Abstract
Serum paraoxonase (PON1) is a high-density lipoprotein (HDL)-associated enzyme that protects lipoproteins, both low-density lipoprotein (LDL) and HDL, against oxidation, and is considered as an antioxidative/anti-inflammatory component of HDL. In this study, PON1 was purified from bovine serum by ammonium sulfate precipitation and hydrophobic interaction chromatography on sepharose-4B-l-tyrosine-1-napthylamine. It was then immobilized on an unmodified Eupergit® C 250 L support. The immobilized PON1 retained a high catalytic activity and showed increased thermal stability compared to the native enzyme.
Introduction
Serum paraoxonase-1 (PON1), also known as aryl esterase, is a calcium-dependent esterase that catalyzes the hydrolysis of various aromatic carboxylic acid esters and several organophosphatesCitation1. Paraoxonase-1 (E.C. 3.1.8.1) is a 43-kDa mammalian enzyme synthesized primarily in the liver and secreted into the bloodCitation2. In human serum, PON1 is closely associated with high-density lipoproteins (HDL)Citation3. Its name was derived from one of the most commonly used in vitro substrates, paraoxonCitation4. In the middle of the twentieth century, PON1 was recognized to have a significant role in the metabolism of xenobioticsCitation5. It also hydrolyzes the toxic oxon metabolites of organophosphorus compounds, thereby providing limited protection against chronic exposure to organophosphatesCitation6. PON1 plays an important physiological role in lipid metabolism; it hydrolyzes oxidized lipids in the form of lipid hydroperoxides generated on lipoproteins (both HDL and LDL), and protects against the development of oxidative stressCitation7. A decreased serum PON1 activity in humans has been well established in metabolic diseases associated with atherosclerosis. More recently, in addition to its role in lipid metabolism, in cardiovascular disease and arteriosclerosis, PON1 has been shown to play a significant role in the metabolism of pharmaceutical drugs. Given the physiological importance of PON1, the metabolic impact of medically important drugs should receive greater studyCitation8.
Although the presence of PON1 in the serum has long been known in ruminantsCitation5, and the first evidence of a physical association of PON1 with lipoproteins was determined in cattle by Kitchen et al.Citation9 and Don et al.Citation10, knowledge about serum PON1 activity in veterinary medicine is still scarceCitation11.
Hydrophobic interaction chromatography (HIC) is a valuable complementary tool for protein purification. However, its complex selectivity behavior and potentially destabilizing effects on marginally stable proteins limit the preparative application of HIC. There are a large number of process variables that can be manipulated to affect protein selectivity, including temperature, pH, salt type, additives, and chromatographic ligand type and density. In particular, interesting and significant selectivity changes, including reversals, have been noted with changes in the salt type and the stationary phaseCitation12.
Many protocols for covalent immobilization of proteins at high yields have been reported. From these cases, much experience has been gained about support activation and protein immobilization methodsCitation13. However, most protocols have some drawbacks with immobilization under mild experimental conditions. A major one is the requirement for large amounts of protein per milliliter of support throughout long-term handling of the activated supports when the immobilization is carried out at an industrial levelCitation14. Compared to other protocols, in our study, epoxy-activated supports seem to be almost ideal systems for maintaining an easy protocol for enzyme immobilization. Epoxy groups are very stable at neutral pH values and this property can give the advantage of a long time period of storageCitation15. Epoxy-activated supports are almost ideal ones for performing easy immobilization application for proteins and enzymes at both laboratory and industrial scale. Furthermore, epoxy-activated supports are able to form very stable covalent linkages with different protein groups (amino, thiol, and phenolic ones) under very mild experimental conditions (e.g. pH 7.0)Citation16.
The immobilization of enzymes inside porous supports may increase the enzyme stability by preventing any intermolecular process (proteolysis, aggregation), and also by preventing the enzyme from interactions with external interfaces (air, oxygen, immiscible organic solvents, etc.)Citation17–19. However, random immobilization may not promote any additional conformational stabilization of immobilized enzymes. In fact, there are some reports in the literature of immobilization procedures giving no effect, or even negative effects, on the stability of some enzymesCitation20. However, it is generally accepted that stabilization should be achieved if the immobilization of each enzyme molecule occurs through several residues, mainly if the reactive groups in the support are extended from its surface via short spacer armsCitation21.
Enzymes can be immobilized on Eupergit® through their different groups (amino, sulfhydryl, hydroxyl, phenolic ones) that could, however, be essential for their catalytic action. Hence, it has often been difficult to achieve stable immobilization of high levels of activity, because either the active site may be blocked from substrate accessibility, multiple point-binding may occur, or the enzyme may be denatured. However, advantages are that Eupergit® beads are not mechanically destroyed in stirred tank reactors, and filtration at the end of the reaction cycle is quick and easy to perform. Eupergit® is suitable for using in fixed bed reactors, too, where the rigid character of the beads allows high linear flow rates. Changes in pH and salt concentration have no effect on matrix swelling. Thus, fixed bed reactors filled with Eupergit® have a constant bed volume. Because of these advantageous properties, we utilized Eupergit® in this study as a support material for PON1.
Materials and methods
Materials
Most materials used in this study, such as Sepharose 4B, l-tyrosine, 1-napthylamine, paraoxon and protein assay reagents, were obtained from Sigma Chem. Co. (Milan, Italy). All other chemicals used were of analytical grade. Eupergit C 250L was a gift from Röhm GmbH & Co., Degussa (Darmstadt, Germany).
Samples
Blood samples were obtained from Turkish cows. The samples were allowed to clot for 2 h at room temperature and then centrifuged at 5000 rpm for 15 min. In order to perform all the paraoxonase activity measurements in the same run, serum samples used in the study were stored at −20 °C for a short period of time (up to five weeks), which is a much shorter period than the storage stability time recommended for human serumCitation22.
Purification of PON1 enzyme from Bovine serum
Ammonium sulfate precipitation
Bovine serum was isolated from 95 ml samples of fresh bovine blood taken in a dry tube. The blood samples were centrifuged at 1500 rpm for 15 min and approximately 45 ml of serum was removed. Ammonium sulfate precipitation was chosen for initial purification of enzyme. The precipitation intervals for PON1 enzyme were 60–80%. The precipitate was collected by centrifugation at 15 000 rpm for 20 min, and then redissolved in 100 mM Tris-HCl buffer pH 8.0. Ammonium sulfate precipitation has been chosen for initial purification step of bovine serum paraoxonase. Subsequently, prior to loading onto hydrophobic interaction column, the precipitate was saturated with 1 M ammonium sulfate in order to improve its efficiency for binding to hydrophobic gel of the column.
Hydrophobic interaction chromatography
The enzyme obtained from bovine serum using ammonium sulfate precipitation was subjected to HIC. The final saline concentration of the PON1 sample was adjusted to 0.5 M ammonium sulfate, prior to loading onto Sepharose 4B derivatized with l-tyrosine-1-napthylamine. The preparation of the hydrophobic support was as follows. CNBr (10%) was added to a 1:1 mixture of Sepharose 4B and water. The mixture was titrated to pH 11 in an ice bath and maintained at that pH for 8–10 min. The reaction was stopped by filtering the gel on a Buchner funnel and washing with cold 0.1 M NaHCO3 buffer at a pH 10. l-Tyrosine was then coupled to the CNBR-activated Sepharose-4B by the addition of saturated l-tyrosine solution in the same buffer by stirring with a magnet for 90 min. In order to remove the excess l-tyrosine from the Sepharose-4B-l-tyrosine gel, the mixture was washed with distilled water. The final hydrophobic gel was obtained by diazotization of 1-naphthylamine and coupling of this compound to the Sepharose-4B-l-tyrosine. The pH was adjusted to 9.5 with 1 M NaOH and, after gentle stirring for 3 h at room temperature, the coupled red Sepharose derivative was washed with 1:l of water and then with 200 ml of 0.05 M Tris-sulfate at pH 7.5. The column was equilibrated with 0.1 M Na2HPO4 buffer at pH 8.0 including 1 M ammonium sulfate. The paraoxonase was eluted with ammonium sulfate gradient (60–80% intervals) using 0.1 M Na2HPO4 buffer with and without ammonium sulfate at pH 8.0. The purified PON enzyme was stored in the presence of 2 mM CaCl2 at 4 °C, in order to maintain activityCitation4.
Total protein determination
The absorbance at 280 nm was used to monitor the protein during the ammonium sulfate precipitation and in the column effluents. Quantitative protein determination was achieved by absorbance measurements at 595 nm according to the method of BradfordCitation23, using bovine serum albumin as a standard.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed in order to verify the purification of PON1. It was carried out in 12% acrylamide gels (with 3% acrylamide stacking gels), containing 0.1% SDS, according to LaemmliCitation24. Visualization of protein bands was carried out by incubating the gel with a Coomassie staining solution.
Immobilization of paraoxonase
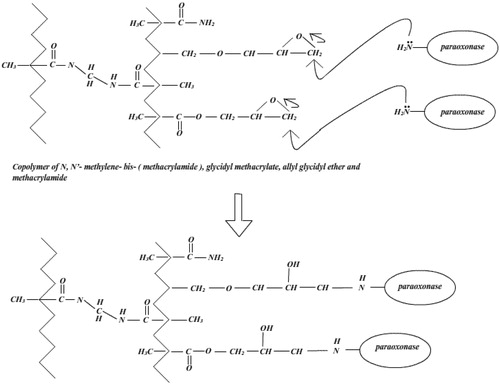
The conventional method for enzyme immobilization on Eupergit® supports involves the direct enzyme coupling to the polymer via its oxirane groups. Unmodified Eupergit® 250 L (50 mg) was incubated with 10 ml of purified PON1. After incubation for 48 h, the beads were collected by vacuum filtration using a glass filter (Whatman), washed with 40 ml of 0.1 M Na2HPO4, at pH 8.0 including 1 M (NH4)2SO4, and then stored at 4 °C until use. As shown in , the epoxy group of the Eupergit C 250L couples with amino groups of the enzyme.
Enzyme activity assay
PON1 enzyme activity toward paraoxon was quantified spectrophotometrically by the method of Gan et al.Citation3. The reaction was followed for 2 min at 37 °C by monitoring the appearance of p-nitrophenol at 412 nm in a Biotek automated recording spectrophotometer (Bad Friedrichshall, Germany). A final substrate concentration of 2 mM was used for the enzyme assay, and all measurements were taken in duplicate and corrected for the non-enzymatic hydrolysis.
Activity immobilization yield
The efficiency of enzyme immobilization was evaluated in terms of enzyme and activity coupling yields. The enzyme coupling yield, ηenz(%), and activity coupling yield, ηact(%), were calculated as follows:
(1)
(2)
where P1 is the amount of immobilized enzyme, P0 the initial amount of enzyme, SA2 the specific activity of immobilized PON1, and SA1 the specific activity of free PON1.
Thermal inactivation assays
Thermal stability assays were performed at three different temperatures (25, 45, and 65 °C) in an aqueous medium (100 mM Tris-HCl, pH 8.0). At these temperatures, the activity of both native and immobilized paraoxonase was measured using paraoxon substrate for a duration of 3 h.
pH-dependent activity
The effects of pH on both native and immobilized enzymes were studied by assaying the preparations at pH values over the range of 6.5–9.0 in 100 mM Tris-HCl buffer.
Determination of Km and Vmax
The absorbances for product generated from paraoxon substrate by native and immobilized enzymes were measured at seven different substrate concentrations (0.5, 1.0, 1.5, 2, 2.5, 3, and 4 mM) at pH 8.0 and a temperature of 37 °C. Km and Vmax values were determined by means of Lineweaver–Burk graphs.
Results and discussion
Access to purified PON1 would afford several benefits. It would greatly facilitate their structural and functional characterization and also to permit examination of their weak, yet potentially most biologically relevant activities, in the complete absence of other serum proteins. This enzyme is bound to HDL, thus its purification could be problematic and generally consists of multistep procedures. The purification of aryl esterase (paraoxonase) from human sera was originally carried out by Gan et alCitation3. Further studies on this enzyme improved the purification proceduresCitation25,Citation26. In our study, bovine serum PON1 was purified by using only two sequential steps: ammonium sulfate precipitation and HIC.
A new hydrophobic gel has been synthesized in order to reduce the number of purification steps required. This hydrophobic gel was designed on the basis of the retained N-terminal hydrophobic signal peptide present on bovine serum PON1. A hydrophobic ligand was obtained by the coupling of 1-napthylamine to a Sepharose-4B gel matrix, which had been provided with an l-tyrosine armCitation4. Our analyses (shown in ) indicated a high purification yield of PON1 under mild experimental conditions.
Table 1. Summary of the purification of bovine serum paraoxonase.
As shown in , our enzyme purification yielded a 337-fold purification from two steps, which compares very favorably with the final specific activity and purification values reported for other purification proceduresCitation27,Citation28. However, different purification protocols have been used for PON enzyme from different sources. Sheep serum PON was purified 330-to-385-fold using ethanol, pH, and ionic strength fractionationCitation29. Rodrigo et al.Citation30 purified the liver PON by 415-fold using hydroxyapatite adsorption, chromatography on DEAE–Sepharose CL-6B, non-specific affinity chromatography on Cibacron Blue 3 GA, and anion-exchange on Mono Q HR 5/5. In addition, liver PON3 has been purified 177-fold using a protocol consisting of seven steps.
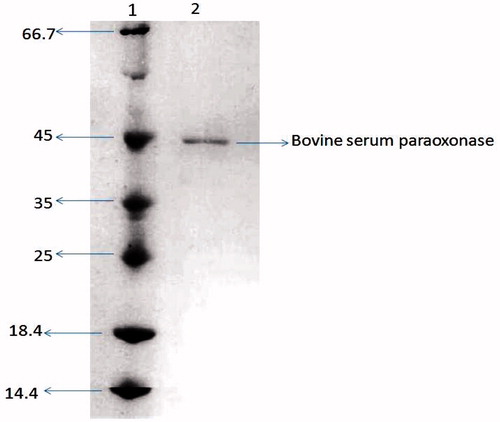
illustrates the final enzyme purification as determined by SDS-PAGE gel electrophoresis (BioRad GmbH, München, Germany). The purified bovine serum PON1 gives a single band on SDS-PAGE with a molecular weight of 45 kDa. Some purification studies indicate differences in the migration of PON bands in SDS-PAGECitation24. Furlong et al.Citation31 demonstrated two PON bands purified from rabbit serum. Sequence analyses have indicated five potential N-glycosylation sites in rabbit PON and four in human PONCitation32. However, the purification protocol used in this studyCitation4 yielded a single 45-kDa band, suggesting that the purification is free from contaminants.
Figure 2. SDS-PAGE of bovine serum PON1. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction. Experimental conditions were as described in the Methods section. Lane 1 contained 3 μg of various molecular-mass standards: Bovine serum albumin (66.7 kDa), ovalbumin (45.0 kDa), lactate dehydrogenase (35 kDa), REase βsp98I (25.0 kDa), β-lactoglobulin (18.4 kDa), and lysozyme (14.4 kDa).
Subsequently, we have designed a procedure for the efficient, covalent immobilization of PON1 to Eupergit® C 250 L via its oxirane groups. The enzyme loading, specific activity, and coupling yields of this immobilization obtained using a fixed ratio between support (50 mg) and enzyme (30 mg) are summarized in .
Table 2. Summary of immobilization of PON1 on the Eupergit C 250 L support.
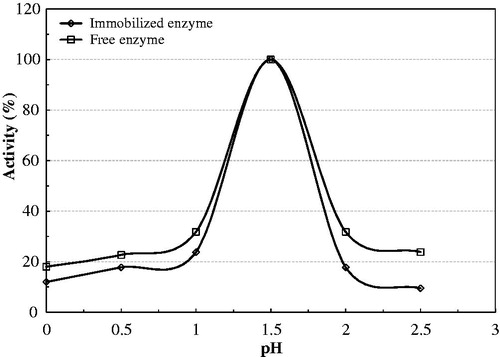
The pH is one of the major parameters capable of shifting enzymatic activities in an aqueous solution. Immobilization of enzyme usually results from a conformational change in the enzyme and a resulting shift in the optimum pH. The effects of pH on the activity of native versus immobilized PON1 are given in . The maximum activity of both the native and immobilized enzymes was observed at pH 8.0. However, as shown in , the enzyme activity of immobilized PON1 is proportionately higher than that of the native enzyme at pH values both below (pH 6.5) and above (pH 9) the optimum pH. Eupergit® C 250 L, as a carrier, thus appears to give an enhanced stabilization effect to bovine serum PON1.
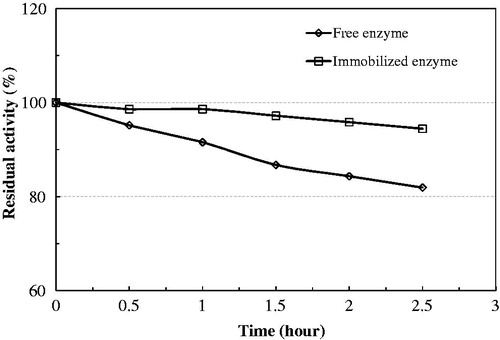
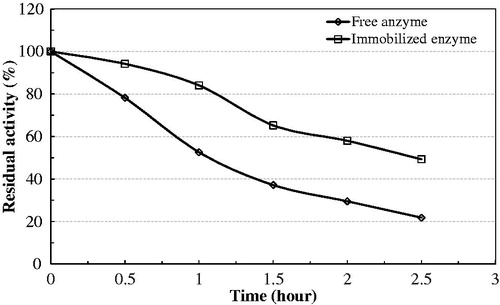
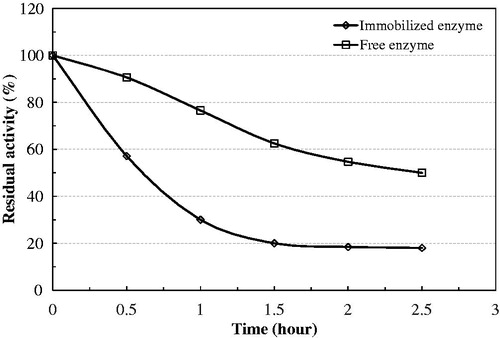
The activity of the soluble enzyme is strongly dependent on temperature (). The activity of native enzyme decreased gradually with temperature, with an optimum temperature of 45 °C. The thermal inactivation of soluble versus immobilized bovine serum PON1 was studied over the temperature range of 25 to 65 °C, at the optimal pH for the catalytic activity (pH 8.0). In general, the immobilization of bovine serum PON1 on Eupergit C protected the enzyme against thermoinactivation, allowing the immobilized enzyme to continue to work in a tougher environment with minimal activity loss. In particular, a significant improvement of the thermal stability of the immobilized enzyme was observed at 65 °C. This stabilization seems to represent an increase in rigidity as a result of a conformational stabilization of the protein. This improved thermostability might be useful in the application of this system at high temperatures, thereby avoiding microbial contamination. Furthermore, at 65 °C, the solubility of both substrate and products is higher.
The initial reaction rates for free versus immobilized bovine serum PON1 were determined at different concentrations of paraoxon substrate ranging from 0.5 to 4 mM. The Michaelis constant (Km) and maximum velocity (Vmax) were calculated from Lineweaver–Burk plots, and the results are shown in .
Table 3. Influence of immobilization process on kinetic constants.
The Km values of the native and immobilized enzymes were calculated as 6.26 and 2.47 mM, respectively. The initial reaction rates for free and immobilized bovine serum paraoxonase were determined at different concentrations of paraoxon ranging from 0.5 to 4 mM. The Michaelis constant (Km) and maximum velocity (Vmax) were calculated from Lineweaver–Burk plots. The results are shown in Table 3. When we compare each enzyme status after immobilization processes according to their kinetic values, we recognized that the native enzyme showed a lower binding. On this point, we can easily say that the above values show the effective interaction between the enzyme and the substrate after this immobilization.
Conclusions
PON1 is also a major anti-atherosclerotic component of HDLCitation33,Citation34. The PON1 gene is activated by PPAR-γ, which increases synthesis and release of PON1 enzyme from the liver, reducing atherosclerosisCitation35. The activities of a wide variety of enzymes are responsible for aspects of pharmaceutical and toxicological metabolism. For example, glucose 6-phosphate dehydrogenase and carbonic anhydrase are known as drug targetsCitation36,Citation37. In recent years, the PON enzyme family, particularly PON1, has also been studied as a potential drug targetCitation38. For further studies on this system, it is useful to have an easy method of enzyme purification together with a stable system for testing enzymatic activities. In this study, we have demonstrated a two-step procedure for purifying PON1 from bovine serum. In addition, a novel immobilization of PON1 by covalent attachment to a Eupergit® C 250 L support yields an active enzyme with an enhanced resistance to thermal and pH denaturation.
Acknowledgements
The authors thank Röhm GmbH & Co., Degussa (Darmstadt, Germany) for providing us with a gift of Eupergit C 250 L. They also thank Dr. Malcolm Lyon for critically reading and helpful discussion of the manuscript.
Declaration of interest
The authors have no conflicts of interest to report for this study. This work was supported by Balıkesir University Research Foundation project 2008/22.
References
- La Du BN, Aviram M, Billecke S, et al. On the physiological role(s) of the paraoxonase. Chem Biol Interact 1999;119-120:379–88
- Hassett C, Richter RJ, Humbert R, et al. Characterisation of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry 1991;30:10141–9
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–6
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–74
- Aldridge WN. Serum esterases 2. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E 600) and its identity with the A-esterase of mammalian sera. J Biochem 1995;53:117–24
- Draganov D, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol 2004;369:78–88
- Mackness B, Durrington PN, Boulton AJ, et al. Serum paraoxonase activity in patients with type 1 diabetes compared to healthy controls. Eur J Clin Invest 2002;32:259–64
- Turk R, Juretić D, Gereš D, et al. Flegar–Meštrić influence of oxidative stress and metabolic adaptation on PON1 activity and MDA level in transition dairy cows. Anim Reprod Sci 2008;108:98–106
- Kitchen BJ, Masters CJ, Winzor DJ. Effects of lipid removal on the molecular size and kinetic properties of bovine plasma arylesterase. Biochem J 1973;135:93–9
- Don MM, Masters CJ, Winzor DJ. Further evidence for the concept of bovine plasma arylesterase as a lipoprotein. Biochem J 1975;151:625–30
- Turk R, Juretic D, Geres D, et al. Serum paraoxonase activity in dairy cows during pregnancy. Res Veter Sci 2005;79:15–18
- Jones TT, Fernandez EJ. Hydrophobic interaction chromatography selectivity changes among three stable proteins: conformation does not play a major role. Biotechnol Bioeng 2004;87:388–99
- Katchalski-Katzir E. Immobilized enzymes-learning from past successes and failures. Trends Biotechnol 1993;11:471–8
- Kennedy JF, Melo EHM, Jumel K. Immobilized enzymes and cells. Chem Eng Prog 1990;45:81–9
- Giacomini C, Irazoqui G, Batista-Viera F, Brena BM. Influence of the immobilization chemistry on the properties of immobilized β-galactosidases. J Mol Catal B Enzym 2001;11:597–606
- Mateo C, Abian O, Fernandez R, Guisan JM. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb Technol 2000;26:509–15
- Bes T, Gomez-Moreno C, Guisan JM, Fernandez-Lafuente R. Selective enzymatic oxidations: stabilization by multipoint covalent attachment of ferredoxin NAD-reductase: an interesting cofactor recycling enzyme. J Mol Catal 1995;98:161–9
- Fernandez-Lafuente R, Rodriguez V, Guisan JM. The coimmobilization of D-amino acid oxidase and catalase enables the quantitative transformation of D-amino acids (D-phenylalanine) into keto acids (phenylpyruvic acid). Enzyme Microb Technol 1998;23:28–33
- Gupta MN. Thermostabilization of proteins. Biotechnol Appl Biochem 1991;14:1–11
- Nanalov RJ, Kamboure MS, Emanuiloda EI. Immobilization and properties of Bacillus stearothermophilus pulanase. Biotechnol Appl Biochem 1993;18:409–16
- Desmukth SS, Duta M, Choudohury S, Shanker V. Preparation and properties of glucose isomerase immobilized on indon 48-R. Appl Biochem Biotechnol 1993;42:95–104
- Brackley M, Carro-Ciampi G, Stewart DJ, et al. Stability of the paraoxonase phenotyping ratio in collections of human sera with differing storage times. Res Commun Chem Pathol Pharmacol 1983;41:65–78
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51
- Laemmli UK. Cleavage of structual proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685
- Adler A, Disteche CM, Omiecinski CJ, et al. Human and rabbit paraoxonases: purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxification. Chem Biol Interact 1993;87:35–48
- Rodrigo L, Gil F, Hernandez AH, et al. Purification and characterization of paraoxon hydrolyse from rat liver. Biochem J 1997;321:595–601
- Furlong CE, Costa LG, Hasett C, et al. Human and rabbit paraoxonases: purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxification. Chem Biol İnteract 1993;87:35–48
- Beltowski J, Wojcicka G, Jamroz A. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) or tissue paraoxonase 1 and plasma platelet activating factor acetylhydrolase activities. J Cardiovasc Pharmacol 2004;43:121–7
- Main AR. The role of A-esterase in the acute toxicity of paraoxon, TEPP and parathion. Can J Biochem Physiol 1956;34:197–216
- Rodrigo L, Gil F, Hernandez AF, et al. Purification and characterization of paraoxon hydrolyse from Rat Liver. J Biochem 1997;321:595–601
- Furlong CE, Richter RJ, Chapline C, Crabb JW. Purification of rabbit and human serum paraoxonase. Biochemistry 1991;30:10133–40
- Blatter-Garin MC, Kalix B, De Pre S, James RW. Aspirin use is associated with higher serum concentrations of the antioxidant enzyme, paraoxonase-1. Diabetologia 2003;46:593–4
- Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol 2005;15:261–7
- Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: is the gene or the protein more important? Free Radic Biol Med 2005;37:1317–23
- Khateeb J, Gantman A, Kreitenberg AJ, et al. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: a role for PPAR-gamma pathway. Atherosclerosis 2010;8:119–25
- Supuran CT. Carbonic anhydrases as drug targets – an overview. Curr Top Med Chem 2007;7:825–33
- Gupte SA. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr Opin Investig Drugs 2008;9:993–1000
- Akkemik E, Budak H, Ciftci M. Effects of some drugs on human erythrocyte 6-phosphogluconate dehydrogenase: an in vitro study. J Enzyme Inhib Med Chem 2010;25:476–9