213 (100). Anal. Calcd. For C16H12N3O3S (345.35): C, 55.65; H, 5.50; F, 5.05; N 12.17; S, 9.28. Found: C, 55.38; H, 5.22; F, 5.19; N, 12.51; S, 9.52.
2-Cyano-3-(2,4-dinitrophenyl)-N-(4-sulfamoylphenyl)acrylamide (3)
Yield % 88; m.p. 215.6 °C; IR (KBr, cm−1): 3416, 3372, 3286 (NH, NH2), 3096 (CH arom.), 2976, 2836 (CH aliph.), 2218 (C≡N), 1696 (C=O), 1378, 1161 (SO2). 1H-NMR in (DMSO-d6) δ: 7.1–8.4 [m, 9H, Ar-H+ SO2NH2], 8.7 [s, 1H, CH], 11.3 [s, 1H, NH; D2O-exchangeable]. 13C-NMR in (DMSO-d6) δ: 109.0, 116.7, 118.8, 120.6 (2), 128.1 (2), 128.7, 130.8, 135.9, 138.4, 141.2, 146.7, 150.1, 155.2, 165.3. Anal. Calcd. For C16H11N5O7S (417.35): C, 46.05; H, 2.66; N, 16.78; S, 7.98. Found: C, 46.36; H, 2.43; N, 16.47; S, 7.92.
2-Cyano-5-(4-(dimethylamino)-N-(4-sulfamoylphenyl)penta-2,4-dienamide (4)
Yield % 89; m.p. 240.2 °C; IR (KBr, cm−1): 3360, 3315, 3272 (NH, NH2), 3091 (CH arom.), 2946, 2860 (CH aliph.), 2212 (C≡N) 1676 (C=O), 1377, 1156 (SO2). 1H-NMR in (DMSO-d6) δ: 3.0 [s, 6H, N(CH3)2], 6.6,6.9 [2d, 2H, CH=CH; J = 7.0, 7.1 Hz], 7.5 [s, 1H, CH], 7.7–8.0 [m, 10H, Ar-H + SO2NH2], 10.3 [s, 1H, NH; D2O-exchangeable]. 13C-NMR in (DMSO-d6) δ: 40.1 (2), 96.7, 112.4 (2), 115.8, 122.1 (2), 122.8, 126.4, 126.8 (2), 129.3 (2), 130.6, 138.9, 141.5, 149.7, 164.2. Anal. Calcd. For C20H20N4O3S (396.46): C, 60.59; H, 5.08; N, 14.13; S, 8.09. Found: C, 60.31; H, 5.37, N, 13.84; S, 7.08.
3-(3-Bromophenyl)-2-cyano-N-(4-sulfamoylphenyl)acrylamide (5)
Yield % 86; m.p. 167.2 °C; IR (KBr, cm−1): 3412, 3391, 3212 (NH, NH2), 3095 (CH arom.), 2971, 2836 (CH aliph.), 2218 (C≡N), 1685 (C=O), 1328, 1155 (SO2). 1H-NMR (DMSO-d6) δ: 7.0–7.9 [m, 10H, Ar-H + SO2NH2], 8.9 [s, 1H, CH], 10.6 [s, 1H, NH, D2O-exchangeable]. 13C-NMR (DMSO-d6) δ: 109.0, 115.8, 121.1 (2), 126.2, 126.5, 127.3 (2), 130.3, 130.8, 131.8, 137.5, 138.3, 141.3, 152.6, 170.3. Anal. Calcd for C16H12BrN3O3S (406.25): C, 47.30; H, 2.98; N, 10.34. Found: C, 47.62; H, 2.66; N, 10.12.
3-(4-Bromophenyl)-2-cyano-N-(4-sulfamoylphenyl)acrylamide (6)
Yield % 79; m.p. 233.5 °C; IR (KBr, cm−1): 3390, 3309, 3287 (NH, NH2), 3100 (CH arom.), 2992, 2920 (CH aliph.), 2219 (C≡N) 1689 (C=O), 1336, 1156 (SO2). 1H-NMR in (DMSO-d6) δ: 7.0–7.9 [m, 10H, Ar-H + SO2NH2], 8.4 [s, 1H, CH], 11.2 [s, 1H, NH, D2O-exchangeable]. 13C-NMR in (DMSO-d6) δ: 108.2, 115.1, 119.8 (2), 121.7, 128.4 (2), 129.6 (2), 130.7 (2), 133.8, 136.7, 140.8, 155.1, 164.6. Anal. Calcd. for C16H12 BrN3O3S (406.25): C, 47.30; H, 2.98; N, 10.34; S, 7.89. Found: C, 47.67; H, 2.65; N, 10.08; S, 7.58.
2-Cyano-3-(2-methoxynaphthalen-2-yl)-N-(4-sulfamoylphenyl)acrylamide (7)
Yield % 69; m.p. 104.5 °C; IR (KBr, cm−1): 3364, 3310, 3206 (NH, NH2), 3066 (CH arom.), 2980, 2942 (CH aliph.), 2212 (C≡N), 1652 (C=O), 1376, 1156 (SO2). 1H-NMR (DMSO-d6) δ: 4.0 [s, 3H, OCH3], 7.0–8.0 [m, 12H, Ar-H + SO2NH2], 9.1 [s, 1H, CH], 10.7 [s, 1H, NH, D2O-exchangeable]. 13C-NMR (DMSO-d6) δ: 56.2, 109.7, 110.2, 115.4, 121.2 (2), 123.3, 126.5 (3), 127.3 (2), 127.9, 128.5, 129.7, 133.8, 137.9, 139.3, 155.8, 160.5, 164.0. Anal. Calcd for C21H17N3O4S (407.44): C, 61.90; H, 4.21; N, 10.31. Found: C, 61.59; H, 4.44; N, 10.02.
3-Oxo-N-(4-sulfamoylphenyl)-3H-benzo[f]chromene-2-carboxamide (8)
To a solution of 1 (2.11 g, 0.01 mol) in acetic anhydride (15 mL), 2-hydroxy-1-naphthaldehyde (1.72 g, 0.01 mol) and fused sodium acetate (0.8 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 2 h, and the obtained solid was crystallized from acetic acid to give compound 6. Yield % 79; m.p. 287.1 °C; IR (KBr, cm−1): 3343, 3309, 3275 (NH, NH2), 3076 (CH arom.), 1700, 1673 (2C=O), 1374, 1184 (SO2). 1H-NMR in (DMSO-d6); δ: 7.4–8.1 [m, 12H, Ar-H + SO2NH2], 8.5 [s, 1H, CH], 10.3 [s, 1H, NH, D2O-exchangeable]. 13C-NMR in (DMSO-d6) δ: 116.3, 116.9, 118.2, 120.7 (2), 121.4, 122.6, 125.2, 127.3 (2), 128.2 (2), 131.7 (2), 135.7 (2), 142.6, 154.8, 168.9, 172.0. 15N-NMR (DMSO-d6) δ: 114.73, 207.57 [2s, 2N, NHC=O, SO2NH2]. Mass m/z (rel.int.): M+ + 2 396 (0.19), M+ + 1 395 (0.3), M+ 394 (0.6 %), 77 (100). Anal. Calcd. for C20H14N2O5S (394.40): C, 60.91; H, 3.58; N, 7.10; S, 8.13. Found: C, 61.13; H, 3.81; N, 7.42; S, 8.49.
Biological evaluation
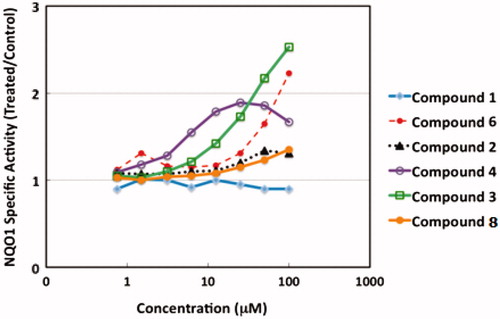
Hepa1c1c7 cells were maintained in a humidified atmosphere at 37 °C, 5% CO2 in α-MEM supplemented with 10% (v/v) fetal bovine serum that had been heat- and charcoal-inactivated. For the NQO1 assay, cells (104 per well) were grown in 96-well plates for 24 h. The medium was then replaced with inducer-containing fresh medium, and the cells were grown in the presence of inducers for a further 48 h. There were eight replicates of each treatment of eight different concentrations of inducers ranging from 0.75 to 100 µM. All compounds were dissolved in DMSO. The final concentration of the solvent in the medium was 0.1% (v/v) in all wells. At the end of the treatment period, cell lysates were prepared in digitonin and the specific activity of NQO1 was determined using menadione as a substrate. The Concentration which Doubles the specific activity of NQO1 (CD value) was used as a measure of inducer potency. Mean values for the eight replicate wells are shown for each data point. The standard deviation for each data point was <5% of the value.
Molecular modeling study
The molecular model of each of the new (cyano)enone sulfonamide derivatives was built in the dissociated form, using standard bond lengths and angles, with the MOE software suite version 10.2008. Following geometry optimization, a systematic conformational search was carried out to an RMS gradient of 0.01 Å with energy minimization of the resultant conformations employing the ConfSearch module implemented in MOE. All molecular mechanics computations were performed with the Merck Force Field (MMFF94s). To assess the ability of our compounds to access and potentially block the Nrf2-binding site of Keap1, a molecular docking study was performed employing the crystallographic structure of Keap1 obtained from the Protein Data Bank (PDB ID: 4IQK). Missing hydrogens were added to the enzyme and partial charges were calculated. After removing the co-crystallized inhibitor, validation followed by docking of the compounds was carried out using MOE software suite version 10.2008. The target protein was kept rigid, while the ligands were left free to explore the conformational space inside the enzyme cavity; 200 separate docking simulations were run using default parameters and the conformations were chosen based on the combination of S score data, E conformation and appropriate fitting with the relevant amino acids in binding pocket.
Results and discussion
Chemistry
To address the aim of this work of designing and synthesis of a new series of sulfonamides having the biologically active (cyano)enone Michael acceptor moiety, the synthesis protocols depicted in () were adopted for preparation of the target compounds. 2-Cyano-N-(4-sulfamoylphenyl)acetamide 1Citation24, a highly reactive intermediate, was the key starting material for this study. Thus, condensation of compound 1 with aromatic aldehydes in refluxing absolute ethanol in the presence of a catalytic amount of piperidine afforded the corresponding sulfonamide derivatives 2–7 (). The structures of the products were assigned on the basis of their analytical and spectral data. First, the IR spectra of the reaction products showed in each case four absorption bands corresponding to NH and NH2 functions in the region 3416–3206 cm−1, in addition to a carbonyl absorption band in the region 1696–1652 cm−1, absorption bands due to C≡N in the region 2219–2205 cm−1, and SO2 functions in the region 1396–1156 cm−1. Moreover, 1H-NMR spectra of compounds 2–7 in (DMSO-d6) revealed a singlet signal that was exchangeable with D2O in the region 10.3–11.3 ppm corresponding to a NH group. Also, 19F-NMR spectrum of compound 2 revealed one signal at −130.25 ppm consistent with a fluorine atom attached with the phenyl ring confirming the stability of the fluorine atom to the reaction conditions. On the other hand, reaction of compound 1 with 2-hydroxy-1-naphthaldehyde in acetic anhydride containing anhydrous sodium acetate furnished the corresponding 3-oxo-N-(4-sulfamoylphenyl)-3-H-benzo[f]chromene-2-carboxamide 8 (). The structure of 8 was confirmed on the basis of elemental analysis and spectral data. IR spectrum showed bands at 3343, 3309, 3275 cm−1 corresponding to NH, NH2; 1700, 1673 cm−1 pertaining to 2 C=O groups and finally peaks at 1374, 1184 cm−1 referring to the SO2 group. 1H-NMR spectrum in (DMSO-d6) revealed a signal at 8.5 ppm due to the CH group of chromene, and another at 10.3 ppm corresponding to the NH group. Finally, 15N-NMR spectrum of compound 8 exhibited two signals at 114.73 and 207.57 ppm assigned to NHC=O (amide) and SO2NH2 (sulfonamide), respectively. These assignments fully support and affirm the proposed structure.
Biological evaluation
The ability of the new (cyano)enone sulfonamide derivatives to induce NQO1 was evaluated in murine Hepa1c1c7 cells. The Concentration which Doubles the specific activity of NQO1 (CD value) was used as a measure of inducer potency. Mean values for the eight replicate wells are shown for each data point ( and ). As expected, the parent compound 1 which lacks the electrophilic Michael acceptor functionality was inactive. On the other hand, compound 8, which has an enone Michael acceptor moiety within its heterocyclic ring, was weakly active, causing a 1.35-fold increase in activity at 100 µM, the highest concentration tested. With regards to the activity displayed by cyanoenone derivatives 2–7, it was evident that incorporation of this more electrophilic functionality within the molecules significantly increased their inducer activity. Among these analogues, the potency of the fluorophenyl derivative 2 was the lowest, causing a 1.3-fold increase in enzyme activity at a concentration of 50 µM. Compound 2 was slightly toxic, and the protein concentration of lysates of cells that had been exposed to 100 µM compound 2 for 48 h was 20% lower than that of vehicle-treated cells. The dinitrophenyl derivative 3 showed a very promising potency reaching a CD value at a concentration of 40 µM which is the lowest amongst the tested compounds. While the dimethylamino derivative 4 was the most potent in the series at lower concentrations, inducing NQO1 by 1.5-fold at a concentration of 5 µM, and nearly doubling (1.9-fold) the specific activity of the enzyme at a concentration of 22.5 µM, it did not maintain the same behavior at higher concentrations. Moreover, 4 was also the most toxic among all the active derivatives where the protein concentration of cell lysates treated with 22.5 µM was 50% lower than that of vehicle-treated cells, and at a concentration of 100 µM, the cytotoxicity was 68%. Coming next was the 4-bromophenyl counterpart 6 which had a CD value of 75 µM. No signs of toxicity were observed for compounds 1, 3, 6 and 8 at concentrations up to 100 µM.
Table 1. NQO1 inducer activity of sulfonamide derivatives.
Molecular modeling study
It is well established that Keap1 binds to Nrf2 promoting its degradation, resulting in low levels of cytoprotective gene products. Several small molecule compounds were reported to specifically bind to the Keap1 Kelch domain and antagonize its activityCitation25,Citation26. To assess the ability of our compounds to access and potentially block the Kelch domain of Keap1, a molecular docking study was performed employing the crystal structure obtained from the Protein Data Bank (PDB ID: 4IQK). It was found that the best compound in this study, the cyanoenone sulfonamide 3, also displayed the best binding with the Kelch domain of Keap1. This was demonstrated through observation of two hydrogen bonds, one pi-cationic and one pi–pi stacking interactions between 3 and the active site essential amino acids with an overall S score of – 9.6201 Kcal/mol () compared to – 10.8896 Kcal/mol for the co-crystallized ligand ().
Figure 2. Left panel: 3D docking of 3 (green) in comparison with a known ligand (magenta); Right panel: 2D ligand interaction of 3 with Keap1 binding site amino acids (S = −9.6201 Kcal/mol) (refer to the on-line article for colored figures).
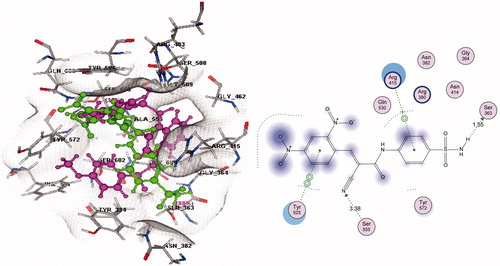
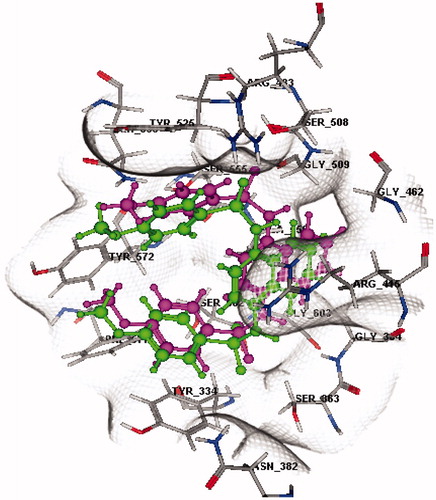
Figure 3. 3D docking validation of re-docked ligand (magenta) compared to known ligand (green) in the binding pocket of Keap1 (rmsd = 1.0749 Å, S = −10.8896 Kcal/mol) (refer to the on-line article for colored figures).
Previous studies have shown that electrophilic olefins react reversibly with nucleophilic sulfhydryl groups, including cysteine residues in Keap1 and other proteins, and that the presence of a nitrile group increases the reactivity of the olefin and the inducer potencyCitation27,Citation28. Here we show that incorporation of a cyanoenone functionality within the structure confers a new biological function – that of induction of the cytoprotective enzyme NQO1. Thus, by analogy with the cyclic cyano enones which covalently bind to cysteine residues of Keap1 and induce expression of Nrf2 target genesCitation17,Citation18, we propose that the same mechanism is involved in the observed upregulation of NQO1 by cyanoenone-containing sulfonamides. Importantly, because the reaction of a cyanoenone moiety with a sulfhydryl group is readily reversible, our findings illustrate the notion that induction of cytoprotective responses can be achieved by covalent binding, but without permanent modification (and possibly destruction) of the sensor protein. Such a mode of action also minimizes the possibility of depletion of glutathione, the principal endogenous antioxidant in the cell, thereby avoiding non-specific cytotoxic effects.
Conclusion
In conclusion, the present study aiming to synthesize and evaluate the NQO1 inducer activity of novel sulfonamide derivatives carrying the biologically active cyanoenone moiety gave good insights about the molecular requirements for developing such compounds. While the cyanoenone derivatives 2–7 displayed higher activity than the enone derivative 8, the most promising cyanoenone was the dinitrophenyl analogue 3 which caused a dose-dependent induction of NQO1 with a CD value of 40 µM.
Declaration of interest
The authors declare that they have no conflict of interest. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-302. We also thank Cancer Research UK (C20953/A10270) for financial support.
References
- Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob 2008;7:Art No. 17 . doi:10.1186/1476-0711-7-17
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Sondhi SM, Rani R, Gupta PP, et al. Synthesis, anticancer, and anti-inflammatory activity evaluation of methanesulfonamide and amidine derivatives of 3,4-diaryl-2-imino-4-thiazolines. Mol Div 2009;13:357–66
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002;2:55–75
- Supuran CT, Vullo D, Manole G, et al. Designing of novel carbonic anhydrase inhibitors and activators. Curr Med Chem Cardiovasc Hematol Agents 2004;2:49–68
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem 2003;10:925–53
- Magda A, Massoud MA, Tantawy AS, et al. Synthesis and biological evaluation of new unsaturated derivatives of cyclic compounds as potent antioxidant agent. Der Pharma Chemica 2012;5:1785--97
- Kalra S, Knatko EV, Zhang Y, et al. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): implications for protection against UV radiation during thiopurine therapy. Cancer Prev Res 2012;5:973–81
- Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev 2004;36:639–54
- Dinkova-Kostova AT, Talalay P. Nad(P)H:quinone acceptor oxidoreductase 1 (Nqo1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 2010;501:116–23
- Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997;236:313–22
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-Are pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116
- Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011;16:123–40
- Kobayashi M, Li L, Iwamoto N, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 2009;29:493–502
- McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci USA 2010;107:18838–43
- Dinkova-Kostova AT, Talalay P, Sharkey J, et al. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem 2010;285:33747–55
- Honda T, Yoshizawa H, Sundararajan C, et al. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J Med Chem 2011;54:1762–78
- Zheng S, Santosh Laxmi YR, David E, et al. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J Med Chem 2012;55:4837–46
- Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 2012;18:337–47
- Al-Said MS, Bashandy MS, Al-Qasoumi SI, Ghorab MM. Anti-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur J Med Chem 2011;46:137–41
- Al-Said MS, Ghorab MM, Al-Dosari MS,Hamed MM. Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem 2011;46:201–7
- Alqasoumi SI, Al-Taweel AM, Alafeefy AM, et al. Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 2010;45:1849–53
- Ghorab MM, Ragab FA, Heiba HI, et al. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur J Med Chem 2010;45:3677–84
- Alafeefy AM, Isik S, Abdel-Aziz HA, et al. Carbonic anhydrase inhibitors: benzenesulfonamides incorporating cyanoacrylamide moieties are low nanomolar/subnanomolar inhibitors of the tumor-associated isoforms IX and XII. Bioorg Med Chem 2013;21:1396–403
- Marcotte D, Zeng W, Hus JC, et al. Small molecules inhibit the interaction of Nrf2 and the Keap1 Kelch domain through a non-covalent mechanism. Bioorg Med Chem 2013;21:4011–19
- Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-Are pathway as potential preventive and therapeutic agents. Med Res Rev 2012;32:687–726
- Couch RD, Browning RG, Honda T, et al. Studies on the reactivity of Cddo, a promising new chemopreventive and chemotherapeutic agent: implications for a molecular mechanism of action. Bioorg Med Chem Lett 2005;15:2215–19
- Serafimova IM, Pufall MA, Krishnan S, et al. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol 2012;8:471–6