Abstract
Panax ginseng Meyer has been shown to be effective in mitigating various diseases. Protopanaxadiols (PPD) and protopanaxatriols (PPT), which are the main constituents of ginseng, have been shown to impact obesity. Therefore, we selected several important ginsenosides to perform our docking study and determine if they had binding affinity with the peroxisome proliferator activated receptor gamma (PPARγ), which is a major transcription factor in adipocytes. Among them, only a few ginsenosides demonstrated binding affinity with PPARγ. Other than ginsenoside F2 rest of them were previously reported by the researchers in experimental study in case of obesity cell line 3T3-L1 adipocyte. In few recent studies, it was reported that F2 has protective effects on malignant brain tumors as well as anti-cancer activity in breast cancer. Therefore, we felt it was important to focus on F2 when considering obesity. Our study focused on this ginsenoside and analyzed its impact on 3T3-L1 adipocytes. Following the molecular interaction studies, further experimental studies were carried out and demonstrated that ginsenoside F2 when treated with different doses reduces the level of lipid accumulated by the 3T3-L1 cell line during adipogenesis. Reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative real-time PCR results showed reduction in PPARγ and perilipin gene expression levels compared to that of differentiated adipocytes without any treatment. So considering the binding with a major adipocyte transcription factor and the performed experiments, we suggest that ginsenoside F2 may reduce obesity via the inhibition of adipogenesis in the 3T3-L1 cell line.
Introduction
Obesity is identified as an excess accumulation of body fat, which may be due to over eating, low physical activity or environmental or genetic conditions. It is a chronic medical disease that can lead to several other diseases, including diabetes, heart disease, stroke, arthritis and even some cancers. It is predicted that obesity will reach to an alarming level in the near future if it is not considered a vital issue. Therefore, it has become necessary to discover methods to reduce obesity in order to maintain a healthy life. Until now, several drugs have been shown to treat obesity. However, many of them have later been found to cause various side effects. Thus, to avoid unwanted side effects, researchers have driven their attention to naturally available compounds that may possess anti-obesity effectsCitation1.
Among the many medicinal herbs, Panax ginseng is one of the most popular oriental medicinal plants that have been used for thousands of years in China, Korea, Japan and many other countries. Ginseng saponins, also known as ginsenosides are being regarded as the principal components responsible for the pharmacological activities of ginseng. They possess multiple pharmacological actions linked to several diseases that include the central nervous system (CNS), cardiovascular, endocrine and immune systems. These ginsenosides are divided into two parts which are PPD and PPT. Where PPD types include Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2 and PPT types includes Re, Rf, Rg1, Rg2 and Rh1Citation2,Citation3. Researchers have already focused on ginsenosides to investigate their impact on adipocytes, which are the building blocks of obesity for example, Rb1, Rh1, Rg3, Rd and Rh2Citation4–8. Among the PPD or PPT type ginsenosides, we considered 12 saponins (Rb1, Rb2, Rg1, Rg2, Rg3, Rc, Rd, Re, Ro, Rh2, Ck and F2) to carry out the molecular docking study, and then for further investigation only those ginsenosides were selected that demonstrated an interaction with the targeted protein by the formation of hydrogen bonds. After the molecular docking studies, some of the ginsenosides possessed binding affinity with the protein but some did not. Among the selected ginsenosides, it was discovered that all had experimental evidences with the 3T3-L1 adipocyte cell line, except for ginsenoside F2. Ginsenoside F2 was produced by the hydrolysis of protopanaxadiol type saponin mixture by various glycosidesCitation9. It has been reported that F2 could be a new potential chemotherapeutic drug for glioblastoma multiforme (GBM) treatment by inhibiting the growth and invasion of cancerCitation10. Another report suggested that F2 initiates an autophagic progression in breast cancer stem cells (CSCs), where treatment with an inhibitor of autophagy enhanced F2-induced cell deathCitation11. From the studies, it was obvious that F2 was an important saponin of ginseng and it should also be considered to study on other diseases. Therefore, we chose F2 to find out its activity on obesity.
Materials and methods
Compounds fishing and molecular interaction studies
A computational method of molecular docking used for identification of novel molecules from natural products is an effective approachCitation12. In order to identify molecular interactions between PPARγ and ginsenosides from the Korean Panax ginseng medicinal plant, this study was proposed. The X-ray crystal structure of PPARγ (PDB ID: 2ATH)Citation13 was obtained from the Protein Data Bank (PDB)Citation14 with a co-crystallized compound of 3EA. The resolution of PPARγ was 2.28 Å. Initially, the co-crystallized ligand was extracted from the PDB environment and considered as being without ligand. A total of 12 ginsenosides (Rb1, Rb2, Rg1, Rg2, Rg3, Rc, Rd, Re, Ro, Rh2, Ck and F2) were obtained from our own in-house Panax ginseng saponins database. The PPARγ residues were considered as those binding 3EA. Previous studies also confirmed that TYR473, HIS449, SER289 and HIS323 residues are important for PPARγ inhibitionCitation13. In case of PPARγ, water molecules were removed, hydrogen atoms were added, and then the complex was optimized using the Autodock wizardCitation15–17. The molecular docking program Autodock 4.2.3 was used for identification of binding possibilities between PPARγ and ginsenosides. This program was followed by Lamarckian genetic algorithm (LGA)Citation18 for producing a more accurate binding modelCitation19,Citation20. In addition, the known PPARγ inhibitor 3EA (http://www.drugbank.ca/drugs/DB07053), which is commercially available, was re-docked to verify the model reproducibility. The selected crucial amino acids of PPARγ were considered to perform docking simulation. The detailed docking simulation was described in our previous experimentCitation21 and the results were analyzed by Chimera 1.6.1.
Furthermore, the computational program PASS was used for predicting biological activity spectrum based chemical structure formulaCitation22,Citation23. This technology was employed with our selected ginsenosides for identifying the type of biological activity present related to obesity. In addition, this method produced a list of biological activities, along with probability of activity (Pa) and probability of inactivity (Pi) values.
Experimental study
Reagents
Insulin and isobutylmethylxanthine (IBMX) were purchased from Wako. Dexamethasone and Oil Red O staining reagent were purchased from Sigma Chemical Co. (MO). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Welgene, Newborn calf serum was obtained from Gibco, and antibiotic solution was purchased from Biological Industries. Ginsenoside F2 product no. A0449 was purchased from Chengdu Must Bio-Technology Co., Ltd (Wuhou, Chengdu, China) with the purity of ≥98% by HPLC.
Cell culture
Mouse embryo fibroblast 3T3-L1 cells were obtained from the American Type Culture Collection (ATCC; VA) and incubated in DMEM, containing 10% BCS and 1% AB, at 37 °C with a 5% CO2 atmosphere. To induce differentiation three days after confluence, which was considered day 0, the pre-adipocytes were cultured in the differentiation medium (DM), which contained: DMEM, 10% BCS, 1% AB and DMI (1 µM dexamethasone, 0.5 mM IBMX and 10 µg/ml insulin) from day 0 to day 3. Subsequently, the medium was switched to growth medium containing DMEM with 10% BCS and 10 µg/ml insulin which was replaced on days 3, 5 and 7. The medium contained ginsenoside F2 from day 0 at the concentrations of 10, 50 and 100 µM.
MTT assay
Cells were cultured in 96-well plates at a density of 1 × 104 cells/100 µl/well. After an 18 h incubation, cells were replaced in DMEM containing 10% BCS and ginsenoside F2 at concentrations of 10, 50 and 100 µM for 48 h. Subsequently, MTT regent was added to the medium and allowed to incubate for 4 h at 37 °C. All liquid was removed from the wells and 100 µl of DMSO was added to each well and then incubated for another hour. Finally, the resultant OD was measured at 570 nm using an ELISA reader.
Oil-Red O staining
Cells were cultured in 24-well plates for differentiation. Oil Red O staining was performed on day 8 of differentiation. Cells were washed with phosphate-buffered saline (PBS), and fixed with 10% formalin for 1 h. The cells were then washed with 60% isopropanol and stained with Oil Red O solution for 10 min. Excess stain was washed away several times with sterile water and cells were dried for imaging. Finally, the stained lipid droplets of the Oil Red O solution were dissolved using 100% isopropanol and quantified via absorbance at 520 nm.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Cells were differentiated and on day 8 total RNA was extracted from the cells with or without treatment of ginsenoside F2. A total RNA extraction kit (Intron Biotechnology, Korea) was used for this purpose. cDNA synthesis for PPARγ and perilipin was done with 1 µg of total RNA using a kit (Thermo Scientific, Lithuania). Primer sequences for the markers are as follows: PPARγ, 5′-ATGGGTGAAACTCTGGGAGATT-3′ for forward, 5′-AGCTTCAATCGGATGGTTCTT-3′ for reverse, Perilipin, 5′-GATCGCCTCTGAACTGAAGG-3′ for forward, 5′-CCTCTGCTGAAGGGTTATCG-3′ for reverse, Beta actin, 5′-ATGAAGTGTGACGTTGACATCC-3′ for forward, 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′ for reverse. The reaction conditions were as follows: denaturation at 94 °C for 30 s; annealing at 60 °C (PPARγ and Beta actin), 55 °C (Perilipin), and extension at 72 °C for 1 min. Thirty cycles were carried out and the resulting PCR products were electrophoresed on a 1% agarose gel and visualized with Image J software.
Real time polymerase chain reaction
Real time reverse transcription PCR (qRT-PCR) was performed using a real-time rotary analyzer (Rotor-Gene 6000; Corbet Life Science, Australia) and 1 µg of cDNA in a 10 µl reaction volume, using SYBR® Green SensiMix Plus Master Mix (Quantace, England), with gene specific primers. PPARγ, 5′-ATGGGTGAAACTCTGGGAGATT-3′ for forward, 5′-AGCTTCAATCGGATGGTTCTT-3′ for reverse, perilipin 5′-GATCGCCTCTGAACTGAAGG-3′ for forward, 5′-CTTCTCGATGCTTCCCAGAG-3′ for reverse, beta actin, 5′-ATGAAGTGTGACGTTGACATCC-3′ for forward, 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′ for reverse. The PCR conditions for each of the 40 cycles were 95 °C for 10 s, 60 °C for 10 s and 72 °C for 20 s. Beta actin was used for comparison purposes.
Statistical analysis
All data were presented as mean ± standard error (S.E.) and all experiments were independently performed three times. The mean values of the treatment groups were compared with untreated groups using Student’s t test. Statistical significance was assigned at *p < 0.05, **p < 0.005 and ***p < 0.0005.
Results and discussion
Molecular interaction results of PPARγ with ginsenosides
Molecular docking studies were carried out with the 12 ginsenosides and the adipocyte protein, PPARγ, which has the most notable function of adipose tissue development. It is responsible for the responses of other major genes involved in mature adipocyte formationCitation24 and adipogenesis is associated with the stimulation of PPARγ. All the results were revalidated using Xscore program (). Analysis of the docking result suggested that Ginsenoside F2 formed one hydrogen bonds with Tyr473 yielding a binding affinity of −9.31 kcal/mol at the distance of 3.36 Å. The oxygen atom of the active site residue Tyr473 formed the bonds with the oxygen atom present in ginsenoside F2. Some more hydrogen bonds were formed with different amino acid residues which are Glu471, Pro467, Gln470 and Asp475. The docking result of Compound K showed the formation of two hydrogen bonds with a binding affinity of −11.72 kcal/mol. The nitrogen group of the amino acid active site residue His323 formed a bond with the O–H group of the glucose molecule in the compound K at the distance of 2.72 Å. Another hydrogen bond was formed with the amino acid active site residue Ser289 and oxygen atom of compound at 2.7 Å distance. One more hydrogen bond was formed with Cys285 amino acid residue. The docked result showed that ginsenoside Rg3 formed two hydrogen bonds, which interacted with the oxygen atom of the amino acid yielding a binding affinity of −12.61 kcal/mol where the active site residues were Tyr473 at the distance of 2.57 Å and His449 at the distance of 1.37 Å. Rg3 also formed some more hydrogen bond with Arg288, Ser342 residues respectively. Analysis of the ginsenoside Re docking result showed that one hydrogen bond was formed with the Ser 289 active site residue with binding energy −13.21 kcal/mol at the distance of 2.21 Å. Several hydrogen bonds were formed other than the active sites that are Gly258, Ser342, Cys285 and Phe363. The control compound 3EA showed interactions at four active site residues Ser289, His323, His449 and Tyr473, resulting in a binding affinity of −10.0 kcal/mol. From the analyzed docking result described above suggested that among the 12 ginsenosides evaluated, only four ginsenosides which are F2, Ck, Rg3 and Re formed hydrogen bonds in active side residues of PPARγ having binding affinity. The obtained ginsenosides complex structures of F2, Ck, Rg3 and Re docking interactions, including the hydrogen bond formation, can be visualized in the . The docking simulation of PPARγ with these four ginsenosides resulted in the formation of hydrogen bonds at their active sites with good binding affinity. Among them compound K, Re and Rg3 already has there experimental evidences on 3T3-L1 cell line. It was shown that they worked as anti-obese and anti-diabetic compounds. Ck in 3T3-L1 cell line inhibited PPARγ that resulted in inhibition of adipocyte differentiation. Where else Re and Rg3 demonstrated the evidence of glucose uptake in mature 3T3-L1 cells. Ginsenoside Rg3 was studied on PPARγ that proved to work as anti-obese compound in 3T3-L1 cell lineCitation6,Citation25,Citation26. Therefore, this manuscript shows that, although we selected 12 ginsenosides at the beginning from that considering the compounds having binding interaction, we selected only F2 for experimental studies, which may be used for PPARγ inhibition to confirm our Insilco hypothesis. In addition, the computational program PASS was used for predicting biological activity spectrum. It shows all four ginsenosides supported probability of active Pa > 0.7 as the transcription factor inhibitor ().
Figure 1. Ginsenosides and amino acid residue hydrogen bond formation. Complex structures of (a) F2, (b) Ck, (c) Rg3 and (d) Re with the PPARγ active site residue after bond formation.
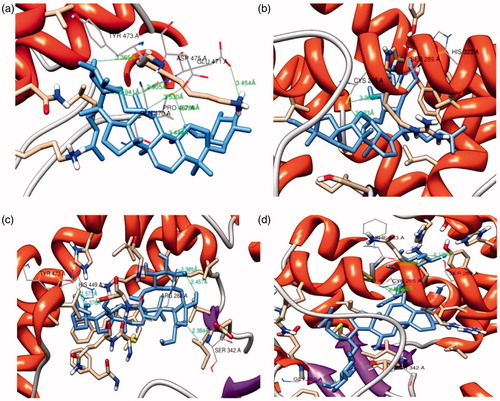
Table 1. Binding affinity prediction of PPARγ with ginsenoside F2, Ck, Rg3 and Re.
Table 2. Predicted biological activity (Pa > 0.7) of the selected ginsenosides from Panax ginseng.
Cell viability
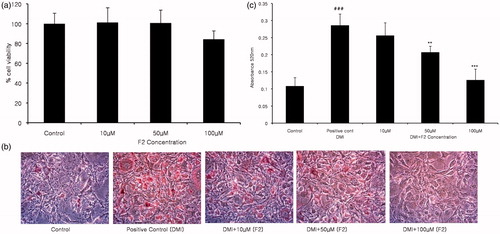
To investigate whether ginsenoside F2 was toxic to cells, we first performed a cell viability assay at different ginsenoside F2 concentrations: 10, 50 and 100 µM for 48 h. The results showed that at the noted concentrations, F2 was not remarkably toxic to the 3T3-L1 cell line (). Therefore, further experiments were carried out considering the same F2 concentrations.
Figure 2. The effect of ginsenoside F2 on 3T3-L1 adipocyte differentiation. The measurement of the cytotoxic activity of compound F2 at different concentration was done by MTT assay after treatment for 48 h (a). Lipid content was visualized by Oil Red O staining on day 8 of differentiation at the concentrations of 10, 50 and 100 µM (b), followed by lipid content evaluation by absorbance measurement (c). The data showed statistical significance ###p < 0.0005 between the control and positive control group, while **p < 0.005 was noted between positive and treated groups. F2 at 100 μM concentration showed to be extreme significantly different ***p < 0.0005 compared to the DMI treated positive control.
Inhibition of lipid content by Oil Red O staining
Next Oil red O staining assay was done to examine the efficacy of ginsenoside F2 on the adipogenesis of 3T3-L1 cells, which suggested that ginsenoside F2 could potentially inhibit the adipogenesis process. Oil Red O staining and the triglyceride content were measured. From the stained pictures, it is clearly visible that the cells accumulated lipid in the DMI supplemented wells, while the cells receiving DMI along with ginsenoside F2 contained less amount of lipid in a dose dependent manner (). To measure triglyceride content, the collected stained lipid was measured. Ginsenoside F2, at the concentrations of 50 and 100 µM accumulated less lipid content by 27 and 55%, respectively, compared to that of the differentiated positive control (). This result conveys that, at these concentrations, the adipocyte differentiation process was blocked.
Down-regulation of PPARγ and perilipin gene expression and quantification
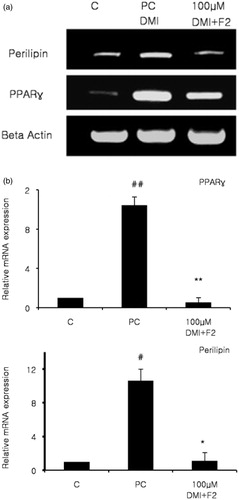
Finally to evaluate adipocyte-specific gene expression, we selected two genes, PPARγ and perilipin, that were closely related with the lipid contents inside adipocytes. Perilipin is a lipid droplet associated proteinCitation27 and the occurrence of these proteins in tissues denotes triacylglycerol metabolism. Perilipin is located directly on the surface layer of every differentiating 3T3-L1 adipocyte, and surrounds the core triacylglycerol of intracellular lipid droplets of these adipocytes that may be in culture, or sourced from white and brown adipose tissueCitation28. Thus it was clear that these two markers play separate but crucial roles in adipogenesis, and the expression of these two proteins was investigated and mRNA expression was quantified. 3T3-L1 cells were differentiated and treated, then the expression profile of the adipocyte-specific genes was investigated by RT-PCR and quantitative real time PCR. DMI treated adipocytes demonstrated increased marker expression compared to the normal control group, which did not receive DMI and ginsenoside. PPARγ and perilipin expressions were down-regulated when treated with ginsenoside F2, which also contained DMI (). Treatment with ginsenoside F2 reduced the mRNA levels of PPARγ and perilipin by 95 and 89% respectively (). Therefore, gene expression studies suggest that adipogenesis was inhibited by blocking the associated genes via ginsenoside F2 treatment.
Figure 3. Effects of ginsenoside F2 on adipocyte markers were evaluated. At day 8 of differentiation total RNA was isolated. Control groups were treated with the normal medium, while the positive control received differentiation medium (DMI). Treated groups received differentiation medium with different F2 concentration (DMI + F2), while expression levels were evaluated by RT-PCR and visualized via gel (a). Gene expression levels were quantified using qRT-PCR and results analyzed (b). Presented data were statistically significant ##p < 0.005 between control and positive control, also **p < 0.005 compared between positive control and F2 concentration for PPARγ. #p < 0.05 compared with the normal control group and positive control, *p < 0.05 compared with DMI treated positive control and DMI and F2 treated for Perilipin.
Obesity is associated with the accumulation of excess fat in adipocytes. The process of adipogenesis includes morphological changes, interruption of cell growth, expression of many lipogenic enzymes and large scale of lipid accumulationCitation29. Therefore, blocking this process will help inhibit the formation of mature fat cells. In order to inhibit adipocyte differentiation, other researchers have also shown interest in blocking PPARγCitation7,Citation8,Citation24. In our study, we started with 10 µM F2 to demonstrate its efficacy on adipocyte differentiation, while 100 µM ginsenoside F2 nearly blocked the differentiation process.
Conclusions
Molecular interaction studies describe the first 12 ginsenosides selected, but among them, only four ginsenoside had hydrogen bond formation with the active side residues of the adipogenic protein. From those, we selected four ginsenosides, three of them were previously discussed so we chose ginsenoside F2 for our experimental investigation. F2 had good binding affinity with PPARγ, the major transcription factor for adipocyte differentiation. Therefore the confirmation of the in silico hypothesis experimental studies were carried out demonstrated that ginsenoside F2 possesses anti-obesity activity. The in vitro analysis clearly showed that F2 treated cells contained fewer lipids compared to not treated cells and also down-regulated the adipocyte markers, which successfully inhibited adipocyte differentiation. These evidences supports that ginsenoside F2 derived from Panax ginseng might be used to prevent obesity with the aid of its anti-obesity activity.
In conclusion, we can state that, this was the first study to show PPARγ inhibitory mechanism with molecular interactions that includes experimental evidences of ginsenoside F2 on the 3T3-L1 adipocyte cell line and molecular level.
Declaration of interest
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea (iPET # 309019-3). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Yun JW. Possible anti-obesity therapeutics from nature – a review. Phytochemistry 2010;71:1625–41
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 1999;58:1685–93
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 1997;54:1–8
- Wenbin S, Ying Y, Boren J, et al. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci 2007;80:618–25
- Gu W, Kim KA, Kim DH. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol Pharm Bull 2013;36:102–7
- Hwang JT, Lee MS, Sung MJ, et al. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-γ signal pathways. Phytother Res 2009;23:262–6
- Kim MS, Lee MS, Kim SH, et al. Anti-obesity Effects of Ginsenoside Rd via AMPK and PPAR Gamma. Korean J Biotech Bioeng 2007;22:341–4
- Hwang JT, Kim SH, Lee MS, et al. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun 2007;364:1002–8
- Ko SR, Suzuki Y, Suzuki K, et al. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull 2007;55:1522–7
- Shin JY, Lee JM, Shin HS, et al. Anti-cancer effect of ginsenoside F2 against glioblastoma multiforme in xenograft model in SD rats. J Ginseng Res 2012;36:86–92
- Mai TT, Moona JY, Song YW, et al. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett 2012;321:144–53
- Ma DL, Chana DSH, Leung CH. Molecular docking for virtual screening of natural product databases. Chem Sci 2011;2:1656--65
- Sohn YS, Lee Y, Park C, et al. Pharmacophore identification for peroxisome proliferator-activated receptor gamma agonists. Bull Korean Chem Soc 2011;32:201--7
- Berman HM, Westbrook J, Feng Z, et al. The protein data bank. Nucleic Acids Res 2000;28:235–42
- Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit 1996;9:1–5
- Jones G, Willett P, Glen RC, et al. Development and validation of a genetic algorithm for flexible docking. J Mol Biol 1997;267:727–48
- Rarey M, Kramer B, Lengauer T, Klebe G. A fast flexible docking method using an incremental construction algorithm. J Mol Biol 1996;261:470–89
- Morris GM, Goodsell DS, Halliday RS. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998;19:1639–62
- Park H, Lee J, Lee, S. Critical assessment of the automated AutoDock as a new docking tool for virtual screening. Prot Struct Funct Bioinform 2006;65:549–54
- Bursulaya BD, Totrov M, Abagyan R, Brooks CL III. Comparative study of several algorithms for flexible ligand docking. J Computer-Aided Mol Des 2003;17:755–63
- Sathishkumar N, Sathiyamoorthy S, Ramya M, et al. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J Enzyme Inhibit Med Chem 2012;27:685–92
- Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: prediction of activity spectra for biological active substances. Bioinform Appl Notes 2000;16:747–8
- Lagunin A, Filimonov D, Poroikov V. Multi-targeted natural products evaluation based on biological activity prediction with PASS. Curr Pharm Des 2010;16:1703–17
- Farmer SR. Regulation of PPARγ activity during adipogenesis. Int J Obes 2005;29:S13–16
- Park D, Yoon M. Compound K, a novel ginsenoside metabolite, inhibits adipocyte differentiation in 3T3-L1 cells: involvement of angiogenesis and MMPs. Biochem Biophys Res Commun 2012;422:263–7
- Lee OH, Lee HH, Kim JH, Lee BY. Effect of ginsenosides Rg3 and Re on glucose transport in mature 3T3-L1 adipocytes. Phytother Res 2011;25:768–73
- Kovsan J, Ben-Romano R, Souza SC, et al. Regulation of adipocyte lipolysis by degradation of the perilipin protein. J Biol Chem 2007;282:21704–11
- Blanchette-Mackie EJ, Dwyer NK, Barber LT, et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 1995;36:1211–26
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 2000;16:145–71
