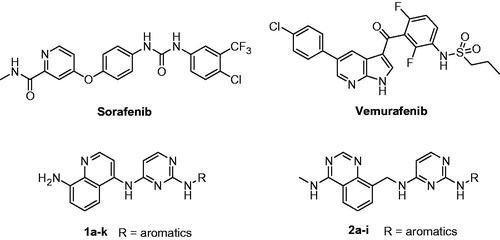
Abstract
Synthesis of a new series of quinolinylaminopyrimidines 1a–k and quinazolinylmethylaminopyrimidines 2a–i containing aminoquinoline and aminoquinazoline as hinge regions is described. Their in vitro antiproliferative activities against A375P human melanoma cell line were tested. Among them, compounds 1h and 1k exhibited the highest antiproliferative activities against A375P cell line with IC50 values in sub-micromolar scale. Compounds 1i, 2b and 2g showed similar potency against A375P to Sorafenib as a reference compound. The representative compound 1h showed high, dose-dependent inhibition of MEK and ERK kinases.
Introduction
Melanoma is a malignant tumor that arises from melanocytic cells and primarily involves the skin. Exposure to solar ultraviolet irradiation, fair skin, dysplastic nevi syndrome, and a family history of melanoma are major risk factors for melanoma development. Melanomas can metastasize either by the lymphatic or by the hematogenous routeCitation1. Metastatic melanoma is a particularly aggressive form of cancer that has a very poor prognosis, and is resistant to standard anticancer therapies. Early stage melanoma (stage I/II) can be cured surgically with more than 95% success rate. But melanoma metastasizing to major organs (stage IV) is virtually incurableCitation2. Patients with advanced melanoma have a median survival time of less than one year, and the estimated five-year survival rate is less than 15%Citation3,Citation4. With the rapid incidence of melanoma in the United States and other developed countries, there is an urgent need to develop more effective drugsCitation5–7. The RAS-RAF-MEK-ERK signaling pathway (ERK pathway) plays an important role in tumorigenesis and cancer progressionCitation8. Sorafenib (Nexavar®, ) targets ERK pathway. It inhibits basal phosphorylation of ERK (p-ERK) in numerous cancer cell lines in vitro, including melanoma cell lines, independent of their K-RAS and b-RAF mutational statusCitation9. Vemurafenib (PLX4032, Zelboraf®, ) is another drug which targets ERK pathway through inhibition of V600E-B-RAF kinase. In 2011, it was approved by the U.S. Food and Drug Administration (FDA) for treatment of late-stage melanomaCitation10. So, inhibition of ERK signaling pathway is a very potential avenue for treatment of melanoma.
Numerous compounds with new scaffolds consisting of hinge, spacer, and tail regions have recently reported as potential antiproliferative agents against melanoma cell linesCitation11–22. In this work, a new series of quinolinylaminopyrimidines 1a–k and quinazolinylmethylaminopyrimidines 2a–i containing aminoquinoline and aminoquinazoline as hinge regions was designed and synthesized (). Their in vitro antiproliferative activities were tested over A375P human melanoma cell line. And MEK and ERK kinases inhibitory activity for the representative compound 1h is reported.
Experimental
Chemistry
1H NMR spectra were recorded on a Bruker Avance 400 spectrometer using DMSO-d6 containing tetramethylsilane as an internal standard. LC-MS spectra were determined on a Waters Quattro Micro System. The liquid chromatography high-resolution mass spectra (LC-HRMS, electron spray ionization) experiments were performed with Synapt G2 (Waters MS Technology, Manchester, Q-TOF MS, UK) mass analyzer. Data were acquired in the positive ion mode. Calibration was performed with an external calibration mixture immediately prior to analysis. Column chromatography was carried out using silica gel (230–400 mesh). Solvents and liquid reagents were transferred using hypodermic syringes. Purity % of all the target compounds were determined by LC-MS and found to be > 95%.
4-Chloro-8-nitroquinoline (3)
To a solution of 4-chloroquinoline (2) (5.0 g, 0.031 mmol) in sulfuric acid (23 mL, 0.42 mol), nitric acid (4.5 mL, 0.11 mol) was added dropwise at 0 °C. The mixture was stirred at room temperature for 4 h. Upon completion, the reaction mixture was cooled to 0 °C and neutralized with 1 M NH4OH. The resulting precipitate was collected by filtration, washed with water, and dried to give the title compound 3 (3.6 g, 56%) as a white solid. 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.93 (d, J = 4.59 Hz, 1H), 8.47 (d, J = 8.44 Hz, 1H), 8.08 (d, J = 7.33 Hz, 1H), 7.74 (t, J = 8.02 Hz, 1H), 7.59 (d, J = 6.76 Hz, 1H).
General procedure for the synthesis of pyrimidin-2,4-diamines 5
To a mixture of 2-chloropyrimidin-4-amine (4) (2.0 mmol), (±)-2,2-bis(diphenylphosphino)-1,1'-binaphinyl [(±)-BINAP] (0.20 mmol), palladium (II) acetate (0.20 mmol), and cesium carbonate (0.30 mmol) in toluene, the appropriate aminobenzene (2.1 mmol) was added. The mixture was refluxed under N2 atmosphere for 6 h. Upon completion, the reaction mixture was filtered through a pad of celite. The filtrate was evaporated under reduced pressure, and the resulting residue was purified by column chromatography to afford the corresponding compound 5 (21–60%).
General procedure for preparation of nitroquinolinylaminopyrimidines 6a–k
To a solution of 4-chloro-8-nitroquinoline (3) (0.50 mmol) in DMF, the appropriate pyrimidin-2,4-diamines 5 (0.55 mmol) and NaOtBu (0.75 mmol) were added. After stirring at room temperature for 4 h, the reaction mixture was diluted with saturated aqueous NaHCO3, and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was purified by column chromatography to afford the corresponding compounds 6a–k.
3,4-Dimethylphenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6a)
White solid, yield 15%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.82 (s, 1H), 8.65 (d, J = 6.19 Hz, 1H), 8.25 (d, J = 7.88 Hz, 1H), 8.20 (s, 1H), 7.85 (s, 1H), 7.52 (s, 1H), 7.28–7.22 (m, 5H), 6.22 (s, 1H), 2.24 (s, 3H).
2-Methyl-5-(4-(8-nitroquinolin-4-ylamino)pyrimidin-2-ylamino)benzonitrile (6b)
Brown solid, yield 15%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 9.51 (s, 1H), 8.85 (d, J = 4.32 Hz, 1H), 8.35–8.32 (m, 2H), 8.15–8.11 (m, 2H), 7.82 (s, 1H), 7.64 (d, J = 9.68 Hz, 1H), 7.22 (d, J = 8.64 Hz, 1H), 6.5 (s, 1H), 2.33 (s, 3H).
8-Nitroquinolin-4-yl-N2-(4-trifluoromethylphenyl)pyrimidine-2,4-diamine (6c)
White solid, yield 20%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 9.90 (s, 1H), 9.68 (s, 1H), 8.90 (d, J = 4.82 Hz, 1H), 8.38 (s, 2H), 8.23 (d, J = 5.69 Hz, 1H), 7.91 (d, J = 4.77 Hz, 1H), 7.83 (d, J = 8.45 Hz, 2H), 7.46 (d, J = 8.44 Hz, 1H), 6.55 (d, J = 5.69 Hz, 1H).
8-Nitroquinolin-4-yl-N2-(3-trifluoromethylphenyl)pyrimidine-2,4-diamine (6d)
White solid, yield 15%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.85 (d, J = 4.66 Hz, 1H), 8.54 (d, J = 9.44 Hz, 1H), 8.32 (d, J = 9.44 Hz, 1H), 8.26 (d, J = 5.66 Hz, 1H), 7.96 (s, 1H), 7.85 (s, 1H), 7.63 (d, J = 8.63 Hz, 1H), 7.55 (d, J = 4.71 Hz, 1H), 7.41 (t, J = 7.79 Hz, 1H), 7.29 (d, J = 7.47 Hz, 1H), 6.33 (d, J = 5.64 Hz, 1H).
2-Chloro-4-trifluoromethylphenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6e)
Brown solid, yield 135%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.86 (d, J = 4.82 Hz, 1H), 8.62 (d, J = 8.74 Hz, 1H), 8.45 (d, J = 9.40 Hz, 1H), 8.35 (d, J = 9.40 Hz, 1H), 8.30 (d, J = 5.60 Hz, 1H), 7.85 (s, 1H), 7.66 (s, 2H), 7.57 (d, J = 4.67 Hz, 1H), 7.44 (d, J = 8.74 Hz, 1H), 6.42 (d, J = 5.60 Hz, 1H).
4-Chloro-3-trifluoromethylphenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6f)
White solid, yield 12%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.85 (d, J = 4.68 Hz, 1H), 8.46 (d, J = 9.44 Hz, 1H), 8.32 (d, J = 9.44 Hz, 1H), 8.26 (d, J = 5.64 Hz, 1H), 8.0 (d, J = 2.47 Hz, 1H), 7.82 (s, 1H), 7.63 (d, J = 2.59 Hz, 1H), 7.56 (d, J = 4.72 Hz, 1H), 7.40 (d, J = 8.64 Hz, 1H), 7.16 (s, 1H), 6.34 (d, J = 5.67 Hz, 1H).
4-Fluoro-3-trifluoromethylphenyl)-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6g)
Brown solid, yield 13%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.85 (d, J = 4.75 Hz, 1H), 8.47 (d, J = 9.45 Hz, 1H), 8.30 (d, J = 9.42 Hz, 1H), 8.24 (d, J = 5.66 Hz, 1H), 7.90–7.88 (m, 1H), 7.82 (s, 1H), 7.62–7.59 (m, 1H), 7.55 (d, J = 4.74 Hz, 1H), 7.17–7.12 (m, 2H), 6.32 (d, J = 5.64 Hz, 1H).
3-Chloro-5-trifluoromethylphenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6h)
Brown solid, yield 18%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.85 (d, J = 4.77 Hz, 1H), 8.50 (d, J = 9.42 Hz, 1H), 8.40 (d, J = 9.41 Hz, 1H), 8.27 (d, J = 5.62 Hz, 1H), 7.94 (s, 1H), 7.86 (s, 1H), 7.61 (s, 1H), 7.56 (d, J = 4.76 Hz, 1H), 7.29 (s, 1H), 6.37 (d, J = 5.66 Hz, 1H).
2,4-Bis(trifluoromethyl)phenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6i)
White solid, yield 17%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.85 (d, J = 4.76 Hz, 1H), 8.56 (d, J = 8.79 Hz, 1H), 8.40 (d, J = 9.42 Hz, 1H), 8.30 (s, 1H), 8.29 (d, J = 2.63 Hz, 1H), 8.03–7.99 (m, 2H), 7.87 (s, 1H), 7.70 (d, J = 9.17 Hz, 1H), 7.57 (d, J = 4.76 Hz, 1H), 7.46 (s, 1H), 6.46 (d, J = 5.68 Hz, 1H).
3,5-Bis(trifluoromethyl)phenyl-N4-(8-nitroquinolin-4-yl)pyrimidine-2,4-diamine (6j)
White solid, yield 19%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.86 (d, J = 4.76 Hz, 1H), 8.45 (d, J = 9.43 Hz, 1H), 8.34 (d, J = 9.42 Hz, 1H), 8.30 (d, J = 5.64 Hz, 1H), 8.06 (s, 2H), 7.84 (s, 1H), 7.57 (d, J = 4.76 Hz, 1H), 7.49 (s, 1H), 7.29 (s, 1H), 6.38 (d, J = 5.64 Hz, 1H).
8-Nitroquinolin-4-yl-N2-(3-phenoxyphenyl)pyrimidine-2,4-diamine (6k)
Orange solid, yield 17%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.82 (d, J = 4.75 Hz, 1H), 8.62 (d, J = 9.44 Hz, 1H), 8.32 (d, J = 9.44 Hz, 1H), 8.20 (d, J = 5.60 Hz, 1H), 7.83 (s, 1H), 7.51 (d, J = 4.76 Hz, 1H), 7.48 (t, J = 4.24 Hz, 1H), 7.31–7.26 (m, 2H), 7.22 (d, J = 8.06 Hz, 1H), 7.16 (d, J = 8.31 Hz, 1H), 7.06 (t, J = 7.35 Hz, 1H), 7.01 (d, J = 7.90 Hz, 1H), 6.65 (d, J = 7.80 Hz, 1H), 6.26 (d, J = 5.60 Hz, 1H).
General procedure for preparation of aminoquinolinylaminopyrimidines 1a-k
A mixture of the appropriate nitroquinolinylaminopyrimidines 6a–k and 5% Pd/C in MeOH was stirred in hydrogen atmosphere at room temperature for 1 h. Upon completion, the reaction mixture was filtered through celite. The filtrate was evaporated under reduced pressure, and the resulting residue was purified by column chromatography to afford the corresponding compounds 1a–k.
(2-(3,4-Dimethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1a)
White solid, yield 80%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.82 (s, 1H), 8.66 (s, 1H), 8.26–8.22 (m, 2H), 7.85 (s, 1H), 7.52 (s, 1H), 7.29–7.22 (m, 5H), 6.22 (s, 1H), 2.22 (s, 6H); MS m/z: 357 [M+1]+.
5-(4-(8-Aminoquinolin-4-ylamino)pyrimidin-2-ylamino)-2-methylbenzonitrile (1b)
Brown solid, yield 56%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.59 (d, J = 4.64 Hz, 1H), 8.04 (s, 1H), 8.02 (s, 1H), 7.90 (d, J = 5.88 Hz, 1H), 7.52 (d, J = 8.93 Hz, 1H), 7.45–7.38 (m, 4H), 7.08 (d, J = 8.48 Hz, 1H), 6.76 (s, 1H), 5.88 (d, J = 5.84 Hz, 1H), 4.26 (s, 1H), 2.33 (s, 3H); MS m/z: 368 [M+1]+.
(2-(4-Trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1c)
White solid, yield 75%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.66 (d, J = 4.65 Hz, 1H), 8.03 (d, J = 8.03 Hz, 1H), 7.68 (d, J = 8.45 Hz, 3H), 7.57 (d, J = 8.87 Hz, 1H), 7.51–7.43 (m, 6H), 6.73 (s, 1H), 6.0 (d, J = 3.24 Hz, 1H), 5.23 (s, 2H); MS m/z: 397 [M+1]+.
(2-(3-Trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1d)
White solid, yield 72%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.66 (d, J = 4.42 Hz, 1H), 8.03 (d, J = 4.42 Hz, 1H), 7.94 (s, 1H), 7.70 (d, J = 6.71 Hz, 1H), 7.57 (d, J = 8.82 Hz, 1H), 7.49 (t, J = 8.84 Hz, 2H), 7.43–7.38 (m, 3H), 7.21 (d, J = 6.72 Hz, 1H), 6.74 (s, 1H), 5.97 (d, J = 5.38 Hz, 1H), 5.22 (s, 2H); MS m/z: 397 [M+1]+.
(2-(2-Chloro-4-trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1e)
Pale brown solid, yield 59%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.81 (d, J = 2.76 Hz, 1H), 8.76–8.73 (m, 1H), 8.15–8.10 (m, 2H), 7.64–7.61 (m, 2H), 7.52 (d, J = 4.65 Hz, 1H), 7.44 (q, J = 4.19 Hz, 1H), 7.38 (d, J = 8.58 Hz, 1H), 7.20 (d, J = 8.64 Hz, 1H), 6.54 (s, 1H), 6.05 (d, J = 2.60 Hz, 1H), 5.22 (s, 2H); MS m/z: 431 [M+1]+; HRMS (ESI, positive) calcd for C20H15ClF3N6 [(M+H)+] 431.0921, found 431.0989.
(2-(4-Chloro-3-trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1f)
White solid, yield 60%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.66 (d, J = 4.67 Hz, 1H), 8.05 (d, J = 5.79 Hz, 1H), 8.01 (d, J = 2.58 Hz, 1H), 7.72 (d, J = 2.51 Hz, 1H), 7.57 (d, J = 8.88 Hz, 1H), 7.53–7.48 (m, 2H), 7.34 (d, J = 8.86 Hz, 1H), 7.08 (s, 1H), 6.42 (s, 1H), 5.99 (d, J = 5.73 Hz, 1H), 5.19 (s, 2H); MS m/z: 431 [M+1]+; HRMS (ESI, positive) calcd for C20H15ClF3N6 [(M+H)+] 431.0921, found 431.0997.
(2-(4-Fluoro-3-trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1g)
Pale brown solid, yield 70%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.66 (d, J = 4.76 Hz, 1H), 8.02 (d, J = 7.88 Hz, 1H), 7.90 (d, J = 8.08 Hz, 1H), 7.75–7.72 (m, 1H), 7.58 (d, J = 11.91 Hz, 1H), 7.51 (d, J = 6.48 Hz, 2H), 7.14 (s, 1H), 7.09 (t, J = 12.2 Hz, 1H), 6.58 (s, 1H), 5.98 (d, J = 7.68 Hz, 1H), 5.21 (s, 2H); MS m/z: 415 [M+1]+; HRMS (ESI, positive) calcd for C20H15F4N6 [(M+H)+] 415.1216, found 415.1226.
(2-(3-Chloro-5-trifluoromethylphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1h)
Pale brown solid, yield 50%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 9.64 (s, 1H), 9.01 (s, 1H), 8.68 (d, J = 4.65 Hz, 1H), 8.14 (s, 1H), 8.07 (d, J = 5.77 Hz, 1H), 7.85 (s, 1H), 7.71 (s, 1H), 7.69 (t, J = 2.47 Hz, 1H), 7.41 (d, J = 8.94 Hz, 1H), 7.13 (s, 1H), 6.30 (d, J = 5.81 Hz, 1H), 5.83 (s, 2H); MS m/z: 421 [M+1]+.
(2-(2,4-Bis(trifluoromethyl)phenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1i)
Pale brown solid, yield 48%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.81 (d, J = 1.64 Hz, 1H), 8.80 (d, J = 8.78 Hz, 1H), 8.12 (d, J = 8.28 Hz, 1H), 8.06 (d, J = 5.84 Hz, 1H), 7.82 (s, 1H), 7.61 (d, J = 7.09 Hz, 1H), 7.47–7,42 (m, 2H), 7.36 (d, J = 8.62 Hz, 1H), 7.20 (d, J = 8.57 Hz, 1H), 6.56 (s, 1H), 6.03 (d, J = 5.72 Hz, 1H), 5.26 (s, 2H); MS m/z: 465 [M+1]+; HRMS (ESI, positive) calcd for C21H15F6N6 [(M+H)+] 465.1184, found 415.0611.
(2-(3,5-Bis(trifluoromethyl)phenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1j)
White solid, yield 70%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.76 (d, J = 1.64 Hz, 1H), 8.06 (d, J = 1.61 Hz, 1H), 7.37 (t, J = 4.27 Hz, 1H), 7.32 (d, J = 7.63 Hz, 1H), 7.15 (d, J = 8.10 Hz, 1H), 6.93 (d, J = 1.02 Hz, 1H), 5.0 (s, 2H); MS m/z: 465 [M+1]+; HRMS (ESI, positive) calcd for C21H15F6N6 [(M+H)+] 465.1184, found 415.0611.
(2-(3-Phenoxyphenylamino)pyrimidin-4-yl)quinoline-4,8-diamine (1k)
Brown solid, yield 60%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.62 (d, J = 4.58 Hz, 1H), 7.99 (d, J = 5.66 Hz, 1H), 7.55 (d, J = 9.11 Hz, 2H), 7.46 (t, J = 4.66 Hz, 2H), 7.34 (t, J = 7.56 Hz, 4H), 7.21–7.16 (m, 2H), 7.10 (t, J = 7.21 Hz, 2H), 7.05 (d, J = 8.06 Hz, 2H), 6.61 (d, J = 7.38 Hz, 2H), 5.88 (d, J = 5.60 Hz, 1H), 5.20 (s, 2H); MS m/z: 421 [M+1]+.
8-Methyl-4-quinazolinone (8)
A mixture of 2-amino-3-methylbenzoic acid (7) (25.0 g, 0.165 mol), formamidine acetate (50.0 g, 0.496 mol), and formamide (6.6 mL, 0.165 mol) was heated at 160 °C for 2 h. Upon completion, the reaction mixture was cooled to room temperature and treated with 10% NaOH. After stirring at 60 °C for 20 min, the reaction mixture was neutralized with 1 N HCl at ice bath temperature. The resulting precipitate was collected by filtration, washed with water, and dried to give the title compound 8 (25.4 g, 96%) as a white solid. 1H NMR (400 MHz, DMSO-d6, δ, ppm): 12.16 (s, 1H), 8.50 (s, 1H), 7.94 (d, J = 7.94 Hz, 1H), 7.66 (d, J = 7.38 Hz, 1H), 7.38 (t, J = 7.63 Hz, 1H), 2.51 (s, 3H).
8-Bromomethyl-4-quinazolinone (9)
To a solution of 8-methyl-4-quinazolinone (8) (5.0 g, 31.2 mmol) in CCl4 (150 mL), N-bromosuccinimide (6 g, 34.3 mmol) and AIBN (1.0 g, 6.3 mmol) were added. The mixture was stirred at room temperature for 24 h. Upon completion, the reaction mixture was filtered through a pad of celite. The filtrate was evaporated under reduced pressure, and the resulting residue was triturated with dichloromethane to afford the title compound 9 (5.5 g, 74%) as a pale yellow solid. 1H NMR (400 MHz, DMSO-d6, δ, ppm): 12.42 (s, 1H), 8.23 (s, 1H), 8.10 (d, J = 7.86 Hz, 1H), 7.96 (d, J = 6.45 Hz, 1H), 7.50 (t, J = 7.65 Hz, 1H), 5.06 (s, 2H).
8-Bromomethyl-4-chloroquinazoline (10)
To a solution of 8-bromomethyl-4-quinazolinone (9) (3.0 g, 12.5 mmol) in toluene (100 mL), POCl3 (2.92 mL, 31.3 mmol) and N,N-dimethylaniline (2.38 mL, 18.8 mmol) were added. The mixture was refluxed for 4 h and then cooled to room temperature. The excess of POCl3 was removed by distillation under reduced pressure. H2O was carefully added to the residue, and the suspension was extracted with CH2Cl2. The organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography to afford the title compound 10 (2.7 g, 84%) as a white solid. 1H NMR (400 MHz, DMSO-d6, δ, ppm): 9.12(s, 1H), 8.29(d, J = 8.4 Hz, 1H), 8.13(d, J = 7.1 Hz, 1H), 7.76(t, J = 8.0 Hz, 1H), 5.26(s, 2H).
8-Bromomethyl-N-methylquinazolin-4-amine (11)
To a solution of 8-bromomethyl-4-chloroquinazoline (10) (2.0 g, 7.8 mmol) in THF (50 mL), methylamine (40% solution in water, 1.8 mL, 23.4 mmol) was added. The mixture was stirred at room temperature for 12 h. The solvent was removed by distillation under reduced pressure, the residue was extracted with CH2Cl2. The organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography to afford the title compound 11 (1.3 g, 68%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.56 (s, 1H), 8.40 (d, J = 4.18 Hz, 1H), 8.18 (d, J = 1.12 Hz, 1H), 7.90 (d, J = 6.28 Hz, 1H), 7.50 (t, J = 7.42 Hz, 1H), 5.18 (s, 2H), 3.01 (d, J = 4.54 Hz, 3H).
General procedure for preparation of quinazolinylmethylaminopyrimidines 2a–i
To a solution of 8-bromomethyl-N-methylquinazolin-4-amine (11) (0.50 mmol) in DMF, the appropriate pyrimidin-2,4-diamines 5 (0.55 mmol) and NaOtBu (0.75 mmol) were added. After stirring at room temperature for 4 h, the reaction mixture was diluted with saturated aqueous NaHCO3, and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was purified by column chromatography to afford the corresponding compounds 2a–i.
2-Methyl-5-(4-(4-methylaminoquinazolin-8-yl)methylaminopyrimidin-2-ylamino)benzonitrile (2a)
Brown solid, yield 15%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.64 (s, 1H), 8.01(s, 1H), 7.93 (d, J = 5.68 Hz, 1H), 7.63 (d, J = 2.27 Hz, 1H), 7.59 (d, J = 7.24 Hz, 1H), 7.54 (d, J = 8.17 Hz, 1H), 7.49 (d, J = 8.37 Hz, 1H), 7.30 (t, J = 7.52 Hz, 1H), 7.18 (d, J = 8.41 Hz, 1H), 6.25 (s, 1H), 5.89 (d, J = 5.66 Hz, 1H), 5.73 (s, 1H), 4.78 (s, 2H), 3.15 (s, 3H); MS m/z: 397 [M+1]+; HRMS (ESI, positive) calcd for C22H21N8 [(M+H)+] 397.1811, found 397.1877.
2-Chloro-5-trifluoromethylphenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2b)
Brown solid, yield 14%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.68(s, 1H), 8.12 (s, 1H), 7.87 (s, 1H), 7.59 (s, 1H), 7.49 (d, J = 6.0 Hz, 1H), 7.34 (m, 2H), 7.18 (d, J = 8.40 Hz, 1H), 6.01 (s, 1H), 5.61 (s, 2H), 5.30 (d, J = 8.0 Hz, 1H), 3.16 (s, 3H); MS m/z: 460 [M+1]+; HRMS (ESI, positive) calcd for C21H18ClF3N7 [(M+H)+] 460.1186, found 460.1264.
3-Chloro-4-trifluoromethylphenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2c)
White solid, yield 14%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.69 (s, 1H), 7.96 (d, J = 5.6 Hz, 1H), 7.78 (d, J = 2.5 Hz, 1H), 7.62 (d, J = 6.9 Hz, 1H), 7.53 (d, J = 8.1 Hz, 1H), 7.46 (dd, J = 2.5 Hz, 1H), 7.36 (d, J = 2.5 Hz, 1H), 7.34 (d, J = 3.5 Hz, 1H), 5.92 (d, J = 5.7 Hz, 1H), 5.79 (s, 2H), 4.63 (s, 2H), 3.20 (d, J = 5.5 Hz, 3H); MS m/z: 460 [M+1]+.
4-Fluoro-3-trifluoromethylphenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2d)
Brown solid, yield 13%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.63 (s, 1H), 7.92 (d, J = 5.68 Hz, 1H), 7.63 (m, 2H), 7.50 (m, 1H), 7.46 (d, J = 8.21 Hz, 1H), 7.28 (t, J = 7.42 Hz, 1H), 7.06 (d, J = 9.44 Hz, 1H), 6.15 (s, 1H), 5.88 (d, J = 5.71 Hz, 1H), 5.72 (s, 1H), 4.75 (s, 2H), 3.15 (s, 3H); MS m/z: 444 [M+1]+; HRMS (ESI, positive) Calcd for C21H18F4N7 [(M+H)+] 444.1482, found 444.1551.
4-(4-(4-Methylaminoquinazolin-8-yl)methylaminopyrimidin-2-ylamino)-3-trifluoromethylbenzonitrile (2e)
Brown solid, yield 14%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.71 (d, J = 5.84 Hz, 1H), 8.19 (d, J = 9.68 Hz, 1H), 8.10 (d, J = 8.68 Hz, 1H), 7.89 (t, J = 9.16 Hz, 1H), 7.84 (s, 1H), 7.62 (m, 1H), 7.51 (d, J = 2.64 Hz, 1H), 7.46 (s, 1H), 7.42 (d, J = 3.04 Hz, 1H), 7.38 (d, J = 10.6 Hz, 1H), 5.62 (s, 2H), 7.51 (d, J = 9.72 Hz, 1H), 3.21 (s, 1H); MS m/z: 451 [M+1]+; HRMS (ESI, positive) calcd for C22H18F3N8 [(M+H)+] 451.1528, found 451.1608.
(4-Methylaminoquinazolin-8-yl)methyl-N2-(3-nitro-4-trifluoromethylphenyl)pyrimidine-2,4-diamine (2f)
Brown solid, yield 13%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.73 (s, 1H), 8.07 (s, 1H), 8.03 (d, J = 5.2 Hz, 1H), 7.83 (d, J = 8.9 Hz, 1H), 7.56 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.3 Hz, 1H), 7.32 (t, J = 7.8 Hz, 1H), 6.05 (d, J = 5.6 Hz, 1H), 5.94 (s, 1H), 5.84 (s, 1H), 4.77 (s, 2H), 3.22 (d, J = 4.68 Hz, 1H); MS m/z: 471 [M+1]+; HRMS (ESI, positive) calcd for C21H18F3N8O2 [(M+H)+] 471.1427, found 471.1503.
3-Chloro-5-trifluoromethylphenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2g)
Brown solid, yield 12%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.62 (s, 1H), 7.91 (d, J = 5.7 Hz, 1H), 7.58 (d, J = 7.70 Hz, 2H), 7.54 (d, J = 7.12 Hz, 1H), 7.43 (d, J = 8.02 Hz, 1H), 7.27 (s, 1H), 7.16 (t J = 8.85, 1H), 6.51 (d, J = 4.6 Hz, 1H), 5.88 (d, J = 5.71 Hz, 1H), 5.75 (s, 2H), 4.92 (s, 2H), 3.05 (s, 3H); MS m/z: 460 [M+1]+; HRMS (ESI, positive) Calcd for C21H18ClF3N7 [(M+H)+] 460.1186, found 460.1255.
2,4-Bis(trifluoromethyl)phenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2h)
Brown solid, yield 16%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 8.73 (s, 1H), 8.07 (s, 1H), 8.03 (d, J = 5.2 Hz, 1H), 7.83 (d, J = 8.9 Hz, 1H), 7.56 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.3 Hz, 1H), 7.32 (t, J = 7.8 Hz, 1H), 6.05 (d, J = 5.6 Hz, 1H), 5.94 (s, 1H), 5.84 (s, 1H), 4.77 (s, 2H), 3.22 (d, J = 4.68 Hz, 1H); MS m/z: 494 [M+1]+.
2,4-Bis(trifluoromethyl)phenyl-N4-(4-methylaminoquinazolin-8-yl)methylpyrimidine-2,4-diamine (2i)
White solid, yield 15%; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 7.98 (d, J = 5.66 Hz, 1H), 7.87 (s, 2H), 7.63 (d, J = 7.2 Hz, 1H), 7.54 (m, 2H), 7.36 (t, J = 7.6 Hz, 1H), 5.96 (t, J = 5.6 Hz, 1H), 5.86 (s, 1H), 5.75 (s, 2H), 3.2 (d, J = Hz, 3H); MS m/z: 494 [M+1]+.
Evaluation of the antiproliferative activity against A375P human melanoma cell line
A375P cells were purchased from American Type Culture Collection (ATCC, Rockville, MD) and maintained in Dulbecco's modified eagle medium (DMEM, Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS, Welgene, Daegu, Korea) and 1% penicillin/streptomycin (Welgene, Daegu, Korea) in a humidified atmosphere with 5% CO2 at 37 °C. A375P cells were taken from culture substrate with 0.05% trypsin–0.02% EDTA and plated at a density of 5 × 103 cells/well in 96 well plates and then incubated at 37 °C for 24 h in a humidified atmosphere with 5% CO2 prior to treatment with various concentrations (3-fold serial dilution, 12 points) of test compounds. The cells were incubated for 48 h after treatment with the test compounds. The A357P cell viability was assessed by the conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. MTT assays were carried out with CellTiter 96® (Promega) according to the manufacturer's instructions. The absorbance at 590 nm was recorded using EnVision 2103 (Perkin Elmer; Boston, MA). The IC50 was calculated using GraphPad Prism 4.0 software.
Kinase Screening
Immunoblot analysis
For immunoblotting, A375P melanoma cells grown to 70–80% confluence were harvested in RIPA lysis buffer and disrupted by sonication and centrifuged at 12 000 rpm for 15 min. The quantity of protein was determined with DC protein assay kit (Bio-Rad Lab., Hercules, CA). Protein samples were subjected to SDS-PAGE and immunoblotted with the appropriate primary antibody overnight at 4 °C. The protein bands were visualized using chemiluminescence detection kit (Amersham HRP Chemiluminescent Substrates, Amersham Biosciences, Piscataway, NJ) after hybridization with the HRP-conjugated secondary antibody from rabbits or mice. The LAS4000-mini (Fujifilm, Tokyo, Japan) was used for chemiluminescence detection.
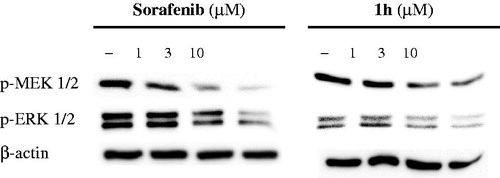
Compound treatment in A375P cells
To assess the effect of the target compounds on the RAF-1/MEK/ERK signaling pathway, A375P cells were treated with the tested compounds and Sorafenib in a dose dependent way (1, 3, and 10 μM) for 24 h and immunoblotted with antibodies against phospho-MEK1/2, ERK1/2 and β-actin, respectively.
Results and discussion
Chemistry
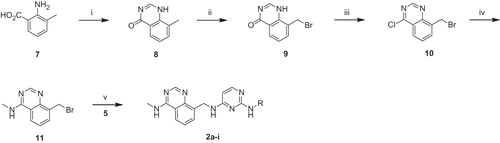
Quinolinylaminopyrimidines 1a–k were synthesized by the pathways illustrated in Scheme 1. Nitration of 4-chloroquinoline (2) using a mixture of nitric acid and sulfuric acid yielded 4-chloro-8-nitroquinoline (3). Nucleophilic substitution of the chloro group of compound 3 with the appropriate pyrimidinyl amines 5 was carried out using sodium t-butoxide to produce compounds 6a–k. Pyrimidinyl amines 5 were obtained by treatment of 2-chloropyrimidin-4-amine (4) with various aromatic amines in the presence of (±)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl [(±)-BINAP], palladium (II) acetate, and cesium carbonate according to the procedure of Buchwald-Hartwig aminationCitation23. Reduction of the nitro group of 6a–k using Pd/C in hydrogen atmosphere gave the title compounds 1a–k.
Scheme 1. Reagents and reaction conditions: (i) HNO3, H2SO4, rt, 4 h; (ii) NaOtBu, DMF, rt, 4 h; (iii) 5% Pd/C, H2, MeOH, rt, 1 h; (iv) Pd(OAc)2, BINAP, CsCO3, toluene, reflux, 6 h.
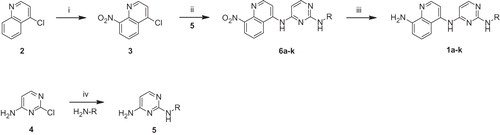
Synthesis of quinazolinylmethylaminopyrimidines 2a–i was carried out by the sequence of reactions shown in Scheme 2. Ring closure of 2-amino-3-methylbenzoic acid (7) with formamidine acetate in formamideCitation24 followed by bromination with N-bromosuccinimide in the presence of AIBN gave the bromomethylquinazolinone 9 in good yield. Compound 11 as a key intermediate was obtained by treatment of 9 with phosphoryl chloride in the presence of N,N-dimethylaniline, and subsequent amination of the resulting chloroquinazoline 10 using methylamine. Coupling of 11 with the appropriate pyrimidinyl amines 5 by the similar procedure as described for the preparation of 6a–k led to the title compounds 2a–i.
Biological activity
The antiproliferative activity of the newly synthesized compounds against A375P human melanoma cell line was tested. The ability of quinolinylaminopyrimidines 1a–k and quinazolinylmethylaminopyrimidines 2a–i to inhibit the growth of A375P cell line is summarized in and . Sorafenib was selected as a reference standard because it has been extensively used in clinical trials for treatment of melanomaCitation5,Citation25. Vemurafenib was also utilized as a second reference standard in this experiment because of its high potency against melanoma cell linesCitation26, and it has been recently approved by the FDA for treatment of advanced melanomaCitation10.
Table 1. Antiproliferative activity of quinolinylaminopyrimidines 1a–k against A375P human melanoma cell line.

Table 2. Antiproliferative activity of quinazolinylmethylaminopyrimidines 2a–i against A375P human melanoma cell line.

In general, compounds 1a–k with quinolinylamino moiety were more potent than compounds 2a-i having quinazolinylmethylamino moiety. This may be attributed to that the spacer length may geometrically permit appropriate fitting of the molecule at the receptor site. As shown in , compounds 1h and 1k with sub-micromolar IC50 values displayed superior antiproliferative activity against A375P human melanoma cell line to Sorafenib, even though their potencies were less than that of Vemurafenib. In addition, compound 1i showed higher potency than Sorafenib. Among all the target compounds, compound 1h possessing 3-chloro-5-trifluoromethylphenyl as a terminal group exhibited the highest potency against A375P cell line with IC50 value of 0.57 μM. Compound 1h with 3,5-disubstituted phenyl group was more potent than compounds 1e and 1f having the different position of substituents. This may be rationalized that the orientation of substituents at receptor site may affect the affinity and potency. As a whole, the structure–activity relationships did not show a well-defined trend. In quinazolinylmethylaminopyrimidine series, compounds 2b and 2g showed similar antiproliferative activities to Sorafenib (). In order to study the mechanism of action, compound 1h with high potency against A375P human melanoma cell line was selected as a representative example of this series to be screened for its inhibitory effects on MEK and ERK kinases (). A375P cell lysate was treated with three different concentrations of the test compound (1, 3, and 10 μM), and its inhibitory activity was compared with that of Sorafenib. The results showed that compound 1h and Sorafenib markedly suppressed phosphorylation of MEK1/2 and ERK1/2 in a dose-dependent manner. Compound 1h inhibited ERK kinase more than MEK kinase at the same concentrations. Therefore, it can be concluded that this compound may inhibit melanoma cell proliferation through ERK kinase inhibition.
Conclusion
A new series of quinolinylaminopyrimidines 1a–k and quinazolinylmethylaminopyrimidines 2a–i was designed and synthesized as a continuation of our ongoing anticancer development research project. The target compounds were evaluated for antiproliferative activities against A375P human melanoma cell line. Compounds 1h and 1k with sub-micromolar IC50 values exhibited superior antiproliferative activity to Sorafenib. The representative compound 1h possessing 3-chloro-5-trifluoromethylphenyl as a terminal group showed significant and dose-dependent ERK kinase inhibitory activity. It can be considered as a promising lead for future development of antiproliferative agents targeting ERK pathway for treatment of melanoma.
Supplementary material available online
Supplemental Material.pdf
Download PDF (74.6 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded by the KIST Institutional Program (Grant No. 2E24760) from Korea Institute of Science and Technology, and by the Creative Fusion Research Program (Grant No. CAP-12-1) from Korea Research Council of Fundamental Science and Technology.
References
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res 2007;17:393–9
- Atallah E, Flaherty L. Treatment of metastatic malignant melanoma. Curr Treat Options Oncol 2005;6:185–93
- Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 1995;181:193–201
- Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncol (Williston Park) 1995;9:1149–58
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature 2007;445:851–7
- Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res 2007;17:117–27
- Koon HB, Atkins MB. Update on therapy for melanoma: opportunities for patient selection and overcoming tumor resistance. Expert Rev Anticancer Ther 2007;7:79–88
- Smith RA, Dumas J, Adnane L, Wilhelm SM. Recent advances in the research and development of RAF kinase inhibitors. Curr Top Med Chem 2006;6:1071–89
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2011/ucm268241.htm
- Nam BS, Kim H, Oh C-H, et al. Aminoquinoline derivatives with antiproliferative activity against melanoma cell line. Bioorg Med Chem Lett 2009;19:3517–20
- Jung M-H, Kim H, Choi W-K, et al. Synthesis of pyrrolo[2,3-d]pyrimidine derivatives and their antiproliferative activity against melanoma cell line. Bioorg Med Chem Lett 2009;19:6538–43
- Kim HJ, Jung M-H, Kim H, et al. Synthesis and antiproliferative activity of pyrrolo[3,2-b]pyridine derivatives against melanoma. Bioorg Med Chem Lett 2010;20:413–17
- Lee J, Kim H, Yu H, et al. Discovery and initial SAR of pyrimidin-4-yl-1H-imidazole derivatives with antiproliferative activity against melanoma cell lines. Bioorg Med Chem Lett 201;20:1573–7
- Yu H, Jung Y, Kim H, et al. 1, 4-Dihydropyrazolo[4,3-d]imidazole phenyl derivatives: a novel type II Raf kinase inhibitors. Bioorg Med Chem Lett 2010;20:3805–8
- Lee J, Nam BS, Kim H, et al. Synthesis of aminoquinazoline derivatives and their antiproliferative activities against melanoma cell line. Bioorg Med Chem Lett 2010;20:5722–5
- El-Gamal MI, Jung M-H, Lee WS, et al. Design, synthesis and antiproliferative activity of new 1H-pyrrolo[3,2-c]pyridine derivatives against melanoma cell lines. Eur J Med Chem 2011;46:3218–26
- Choi W-K, El-Gamal MI, Choi HS, et al. New diarylureas and diarylamides containing 1,3,4-triarylpyrazole scaffold: synthesis, antiproliferative evaluation against melanoma cell lines, ERK kinase inhibition, and molecular docking studies. Eur J Med Chem 2011;46:5754–62
- Kim HJ, Cho HJ, Kim H, et al. New diarylureas and diarylamides possessing acet(benz)amidophenyl scaffold: design, synthesis, and antiproliferative activity against melanoma cell line. Bioorg Med Chem Lett 2012;22:3269–73
- Jung MH, El-Gamal MI, Abdel-Maksoud MS, et al. Design, synthesis, and antiproliferative activity of new 1H-pyrrolo[3,2-c]pyridine derivatives against melanoma cell lines. Part 2. Bioorg Med Chem Lett 2012;22:4362–7
- Cho HJ, El-Gamal MI, Oh C-H, et al. Novel quinolinylaminoisoquinoline bioisosteres of sorafenib as selective RAF1 kinase inhibitors: design, synthesis, and antiproliferative activity against melanoma cell line. Chem Pharm Bull 2013;61:747–56
- Koh EJ, El-Gamel MI, Oh C-H, et al. New diarylamides and diarylureas possessing 8-amino(acetamido)quinoline scaffold: synthesis, antiproliferative activities against melanoma cell lines, kinase inhibition, and in silico studies. Eur J Med Chem 2013;70:10–21
- Ninkovic S, Braganza JF, Collins MR, et al. 6-Substituted 2-heterocyclylamino pyrazine compounds as CHK-1 inhibitors. WO2010-016005 A1
- Orfi L, Wáczek F, Pató J, et al. Improved, high yield synthesis of 3H-quinazolin-4-ones, the key intermediates of recently developed drugs. Curr Med Chem 2004;11:2549–53
- Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a phase II randomised discontinuation trial analysis. Br J Cancer 2006;95:581–6
- Sala E, Mologni L, Truffa S, et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res 2008;6:751–9