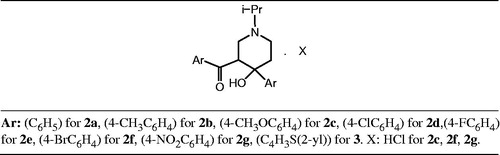
Abstract
Some 4-piperidinol derivatives were synthesized and their cytotoxicity was tested against human hepatoma (Huh7) and breast cancer (T47D) cells. Aryl part was changed as phenyl in 2a, 4-methylphenyl in 2b, 4-methoxyphenyl in 2c, 4-chlorophenyl in 2d, 4-fluorophenyl in 2e, 4-bromophenyl in 2f, 4-nitrophenyl in 2g and 2-thienyl in 3. Compounds were synthesized and reported for the first time by this study except 2a and 2d. Chemical structures were confirmed by 1H NMR, 13C NMR, IR, MS and elemental analyses. Compounds 2a (3.1 times), 2c (3.8 times), 2f (4.6 times), 2g (1.3 times) and 3 (3.2 times) had 1.3–4.6 times higher cytotoxic potency than the reference compound 5-FU against Huh7 cell line while all the compounds synthesized had shown lower activities against T47D cell line than 5-FU. In the light of these results, compounds 2a, 2c, 2f, 2g and 3 may serve as model compounds for further studies.
Introduction
Today, breast cancer is the most common cancer in women and is one of the five common cancers. In the treatment of breast cancer, chemotherapy, endocrine and radiation therapy, and surgery are used in general but emergence of side effects of cytotoxic chemotherapeutics and drug resistance to these drugs are often problems in the course of therapyCitation1. Similarly, hepatocellular carcinoma (HCC) is the fifth most common and the third most deadly cancer disease worldwideCitation2. For most patients with HCC, surgery is the only curative treatment procedure because HCC cells have high resistance to the chemotherapeutic agentsCitation3. In the light of these information mentioned above, novel approaches and cytotoxic agents are urgently needed in the treatment of breast cancer and HCC.
Mannich bases are compounds that attract the attention of researchers. According to the studies carried out, the following activities of Mannich bases have been identified: anticancerCitation4, cytotoxicCitation5–13, anti-inflammatoryCitation14–16, anticonvulsantCitation17,Citation18 and antifungalCitation19,Citation20. Their cytotoxic activities were attributed to the α,β-unsaturated ketone, which is available in the chemical structure of the compound or to the product produced by deamination process in vivo or under simulated conditions in vitroCitation7,Citation21,Citation22.
4-Piperidinol derivatives have been investigated for their biological activities especially in the last decade. Anti-tuberculosisCitation23, anti-inflammatoryCitation24, H3 antagonistCitation25, sodium and calcium channel blockerCitation26, anti-leukemiaCitation27, estrogen receptor modulatorCitation28, liver glycogen phosphorylase inhibitörCitation29, map kinase inhibitorCitation30 and dopamine D2 receptor antagonistCitation31,Citation32 activities were reported in the literatures. Since Mannich bases were known with their remarkable cytotoxic activities in literatureCitation4,Citation13,Citation33, it could be useful to synthesize new chemicals having Mannich base structure for the solution of the problems mentioned above such as breast and liver cancers.
It is an advantage and a good starting point that the cytotoxicities of mono-Mannich bases namely 1-aryl-3-isopropylamino-1-propanone hydrochlorides against Huh7 and T47D cell lines were reported in our previous studyCitation13. It is possible to find new chemical structures which can be drug candidates by the synthesis and investigation of the cytotoxic activities of semi-cyclic mono-Mannich bases having piperidine structure namely 3-aroyl-4-aryl-1-isopropylamino-4-piperidinols. Furthermore, it is also possible to observe the alterations in cytotoxicity depending on chemical structures by comparing the cytotoxicities of the compounds reported here with mono-Mannich bases reported in our previous studyCitation13. Semi-cyclic mono-Mannich bases (piperidines) reported here are non-classic bioisosters of mono-Mannich bases reported beforeCitation13. In addition, there are not any research about cytotoxic activity of 4-piperidinol derivatives on breast cancer and HCC in the literature.
The aims of this study were to synthesize 3-aroyl-4-aryl-1-isopropylamino-4-piperidinols and evaluate their cytotoxicity against human hepatoma and breast cancer cell lines.
Experimental
Chemistry
Chemicals used in synthesis of the compounds in this study were as follows: acetophenone, 4′-methylacetophenone, 4′-nitroacetophenone, 4′-chloroacetophenone, 2-acetylthiophene (Fluka, Steinheim, Switzerland), 4′-methoxyacetophenone, 4′-fluoroacetophenone, 4′-bromoacetophenone, paraformaldehyde (Merck, Darmstadt, Germany), methanol (J.T. Baker, Deventer, Holland) and diethyl ether (Fluka, Steinheim, Switzerland). The 1H and 13C-NMR spectra were recorded at 400 (100) MHz on a Varian Mercury Plus spectrometer (Varian, Palo Alto, CA). Electron ionization mass spectrometry (EI-MS) spectra were recorded on a Thermo-Finnigan’s mass analyzer (Thermo-Finnigan LLC, San Jose, CA). Infrared spectra were obtained for KBr disks on a Mattson 1000 FT-IR (Fourier transform infrared spectroscopy) spectrophotometer (Mattson Instruments, Cambridge, UK). Elemental analyses were carried out with a LECO’s CHNS-932 instrument (LECO Corporation, St. Joseph, MI). Melting points were determined on a BUCHI 530 (Büchi Labortechnik AG, Flawil, Switzerland).
Synthesis
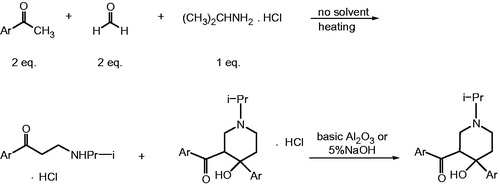
The mixture of suitable ketone, paraformaldehyde and isopropylamine hydrochloride in 2:2:1 mole ratios was stirred and heated without solvent in an oil bath. Mono-Mannich bases type, namely 1-aryl-3-isopropylamino-1-propanone hydrochloride, and piperidinol type compounds, which are a semi-cyclic mono-Mannich base namely 3-aroyl-4-aryl-1-isopropylamino-4-piperidinol hydrochlorides were produced in the reaction medium at the same time. Corresponding mono-Mannich bases were removed from the reaction medium to obtain piperidinol type compounds 2a–2g and 3 synthesized in this study. To obtain piperidinols, 5% sodium hydroxide solution was added to the residue. The reaction content was stirred in water bath at 40 °C. The reaction content, which had fat emulsion view initially, was stirred until a solidification observed (24–96 h), except compound 2c. In the synthesis of compound 2c, any solidification was not observed at the end of period of time. The residue of 2c was filtered on the column containing basic Al2O3 using ethanol as the solvent. The crude compounds of 2a, 2b and 2d were crystallized from methanol; 2e, 2f and 2g were crystallized from chloroform and hexane; 2c and 3 were crystallized from methanol and diethyl ether. The elemental analysis results of the compounds 2c, 2f and 2g were consistent with the hydrochloride salts of the compounds and were within the acceptable limits according to elemental analysis results although 5% sodium hydroxide had been used during the reaction process.
Determination of the cytotoxic activity of the compounds synthesized against human hepatoma cell lineCitation34 and human breast cancer cell line (T47D)
Chemicals were tested with (National Cancer Institute, NCI) anticancer drug screening methodCitation34. Human hepatoma cells (Huh7 cells) (5000 or 10 000 cells) were inoculated into 96-well microtiter plates in 100 µl of standard medium 24 h prior to treatment with inhibitors (compounds). Compounds with various (40–2.5 µM) concentrations were applied in additional 100 µl of cell culture medium. After 72 h of treatment with compounds, cells were fixed by gentle addition of 50 µl of cold 50% (w/v) TCA for 60 min at 4 °C. After fixation, cell culture medium is discarded and washed with distilled water and dried in air. Then, 100 µl of 0.4% sulforhodamine B (SRB) solution were applied to each well and incubated for 10 min at room temperature. Extra unbound dye were washed five times with 200 µl 1% acetic acid and air-dried. SRB dye, which was bound to cellular proteins, solubilized adding 200 µl–10 mM Tris–Base solution and the absorbance was acquired at 515 nm, then IC50 values were calculated as describedCitation34. The figures given were the average of the two independent determinations, which differed by <10%.
3-Benzoyl-4-phenyl-1-isopropyl-4-piperidinol 2a
M.p. 123–124 °C. Yield: 26%. 1H NMR [400 MHz, CDCl3, parts per million (ppm)] δ = 7.87 (br d, 2H, H-2′/6′, J = 7.3 Hz), 7.54 (t, 1H, H-4′, J = 7.3 Hz), 7.51 (d, 2H, H-2′′/6′′, J = 7.7 Hz), 7.41 (t, 2H, H-3′/5′, J = 7.7 Hz), 7.23 (t, 2H, H-3′′/5′′, J = 7.7 Hz), 7.11 (t, 1H, H-4′′, J = 7.3 Hz), 5.15 (d, 1H, OH, J = 2.6 Hz), 4.35 (dd, 1H, H-3, J = 11.0, 2.9 Hz), 2.96–2.77 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.06–2.00 [m, 1H, H-5(a)], 1.84 [br d, 1H, H-5(b), J = 13.9 Hz], 1.10 [d, 6H, CH(CH3)2, J = 6.6 Hz]; 13C NMR (100 MHz, CDCl3, ppm) δ = 204.9, 147.6, 136.2, 134.1, 129.0, 128.6, 128.5, 126.9, 124.8, 73.6, 55.0, 51.3, 48.8, 44.6, 40.4, 18.8, 18.6; MS (EI, 70 eV): m/z 324 (M + 1, 63), 280 (18), 235 (3), 203 (90), 186 (41), 160 (18), 105 (100), 77 (70%); IR (KBr, cm−1): 3453, 3059, 2964, 2831, 1660, 1596, 1578, 1492, 1469, 1447, 1384, 1361, 1335, 1273, 1206, 1172, 1132, 1103, 1068, 1052, 1014, 1001, 958, 899, 759, 709. Calculated for C21H25NO2 (323.43): C, 77.98; H, 7.79; N, 4.33. Found: C, 77.81; H, 8.14; N, 4.38.
3-(p-Methylbenzoyl)-4-(p-methylphenyl)-1-isopropyl-4-piperidinol 2b
M.p. 141–142 °C. Yield: 20%. 1H NMR (400 MHz, CDCl3, ppm) δ = 7.80 (d, 2H, H-2′/6′, J = 8.1 Hz), 7.38 (d, 2H, H-2′′/6′′, J = 8.1 Hz), 7.22 (d, 2H, H-3′/5′, J = 8.1 Hz), 7.03 (d, 2H, H-3′′/5′′, J = 8.1 Hz), 5.21 (d, 1H, OH, J = 2.9 Hz), 4.30 (dd, 1H, H-3, J = 11.2, 3.9 Hz), 2.93–2.75 (m, 5H, CH(CH3)2, 2XH-2, 2XH-6), 2.38 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.03–1.94 [m, 1H, H-5(a)], 1.81 [br d, 1H, H-5(b), J = 13.6 Hz], 1.09 [d, 6H, CH(CH3)2, J = 6.6 Hz]; 13C NMR (100 MHz, CDCl3, ppm) δ = 204.5, 145.1, 144.8, 136.4, 133.8, 129.7, 129.2, 128.8, 124.7, 73.5, 54.9, 50.9, 48.9, 44.7, 40.7, 21.9, 21.1, 18.8, 18.6; MS (EI, 70 eV): m/z 351 (M+, 1), 308 (7), 217 (6), 200 (8), 190 (6), 163 (8), 146 (11), 131 (5), 119 (100), 105 (8), 91 (56), 89 (7), 82 (5), 71 (10), 64 (17), 56 (19%); IR (KBr, cm−1): 3449, 3025, 2964, 2921, 2831, 1658, 1605, 1570, 1512, 1458, 1407, 1384, 1361, 1333, 1302, 1273, 1258, 1203, 1178, 1071, 1014, 1002, 957, 902, 811, 738. Calculated for C23H29NO2 (351.48): C, 78.59; H, 8.32; N, 3.99. Found: C, 78.10; H, 8.59; N, 3.99.
3-(p-Methoxybenzoyl)-4-(p-methoxyphenyl)-1-isopropyl-4-piperidinol hydrochloride 2c
Yellow oil. Yield: 40%. 1H NMR (400 MHz, CDCl3, ppm) δ = 7.87 (br d, 2H, H-2′/6′, J = 9.0 Hz), 7.39 (br d, 2H, H-2′′/6′′, J = 9.0 Hz), 6.88 (br d, 2H, H-3′/5′, J = 9.0 Hz), 6.75 (br d, 2H, H-3′′/5′′, J = 9.0 Hz), 5.30 (d, 1H, OH, J = 2.6 Hz), 4.24 (dd, 1H, H-3, J = 10.8, 3.9 Hz), 3.84 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 2.91–2.78 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.09–1.90 [m, 1H, H-5(a)], 1.80 [br d, 1H, H-5(b), J = 13.9 Hz], 1.09 [d, 6H, CH(CH3)2, J = 6.6 Hz]; 13C NMR (100 MHz, CDCl3, ppm) δ = 203.3, 164.4, 158.4, 140.0, 131.0, 129.3, 126.0, 114.2, 113.7, 73.3, 55.7, 55.3, 54.9, 50.7, 49.0, 44.7, 40.6, 18.8, 18.6; MS (EI, 70 eV): m/z 383 (M+, 1), 162 (34), 135 (100), 119 (2), 107 (11), 92 (8), 77 (16), 64 (6%); IR (KBr, cm−1): 3434, 2962, 2839, 2670, 2578, 2462, 2049, 1981, 1909, 1780, 1667, 1660, 1651, 1600, 1574, 1514, 1463, 1456, 1423, 1384, 1306, 1259, 1232, 1174, 1119, 1071, 1031, 999, 972, 842, 759. Calculated for C23H30ClNO4 (419.94): C, 65.78; H, 7.20; N, 3.34. Found: C, 65.46; H, 7.29; N, 3.36.
3-(p-Chlorobenzoyl)-4-(p-chlorophenyl)-1-isopropyl-4-piperidinol 2d
M.p. 135–137 °C. Yield: 16%. 1H NMR (400 MHz, CDCl3, ppm) δ = 7.80 (d, 2H, H-2′/6′, J = 8.4 Hz), 7.40 (d, 4H, H-3′/5′, H-2′′/6′′, J = 7.7 Hz), 7.19 (d, 2H, H-3′′/5′′, J = 8.4 Hz), 5.06 (d, 1H, OH, J = 2.2 Hz), 4.24 (dd, 1H, H-3, J = 10.6, 3.3 Hz), 2.90–2.75 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 1.99–1.92 [m, 1H, H-5(a)], 1.79 [br d, 1H, H-5(b), J = 13.9 Hz], 1.09 (d, 3H, CH3, J = 6.2 Hz], 1.08 (d, 3H, CH3, J = 6.6 Hz); 13C NMR (100 MHz, CDCl3, ppm) δ = 203.4, 146.0, 141.0, 134.3, 132.9, 129.9, 129.5, 128.7, 126.3, 73.4, 55.0, 51.2, 48.8, 44.3, 40.3, 18.8, 18.4; MS (EI, 70 eV): m/z 392 (M + 1, 8), 348 (4), 303 (2), 252 (2), 238 (21), 226 (87), 210 (40), 194 (8), 182 (14), 167 (20), 149 (4), 139 (37), 111 (100), 98 (12), 85 (7), 72 (69), 56 (93%); IR (KBr, cm−1): 3455, 2965, 2831, 1662, 1588, 1569, 1489, 1469, 1401, 1384, 1362, 1308, 1272, 1204, 1172, 1093, 1011, 979, 900, 841, 827. Calculated for C21H23Cl2NO2 (392.32): C, 64.29; H, 5.91; N, 3.57. Found: C, 63.05; H, 5.85; N, 3.58.
3-(p-Fluorobenzoyl)-4-(p-fluorophenyl)-1-isopropyl-4-piperidinol 2e
M.p. 113–115 °C. Yield: 24%. 1H NMR (400 MHz, CDCl3, ppm) δ = 7.89 (dd, H-2′/6′, 2H, 3JH,H = 8.6 Hz, 4JH,F = 5.3 Hz), 7.43 (dd, 2H, H-2′′/6′′, 3JH,H = 8.6 Hz, 4JH,F = 5.3 Hz), 7.09 (t, 2H, H-3′/5′, 3JH,H = 3JH,F = 8.6 Hz), 6.90 (t, 2H, H-3′′/5′′, 3JH,H = 3JH,F = 8.6 Hz), 5.10 (d, 1H, OH, J = 2.6 Hz), 4.23 (dd, 1H, H-3, J = 10.2, 3.3 Hz), 2.91–2.76 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.01–1.95 [m, 1H, H-5(a)], 1.80 [br d, 1H, H-5(b), J = 13.9 Hz], 1.09 [d, 6H, CH(CH3)2, J = 6.6 Hz]; 13C NMR (100 MHz, CDCl3, ppm) δ = 203.2, 166.5 (d, 1JC–F = 257 Hz), 161.8 (d, 1JC–F = 245 Hz), 143.3, 132.6, 131.3 (d, 3JC–F = 9 Hz), 126.5 (d, 3JC–F = 8 Hz), 116.3 (d, 2JC–F = 22 Hz), 115.2 (d, 2JC–F = 21 Hz), 73.4, 55.0, 51.4, 48.8, 44.4, 40.4, 18.8, 18.5; MS (EI, 70 eV): m/z 360 (M + 1, 41), 342 (5), 316 (51), 281 (3), 271 (8), 221 (36), 204 (49), 194 (62), 178 (20), 151 (28), 123 (100), 109 (14), 95 (40), 75 (8), 56 (24%); IR (KBr, cm−1): 3453, 2966, 2832, 1663, 1598, 1508, 1470, 1409, 1385, 1362, 1299, 1273, 1222, 1204, 1173, 1158, 1096, 1070, 1013, 979, 958, 902, 847, 833, 773. Calculated for C21H23F2NO2 (359.41): C, 70.18; H, 6.45; N, 3.90. Found: C, 69.75; H, 6.43; N, 3.88.
3-(p-Bromobenzoyl)-4-(p-bromophenyl)-1-isopropyl-4-piperidinol hydrochloride 2f
M.p. 212–214 °C. Yield: 14%. 1H NMR (400 MHz, CDCl3, ppm) δ = 8.02 (d, 2H, H-2′/6′, J = 8.4 Hz), 7.61 (d, 2H, H-2′′/6′′, J = 8.4 Hz), 7.43 (d, 2H, H-3′/5′, J = 8.4 Hz), 7.36 (d, 2H, H-3′′/5′′, J = 8.4 Hz), 5.78 (dd, 1H, H-3, J = 12.1, 3.7 Hz,), 5.01 (d, 1H, OH, J = 2.6 Hz), 3.51–3.31 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.91–2.84 [m, 1H, H-5(a)], 1.94 [br d, 1H, H-5(b), J = 14.3 Hz], 1.53 (d, 3H, CH3, J = 6.6 Hz), 1.44 (d, 3H, CH3, J = 6.6 Hz); 13C NMR (100 MHz, CDCl3, ppm) δ = 201.2, 143.2, 133.0, 132.3, 132.0, 131.3, 130.9, 126.6, 122.0, 72.2, 58.5, 47.7, 46.1, 43.7, 36.4, 17.6, 16.3; MS (EI, 70 eV): m/z 484/482/480 (M + 1, 1,2,1), 440/438/436 (3,6,3), 283/281 (21,23), 256/254 (27,29), 212/210 (28,30), 185/183 (94,100), 156/154 (31,33), 132 (18), 98 (10), 72 (28), 56 (39%); IR (KBr, cm−1): 3279, 2977, 2507, 1675, 1584, 1485, 1398, 1285, 1254, 1228, 1210, 1179, 1155, 1071, 1001, 973, 897, 864, 824, 750. Calculated for C21H24Br2ClNO2 (517.68): C, 48.72; H, 4.67; N, 2.71. Found: C, 48.74; H, 4.70; N, 2.83.
3-(p-Nitrobenzoyl)-4-(p-bromophenyl)-1-isopropyl-4-piperidinol hydrochloride 2g
M.p. 118–120 °C. Yield: 11%. 1H NMR (400 MHz, CDCl3, ppm) δ = 8.27 (br d, 2H, H-3′/5′, J = 9.0 Hz), 8.10 (br d, 2H, H-3′′/5′′, J = 9.0 Hz), 8.03 (br d, 2H, H-2′/6′, J = 9.0 Hz), 7.67 (br d, 2H, H-2′′/6′′, J = 9.0 Hz), 4.92 (br s, 1H, OH), 4.39 (dd, 1H, H-3, J = 10.8, 3.5 Hz), 2.98–2.80 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.05–1.97 [m, 1H, H-5(a)], 1.84 [br d, 1H, H-5(b), J = 13.9 Hz], 1.10 (d, 3H, CH3, J = 6.2 Hz), 1.09 (d, 3H, CH3, J = 6.6 Hz); 13C NMR (100 MHz, CDCl3, ppm) δ = 202.8, 154.6, 151.2, 147.2, 140.0, 129.6, 125.9, 124.4, 123.9, 73.8, 55.1, 52.1, 48.6, 44.0, 40.0, 18.8, 18.3; MS (EI, 70 eV): m/z 413 (M+, 1), 177 (29), 160 (8), 150 (100), 131 (21), 120 (23), 104 (48), 92 (25), 76 (40), 65 (6), 55 (59%); IR (KBr, cm−1): 3470, 2966, 2834, 1672, 1603, 1521, 1470, 1347, 1273, 1257, 1201, 1172, 1109, 1069, 1011, 903, 853, 805, 753. Calculated for C21H24ClN3O6 (449.88): C, 56.06; H, 5.38; N, 9.34. Found: C, 56.03; H, 5.49; N, 9.20.
3-(Thiophen-2-yl-carbonyl)-4-(thiophen-2-yl)-1-isopropyl-4-piperidinol 3
M.p. 143–144 °C. Yield: 11%. 1H NMR (400 MHz, CDCl3, ppm) δ = 7.80 (d, 1H, H-3′, J = 3.7 Hz), 7.67 (d, 1H, H-5′, J = 4.8 Hz), 7.12 (dd, 1H, H-4′, J = 4.8, 3.7 Hz), 7.07 (d, 1H, H-5′′, J = 4.6 Hz), 6.91 (br d, 1H, H-3′′, J = 3.3 Hz), 6.82 (dd, 1H, H-4′′, J = 4.6, 3.3 Hz), 5.44 (s, 1H, OH), 4.04 (br d, 1H, H-3, J = 8.8 Hz), 2.98–2.75 [m, 5H, CH(CH3)2, 2XH-2, 2XH-6], 2.05–2.00 [m, 2H, H-5(a), H-5(b)], 1.09 [d, 6H, CH(CH3)2, J = 6.2 Hz]; 13C NMR (100 MHz, CDCl3, ppm) δ = 196.9, 153.4, 143.8, 135.8, 133.5, 128.8, 127.0, 123.9, 122.2, 72.9, 54.9, 54.4, 49.1, 44.4, 41.6, 18.8, 18.6; MS (EI, 70 eV): m/z 335 (M+, 1), 292 (3), 170 (17), 155 (11), 138 (19), 110 (100), 82 (8), 58 (4%); IR (KBr, cm−1): 3425, 3095, 2963, 2827, 1635, 1516, 1468, 1412, 1384, 1361, 1305, 1275, 1261, 1235, 1206, 1166, 1065, 1040, 1004, 954, 852, 793, 725, 699. Calculated For C17H21NO2S2 (335.48): C, 60.86; H, 6.31; N, 4.18; S, 19.12. Found: C, 60.55; H, 6.55; N, 4.18; S, 19.47.
Results
In this study, 4-piperidinol derivative compounds 2a–2g and 3 were synthesized as presented in Scheme 1 for the first time, except compounds 2a and 2d (). Experimental and spectral data of the compounds synthesized are presented at the experimental section. The compounds were obtained with the yield of 11–40%. The chemical structures of synthesized compounds were confirmed with IR, 1H NMR, 13C NMR, MS and elemental analyses data. Purity level of the compound were within 0.4 %, except 2d (calculated C:64.29; found C:63.05).
All the synthesized compounds having the chemical structures of 3-aroyl-4-aryl-1-isopropylamino-4-piperidinols were evaluated against the Huh7 and breast cancer (T47D) cells. The cytotoxicity of the compounds and reference compound 5-fluorouracil (5-FU) are listed in in terms of the inhibitory potencies (IC50, μM).
Table 1. Cytotoxic activities against Huh7 cells and T47D cells of the compounds synthesized.
Discussion
In the literature, compounds 2a and 2d were already synthesizedCitation35,Citation36. Plati et al.Citation34 synthesized some 1,3,4-trisubstituted piperidine derivatives from mono-Mannich bases. In that study, a suspension of 128 g of β-benzoylethylisopropylamine hydrochloride, which is a corresponding mono-Mannich base, was stirred for about an hour with 20 g of sodium hydroxide and 1200 cc of water and allowed to stand until solidification was completed for compound 2a. The solid was crystallized from methanol and acetone in a yield of 85% and the melting point was determined as 123–124 °C. The result of elemental analysis of 2a in literature was found 77.88 for C and 7.65 for HCitation35. The melting point value was in accordance with ours. Although the compound 2d was used in a literature for zinc electroplating process with acidic zinc fluoborate electrolyte, there wasn’t detailed experimental procedure for the synthesis of 2dCitation36. In our experimental procedures, the syntheses of the compounds were carried out using a suitable ketone compound, paraformaldehyde and isopropylamine hydrochloride in 2:2:1 mole ratios, respectively. All reagents were stirred and heated without solvent in an oil bath at ∼130 °C.
It was expected that bis-[β-(p-substituted)benzoyl ethyl]isopropylamine hydrochloride or 1-isopropyl-3-(p-substituted)benzoyl-4-hydroxy-4-(p-substituted)phenyl piperidine hydrochloride can be produced with the experimental procedure used here. But in the experimental condition applied, it was obtained mono-Mannich bases namely 1-(p-substituted)phenyl-3-isopropylamine-1-propanone hydrochlorides and semi-cyclic mono-Mannich bases having piperidine structure namely 1-isoproply-3-(p-substituted)benzoyl-4-hydroxy-4-(p-substituted)phenyl piperidine hydrochlorides before the treatment of alkaline NaOH solution. After removing mono-Mannich base from the reaction medium as described in the literatureCitation13, the residue was treated with NaOH aqueous solution (5%) in a water bath at 40 °C until the solidification observed (24–96 h). It was 72 h for 2a, 24 h for 2b, 96 h for 2c, 24 h for 2d, 24 h for 2e, 48 h for 2f, 24 h for 2g and 48 h for 3. Since there was no solidification in the case of 2d, it was filtered on Al2O3 using EtOH. Our experimental procedure was different than the reported experimental procedure of Plati et al.Citation34. Our experimental procedure provides an advantage to obtain mono-Mannich bases and semi-cyclic mono-Mannich bases which have piperidine structure at the same time. The reason of production of piperidine type semi-cyclic mono-Mannich base instead of bis Mannich base can be attributed to the relatively high temperature applied.
Compounds 2a (3.1 times), 2c (3.8 times), 2f (4.6 times), 2g (1.3 times) and 3 (3.2 times) had higher cytotoxic potency than the reference compound, 5-FU, against Huh7 cells while all the compounds had less cytotoxicity than 5-FU against T47D cells. The cytotoxicities of the compounds 2a–2g and 3 were 0.10–0.75 times of 5-FU. The cytotoxicities of the compounds were 0.50 times for 2a, 0.10 times for 2b, 0.60 times for 2c, 0.15 times for 2d, 0.24 times for 2e, 0.75 times for 2f, 0.25 times for 2g and 0.55 times for 3.
When the effect of the chemical modification on cytotoxic activity was evaluated, it can be said that preparation of piperidinol derivatives had increased the cytotoxicity 1.38–24.7 times comparing with corresponding mono-Mannich baseCitation13. The increase in cytotocity in piperidinols was 1.38 times for 2a, 1.71 times for 2c, 5.85 times for 2d, 8.39 times for 2e, 24.7 times for 2f and 1.5 times for 3 against Huh7 cell line comparing mono-Mannich bases reported before. In our previous study, Huh7 was the only cell line used. In the case of 2b and 2g, cytotoxicity decreased 7.59 times and 1.7 times, respectively comparing the mono-Mannich bases reported earlier.
The increase in cytotoxicity in piperidinols may possibly be with resulted from the production of 1-aryl-2-propen-1-one which was considered the responsible cytotoxic moiety in Mannich bases in high ration in piperidinols. There was no correlation between the cytotoxicities of the compounds and σ, π, values of the substituents and log p values of the compounds (data were not shown).
When the cytotoxicities of the compounds against Huh7 and T47D cell lines were compared, the compounds 2b and 2e had shown more potent cytotoxicity against T47D cell line 2.04 and 1.56 times, respectively. While the other compounds had similar cytotoxicities.
Conclusions
As a result, synthesized compounds having the structure of 3-aroyl-4-aryl-1-isopropylamino-4-piperidinols seem to be potential anticancer candidate which deserve further structural modification and pharmacological evaluations. We reported here 2a–2g and 3 compounds for the first time with their synthesis, spectral analysis and cytotoxicities against Huh7 and T47D cell lines. The compound 2a, 2c, 2f, 2g and 3 seem to be candidate compounds for further synthetic designes and studies.
Declaration of interest
The authors report no conflicts of interest. The authors are responsible for the content and writing of this article.
Acknowledgements
The authors thank to Ataturk University Research Fund (project number 2011/289).
References
- Bange J, Zwick E, Ullrich A. Molecular targets for breast cancer therapy and prevention. Nat Med 2001;7:548–52
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 2007;4:424–32
- Dimmock JR, Kumar P. Anticancer and cytotoxic properties of Mannich bases. Curr Med Chem 1997;4:1–22
- Gul HI, Gul M, Vepsalainen J, et al. Cytotoxicity of some azines of acetophenone derived mono-Mannich bases against Jurkat cells. Biol Pharm Bull 2003;26:631–7
- Gul M, Gul HI, Das U, Hanninen O. Biological evaluation and structure-activity relationships of bis-(3-aryl-3-oxo-propyl)-methylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-methyl-4-piperidinol hydrochlorides as potential cytotoxic agents and their alkylating ability towards cellular glutathione in human leukemic T cells. Arzneimittelforschung 2005;55:332–7
- Gul M, Atalay M, Gul HI, et al. The effects of some Mannich bases on heat shock proteins HSC70 and GRP75, and thioredoxin and glutaredoxin levels in Jurkat cells. Toxicol In Vitro 2005;19:573–80
- Gul HI, Yerdelen KO, Gul M, et al. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm 2007;340:195–201
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 2008;56:1675–81
- Gul HI, Das U, Pandit B, Li PK. Evaluation of the cytotoxicity of some mono-Mannich bases and their corresponding azine derivatives against androgen-independent prostate cancer cells. Arzneimittelforschung 2006;56:850–5
- Gul M, Mete E, Atalay M, et al. Cytotoxicity of 1-aryl-3-buthylamino-1-propanone hydrochlorides against Jurkat and L6 cells. Arzneimmittelforschung 2009;59:364–9
- Mete E, Gul HI, Canturk P, et al. Biological activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols on PC-3 cells and DNA topoisomerase I enzyme. Z Naturforsch C 2010;65:647–52
- Mete E, Gul HI, Cetin-Atalay R, et al. The design and cytotoxic evaluation of some 1-aryl-3-isopropylamino-1-propanone hydrochlorides towards human Huh-7 hepatoma cells. Arch Pharm Chem Life Sci 2011;344:333–9
- Suleyman H, Gul HI, Gul M, et al. Anti-inflammatory activity of bis(3-aryl-3-oxo-propyl)methylamine hydrochloride in rat. Biol Pharm Bull 2007;30:63–7
- Gul HI, Suleyman H, Gul M. Evaluation of the anti-inflammatory activity of N,N′-bis(3-dimethylamino-1-phenyl-propylidene) hydrazine dihydrochloride. Pharm Biol 2009;47:968–72
- Sahin YN, Demircan B, Suleyman H, et al. The effects of 3-benzoyl-1-methyl-4-phenyl-4-piperidinolhydrochloride (C1), indomethacin, nimesulide and rofecoxib on cyclooxygenase activities in carrageenan-induced paw edema model. Turk J Med Sci 2010;40:723–8
- Gul HI, Calis U, Vepsalainen J. Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 2004;54:359–64
- Gul HI, Calls U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl) ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzneimittelforschung 2007;57:133–6
- Gul HI, Sahin F, Gul M, et al. Evaluation of antimicrobial activities of several Mannich bases and their derivatives. Arch Pharm 2005;338:335–8
- Mete E, Ozelgul C, Kazaz C, et al. Synthesis and antifungal activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols. Arch Pharm 2010;343:291–300
- Gul M, Gul HI, Hanninen O. Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 2002;16:107–12
- Gul M, Gul HI, Vepsalainen J, et al. Effect of acetophenone derived Mannich bases on cellular glutathione level in Jurkat cells. A possible mechanism of action. Arzneimittelforschung 2001;51:679–82
- Sun D, Scherman MS, Jones V, et al. Discovery, synthesis, and biological evaluation of piperidinol analogs with anti-tuberculosis activity. Bioorg Med Chem 2009;17:3588–94
- Suleyman H, Gul HI, Asoglu M. Anti-inflammatory activity of 3-benzoyl-1-methyl-4-phenyl-4-piperidinol hydrochloride. Pharmacol Res 2003;47:471–5
- Anderson JT, Campbell M, Wang J, et al. Investigation of 4-piperidinols as novel H3 antagonists. Bioorg Med Chem Lett 2010;20:6246–9
- Annoura H, Nakanishi K, Uesugi M, et al. Synthesis and biological evaluation of new 4-arylpiperidines and 4-aryl-4-piperidinols: dual Na(+) and Ca(2+) channel blockers with reduced affinity for dopamine D(2) receptors. Bioorg Med Chem 2002;10:371–83
- Yang DR, Xue SJ, Wang HF, et al. Synthesis and anti-leukemia activity mensuration of 1-phenethyl-4-hydroxy-4-substituted piperidinium hydrochlorides: structure of bis[1-phenethyl-4-hydroxy-4-(3-fluorophenyl) piperidinium hydrochloride] studied by X-ray and DFT methods. J Mol Struct 2009;929:97–104
- Yadav Y, MacLean ED, Bhattacharyya A, et al. Design, synthesis and bioevaluation of novel candidate selective estrogen receptor modulators. Eur J Med Chem 2011;46:3858–66
- Wang S, Sakamuri S, Enyedy IJ, et al. Molecular modeling, structure–activity relationships and functional antagonism studies of 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketones as a novel class of dopamine transporter inhibitors. Bioorg Med Chem 2001;9:1753–64
- Revesz L, Padova FED, Buhl T, et al. SAR of 4-hydroxypiperidine and hydroxyalkyl substituted heterocycles as novel p38 map kinase inhibitors. Bioorg Med Chem Lett 2000;10:1261–4
- Vangveravong S, Taylor M, Xu J, et al. Synthesis and characterization of selective dopamine D2 receptor antagonists. 2. Azaindole, benzofuran, and benzothiophene analogs of L-741,626. Bioorg Med Chem 2010;18:5291–300
- Grundt P, Husband SLJ, Luedtke RR, et al. Analogues of the dopamine D2 receptor antagonist L741,626: binding, function, and SAR. Bioorg Med Chem Lett 2007;17:745–9
- Kucukoglu K, Gul M, Atalay M, et al. Synthesis of some Mannich bases with dimethylamine and their hydrazones and evaluation of their cytotoxicity against Jurkat cells. Arzneim Forsch Drug Res 2011;61:366–71
- Plati JT, Schmidt RA, Wenner W. 1,3,4-Trisubstituted piperidine derivatives from Mannich bases. J Org Chem 1949;14:873–8
- Page W, Schevey WR, Vander Mey JE. Acidic zinc fluoborate electrolyte for a zinc electroplating process. US Patent No. 3655533, USA; 1972
- Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 2006;6:813–23