Abstract
Context: Tumor acidity represents a major cause of chemoresistance. Proton pump inhibitors (PPIs) can neutralize tumor acidity, sensitizing cancer cells to chemotherapy.
Objective: To compare the anti-tumor efficacy of different PPIs in vitro and in vivo.
Materials and methods: In vitro experiments PPIs anti-tumor efficacy in terms of cell proliferation and cell death/apoptosis/necrosis evaluation were performed. In vivo PPIs efficacy experiments were carried out using melanoma xenograft model in SCID mice.
Results: Lansoprazole showed higher anti-tumor effect when compared to the other PPIs. The lansoprazole effect lasted even upon drug removal from the cell culture medium and it was independent from the lipophilicity of the PPIs formulation.
Discussion: These PPIs have shown different anti-tumoral efficacy, and the most effective at low dose was lansoprazole.
Conclusion: The possibility to contrast tumor acidity by off-label using PPIs opens a new field of oncology investigation.
Introduction
Solid tumors resistance to current therapies continues to be a worldwide emergency, despite the development of new molecules and new anti-cancer strategiesCitation1–5. One of the most fascinating approaches to overcome cancer resistance to therapy is based on the evidence that cancers during their growth form, in a sort of evolutionary “microselection”, a very hostile microenvironment mostly represented by low nutrient supply, hypoxia and extracellular acidity. The ground of this peculiarity of malignant cancers is the so-called “Warburg effect”, that is the ability of cancer cells to use sugar fermentation as a fuel for their growth in either the presence or absence of consistent oxygen levels, thus leading to lactate accumulation outside the cells and the consequent acidification that is a hallmark of malignant cancersCitation6. In fact, tumors have a lower extracellular pH (∼6.7–7.1)Citation7–9 than normal tissues (7.4), that is perniciously maintained by the activity of a wide panel of proton exchangersCitation10, that ultimately lead to a reversed pH gradient between the acidic extracellular microenvironment and the alkaline cytosolCitation10. The way the acid tumor microenvironment negatively affects therapeutic efficacyCitation11–13 depends on the main mechanism of drug entry within target cells, that is based on the chemical nature of the vast majority of therapeutic molecules that are weak basis. These drugs, when ending in a H+ rich compartment, are protonated in their majority with slim chances to enter within the target cells. Moreover, the reverted pH gradient negatively impacts the distribution, uptake and bioavailability of weak base chemotherapeutic drugs within tumor cells, leading to marked chemoresistanceCitation13–15. The approaches proposed to overcome this very efficient mechanism of tumor chemoresistance were based either on systemic bufferingCitation16 or on the use of specific inhibitors of proton exchangersCitation10. Among the various proton exchanger's inhibitors a class of proton pump inhibitors (PPIs), introduced in the clinical practice in 1989, target the gastric H+, K+-ATPase, representing a major medical therapeutic breakthrough in the treatment of peptic ulcers and gastroesophageal reflux diseaseCitation17. These drugs promoted a more rapid healing of the lesions and symptom relief than other anti-acid drugs such as H2 blockersCitation17. PPIs are prodrugs which are attracted to and activated by acidic milieu and this property, combined with their high level of instability, resulted in absence of systemic side effects even at very high dosagesCitation17. After the first molecule, omeprazole, that launched the use of PPIs as kings of peptic disease treatment, a series of new molecules were introduced in the panel list of this class of drugs, including lansoprazole, esomeprazole, rabeprazole and pantoprazole, with some enantiomers as wellCitation17. Over the past years, it has been shown a striking similarity between the gastric H+, K+-ATPase, and the neoplastic vacuolar H+ATPase (V-ATPase), highly expressed by cancer cells, and the use of PPIs as anti-cancer agents in both preclinical and clinical settings is highly supporting the use of this class of drugs in cancer treatmentCitation18–25. Indeed PPIs have shown direct anti-tumor action as well as the ability to counteract the acid tumor microenvironment, resulting in potentiation of the action of chemotherapy agents as well as reinstitution of an active immunity at the tumor siteCitation18–25. PPIs are prodrugs with alkaline properties. The different drugs belonging to this family are converted in cyclic sulfenamides through a pH-dependent mechanism. All PPIs have the same activation rate at pH 1.0Citation26. Pantoprazole (pKa 3.96) and rabeprazole (pKa 4.9) are remarkably different in terms of pKa, but gastric acidity induces the same activation rate of the molecules. The same applies to lansoprazole (pKa 4.01), omeprazole and esomeprazole (pKa 3.97). However, this scenario changes at tumor pH ranging between 6.0 and 7.0, in fact, the higher the precursor's pKa the faster is the conversion from the inactive to the active form: the sulfonamideCitation27. Beside the strictly chemical differences, PPIs differ for some pharmaceutical properties as well, such as bioavailability that have been quantified as 30–40% for omeprazole and esomeprazole, 52% for rabeprazole, 77% for pantoprazole and 80–90% for lansoprazoleCitation28, and their metabolism: differently from other PPIs, rabeprazole's metabolism is mostly extra-hepaticCitation29.
Our group has been involved in multiple in vitro and in vivo preclinical investigations on PPIs anti-tumor activity, as well as their confirmation through clinical trials in both domestic animals and human patientsCitation18–25. However, one major concern, as above mentioned, is the choice of the most suitable among the PPIs for the treatment of cancer patients, since, despite the PPIs belonging to the same class of generic drugs, they have different chemical features. In order to provide new and fruitful information for the use of the most appropriate PPI for cancer patient's treatment but also to newly create a modeling for the generation of new anti-cancer drugs, this study compares both in vitro and in vivo different anti-cancer PPIs in terms of anti-tumor effect.
Methods
Chemicals and reagents
PPIs were purchased as following: omeprazole and esomeprazole from Astra Zeneca (Molndal, Sweden); lansoprazole, rabeprazole and pantoprazole by Sigma-Aldrich (Milan, Italy). All PPIs, excepting lansoprazole dissolved at 20 mM in DMSO, were resuspended at 20 mM in physiologic solution in the absence of direct light and reconstituted immediately prior its use. RPMI 1640 cell-culture medium (BE12-702F), antibiotics (DE17-603E), phosphate-buffered saline (PBS) (BE17-512F), trypsin/EDTA (BE17-171E) and fetal bovine serum (DE14-701F) were obtained from Lonza (Milan, Italy), 0.9% sodium chloride saline solution (Baxter s.p.a., Pisa, Italy). Trypan blue was bought from Alexis Biochemicals (Florence, Italy) and Annexin V-FITC Apoptosis detection kit from Enzo Life Sciences (Lause, Switzerland); 4-nitrophenyl phosphate disodium salt hexahydrate tablets for proliferation assay from Sigma.
Cell lines
Metastatic melanoma Me30966 (supplied by Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy), SaOS2 osteosarcoma and U87 glioblastoma cell lines (all purchased from ATCC, Manassas, VA) were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and antibiotics, at 37 °C in humidified 5% CO2. Experiments were performed in unbuffered (without sodium bicarbonate). All cell lines were negative for mycoplasma contamination, as routinely tested by modified nested polymerase chain reaction (VenorGeM Kit, Minerva biolabs, Berlin, Germany).
Cell lines pH medium and measurement
Acid cell culture medium (pH 6.0) was obtained by the addition of 1 M HCl solution.
Unbuffered cell culture medium was obtained by the removal of sodium bicarbonate allowing the cells to generate their own pH.
Cell culture pH 7.4 was obtained following the manufacturers' instructions.
The pH of all cell culture supernatants were estimated by the use of a pH 123 Microprocessor pH Meter (Hanna Instruments, Milan, Italy).
Cell death assay
Tumor cells were plated at 3–4 × 105 cells/ well in 12-well plates in 1 ml of buffered RPMI medium. After 24 h, the medium was replaced with unbuffered medium. After other 24 h, necessary for cell medium adjustment, cells were treated with doses 50, 75, 100, 150 and 300 μM of PPIs for 48 h. After treatment, cells were collected by pooling cells from the medium (i.e. dead cells) and adherent (live) cells obtained by trypsinization. Cells were washed and resuspended in PBS with 0.4% trypan blue 1:1 (vol/vol) dilution or incubated with AnnexinV-FITC/Propidium Iodide for apoptosis detection (Enzo Life Sciences) as reported in the manufacturer's instruction. Then, cells were analyzed by Flow citometry on a Becton Dickinson FACScalibur using CellQuestPro software (Becton Dickinson System, Milan, Italy). For each sample the total events were acquired in 60 s. All experiments were run in triplicate wells and repeated at least twice.
Cell proliferation assay
Melanoma cells were plated at 1 × 104 cells/ well in 96-well plates in buffered RPMI medium. After 24 hours, the medium was replaced with fresh, unbuffered RPMI medium and cells were treated with doses of 50, 75, 100, and 150 μM of PPIs for 48 h as per cell death analysis. After treatment, cell proliferation was determined using 4-nitrophenyl phosphate disodium salt hexahydrate tablets (Sigma) and the response was evaluated by the 405 nm absorbance measured by a spectrophotometer ELx800 (Bio-Tek Instruments, Inc., Colmar Cedex, France). All experiments were run in triplicate wells and repeated at least twice.
In vivo experiments with PPIs
CB.17 SCID/SCID female mice aged 4–5 weeks (Harlan, Milan, Italy) were kept under specific pathogen free conditions and fed ad libitum. The mice were housed in pathogen-free conditions. Mice were injected subcutaneously in the right flank with 1.0 × 106 human melanoma Me30966 cells in 0.2 ml of saline solution (Baxter s.p.a.). Mice were divided into four experimental groups of five mice each. Once tumors became evident, PPIs were administered, four times per week, by intraperitoneal injection. Specifically, omeprazole and esomeprazole were resuspended in 0.2 ml saline solution, while lansoprazole in 5% DMSO saline solution at the dose of 12.5 mg/kg. Mice in the control group received 0.2 ml of 5% DMSO saline solution. Tumor growth was estimated two times per week with caliper by the following formula: tumor weight (mg) = length (mm) × widthCitation2 (mm)/2Citation30. In this experiment, morbidity was considered as end-point according to standard clinical criteria including oversized tumor (>1.0 cm), weight loss (>20%), rough hair coat and general illnessCitation31. All mice were killed by cervical dislocation at the end of the experiments, within 2 months after the injection of the human tumor cells (following the guidelines of the Istituto Superiore di Sanità/Italian National Institute of Health). The animals used in our experimentation were included in the research protocol ‘‘Comparison in vivo on efficacy of different PPIs in cancer therapy; evaluation of their impact cytotoxic in combination with chemotherapy drugs, and qualitative/quantitative analysis of human tumor exosomes’’ that was approved by the experts from Service for Biotechnology and Animal Welfare and authorized by the Italian Ministry of Health with the Decree nu DM 255/2012-B of 22/10/2012.
Statistical analysis
Differences between treatment groups, both in vitro and in vivo, were analyzed by one way ANOVA and Bonferroni t-test. Data are expressed as mean ± SD and p values reported are two-sided. p Values < 0.05 were considered as statistically significant. Statistical analysis was performed with Sigmastat 3.0 software (San Jose, CA).
Results
Experiments in human tumor cell lines
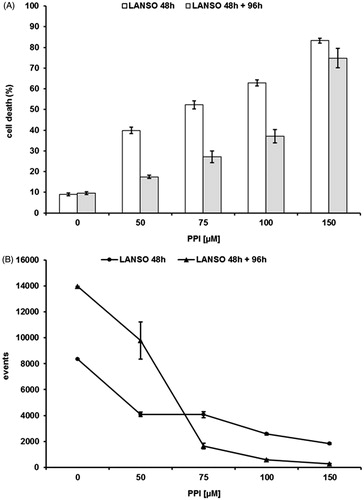
The background of PPIs teaches that differences in term of pKa between the various molecules are impossible to be established at the gastric pH (pH 1.0), while it is much more evident at pH close to the values measured in human tumorsCitation3,Citation7,Citation10,Citation16,Citation17. Thus, the purpose of this first set of experiments was to evaluate the dose ranging of five PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole). The efficacy of these drugs was tested at different Me30966 human melanoma cell culture pH conditionsCitation32: pH 7.4 (equivalent to a condition of mild metabolic or respiratory alkalosis), pH 6.0 (highly acidic condition, superior to the values observed in tumors) and unbuffered pH spontaneously shifting in our experimental in vitro model from 7.4 to 6.7 ± 0.2 (in vitro condition simulating the spontaneous acidification occurring within tumors). In all the experimental conditions, lansoprazole showed the highest anti-tumor effect (i.e. anti-tumor cell cytotoxicity) and this property was more evident in unbuffered conditions (not shown), and the vast majority of the experiments was run in this condition (mean ± SD of dose–response experiments performed in human metastatic melanoma cell line are shown in ). While all the PPI showed a full cytotoxicity at the highest concentration, only omeprazole, esomeprazole and lansoprazole showed a significant effect even at dosages lower than 100 μM, as compared to both pantoprazole and rabeprazole (p < 0.001). However, lansoprazole was the only one showing marked cytotoxicity at 50 μM concentration (p < 0.001) (). To investigate the possible mechanisms underlying PPI-mediated cell death, we performed experiments aimed at evaluating whether PPI-mediated anti-cancer action was mostly exerted through either an apoptosis mediated mechanism or through the induction of necrosis. The results showed that lansoprazole at the lowest effective dose (i.e. 50 μM) induced its effect mostly through the induction of apoptosis (p < 0.001) (). Thus, we performed a series of experiments aimed at verifying the eventual tumor specific activity of PPI, comparing the results obtained with the most effective PPIs (omeprazole, esomeprazole and lansoprazole), in the melanoma cell line with those observed in human tumor cell lines derived from either osteosarcoma or glioblastoma, consistently with the ubiquitous effect that PPI have shown in clinical trials, at least as chemosensitizersCitation21,Citation22,Citation24. In the other tested cell lines, we observed a significant tumor response already at 75 μM concentration of lanzoprazole (p < 0.05) ()Citation7–9,Citation22,Citation33. One of the critical technical problem envisioned in the clinical therapy of cancer is that most drugs must be highly hydrophilic in order to be parenterally administered, while the tumor cell membrane is extremely rich in lipids, thus resulting in slow drug cross-membrane flowCitation34,Citation35. To verify if a major mechanism underlying the increased efficacy of lansoprazole might be its lipophilicity, potentially responsible for an increased drug uptake, as it is for several conventional chemotherapy agentsCitation34–37, we compared its action with that of the liposoluble form of omeprazole, being, together with lansoprazole, the only PPI available in a liposoluble form. Intriguingly, lansoprazole showed again a higher cytotoxic activity as compared to omeprazole, at all the used concentrations, while the liposoluble form of omeprazole appeared to be slightly, but not significantly, more effective that the saline form at 75 and 100 μM concentrations. However, lansoprazole anti-tumor action was again significantly higher than both saline and liposoluble omeprazole formulations (p < 0.05) (). This set of results strongly suggested that lipophilicity did not represent at least the major mechanism underlying lansoprazole anti-tumor effect, mostly supported by the experimental evidence that lansoprazole showed always an increased efficacy when compared to both saline and lipophilic formulations of omeprazole. Interestingly, we have shown that lansoprazole was the most active a concentration, 50 μM, where none of the other PPIs have demonstrated to be effective, in terms of both inhibition of proliferation and cell death induction. Of great interest, time course experiments have shown that lansoprazole effects lasted even after drug withdrawal. In fact, the results of this set of experiments showed that lansoprazole discontinuation after 48 hours of treatment did not allow a quick recovery of cell survival and turn over for the following 96 h (). This is consistent with our clinical observations in domestic animals and humans, where PPIs retain their effectiveness even with a pulse administrationCitation21,Citation22,Citation24.
Figure 1. (A) Cytotoxic effect of rabeprazole (RABE), pantoprazole (PANTO), omeprazole (OME), esomeprazole (ESO) and lansoprazole (LANSO) against Me30966 human melanoma metastatic cells in unbuffered conditions, at different drug dosages and after 48 h of treatment. (B) Apoptosis evaluation of Me30966 human melanoma metastatic cells in unbuffered conditions treated with rabeprazole, pantoprazole, omeprazole, esomeprazole and lansoprazole at different drug dosages and after 48 h of treatment. Columns, mean percentages of cell death of three independent experiments run in triplicate; bars indicate SD. *Indicates p < 0.001.
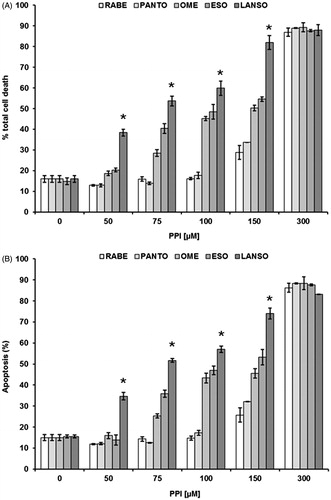
Figure 2. (A) Cytotoxic effect of omeprazole, esomeprazole and lansoprazole against SaOS2 osteosarcoma, (B) U87 glioblastoma in unbuffered conditions, at different drug dosages and after 48 h of treatment. Columns, mean percentages of cell death of three independent experiments run in triplicate; bars indicate SD. *Indicates p < 0.05.
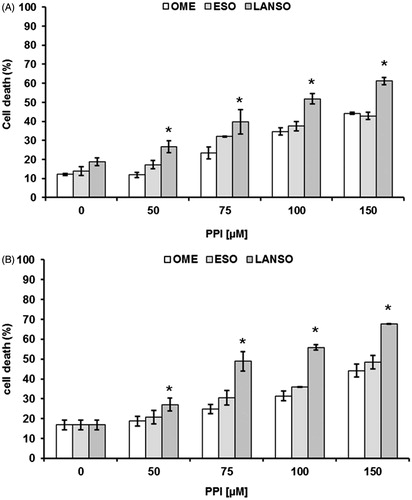
Figure 3. Cytotoxic (A) and proliferative (B) effects of omeprazole, esomeprazole and lansoprazole against human Me30966 melanoma metastatic cells treated in unbuffered conditions for 48 h and at different drug dosages. Columns, mean percentages of cell death of three independent experiments run in triplicate; bars indicate SD. *Indicates p < 0.05.
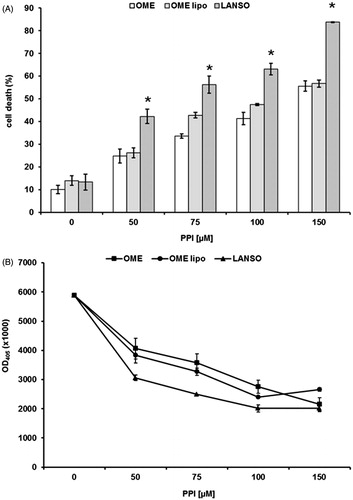
Figure 4. Cytotoxic (A) effects of lansoprazole against human Me30966 melanoma metastatic cells treated in unbuffered conditions, at different drug dosages, for 48 h (dark columns) and for 48 h of treatment and 96 h of recovery in absence of drug (gray columns). Columns, mean percentages of cell death of three independent experiments run in triplicate; bars indicate SD and proliferative (B) proliferative effects lansoprazole against human Me30966 melanoma metastatic cells treated in unbuffered conditions, at different drug dosages, for 48 h (diamond line) and for 48 h of treatment and 96 h of recovery in absence of drug (dotted line).
Experiments in human xenografts
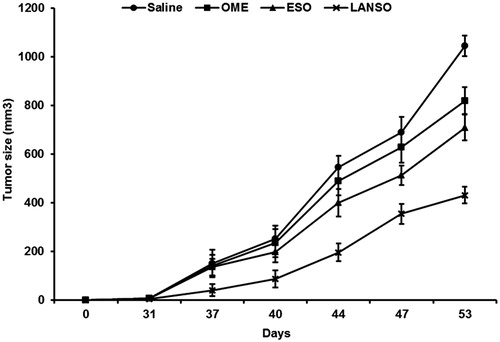
On the basis of the experiments performed in vitro human tumor cell lines, we wanted to obtain a clear proof of concept in in vivo experiments performed in the human tumor xenografts set up in our instituteCitation38. The goal was to have a clear evidence that lansoprazole will be able to control human tumor growth better than the other PPI in a systemic administration regimen. On the basis of the in vitro data we thus compared the effect of lansoprazole administered i.p. to human melanoma xenografts on tumor growth to the effect of omeprazole and esomeprazole; proven to have the highest in vitro efficacy at the lowest doses compared to the other tested PPI and the mostly adopted by our group in clinical studies in humans and domestic animalsCitation21,Citation22,Citation24. All the investigated PPIs were well tolerated even at the highest dose selected (12.5 mg/kg), as analyzed by water and food consumption and body weight preservation. In terms of efficacy, all the PPIs showed significant tumor growth delay compared to the untreated controls; however, in omeprazole this effect was somewhat delayed compared to esomeprazole and lansoprazole. Remarkably, lansoprazole again showed to induce a statistically significant tumor growth inhibition as compared to the other PPIs, in terms of both an early onset and the level of a constant tumor growth inhibition until the end of the in vivo experiment (p < 0.05) (). These data were clear in supporting the use of lansoprazole in the treatment of cancer patients.
Figure 5. In vivo effects of omeprazole (OME), esomeprazole (ESO) and lansoprazole (LANSO) in SCID mice xenografted with human Me30966 melanoma cells. Saline corresponds to the group of control mice. Mice were divided into four experimental groups of five mice each. Once tumors became evident, PPIs were administered (12.5 mg/kg), four times per week, by intraperitoneal injection. Bars indicate SD, p < 0.05.
Discussion
The unraveling of the tumor acid metabolism riddle is providing researchers with new insights on chemoresistance and is unmasking novel targets to be hit by new drugs or unconventional use of already clinically available moleculesCitation8,Citation10–16,Citation18–25. Our current research showed that lansoprazole has great anti-tumor activity both in terms of cytotoxicity and inhibition of cell proliferation. This action is exerted at concentrations much lower than those of the other PPIs that, in order to approximate its efficacy, need to be used at higher concentrations. We had these results in unbuffered condition, allowing a spontaneous acidification of the tumor cell milieu and thus mimicking the tumor microenvironmental acidification, thus further supporting the importance of this study for clinical applications of PPI as anti-neoplastics. The ionization constant cannot explain by itself the observed differences in terms of anti-tumor activity among the different PPI molecules. In fact, accordingly to this chemical property alone, the most effective molecule should be rabeprazole, while our data clearly show that lansoprazole is the most effective against tumors both in vitro and in vivo. The lipophilicity of lansoprazole could represent an hypothesis, but the results of our study showed that lansoprazole was more cytotoxic than omeprazole in either saline or liposoluble formulations. However, there was some difference between the two omeprazole formulations, suggesting that perhaps lipophilicity may have a role, while not a major role, in the lansoprazole anti-tumor activity. This is consistent with experimental and clinical data showing that hydrophilic agents, while handier from the point of view of systemic administration, at the same time suffer a decreased uptake by cancer cells that are highly mutated and frequently show deletion of many transmembrane proteins that act as carriers and potentially as stabilizersCitation34,Citation35. Other factors might help to explain the existing differences between lansoprazole and the other PPIs, such as the n-octanol–water partition coefficient commonly expressed as log P. This parameter is an index of a substance hydrophilic/hydrophobic affinity. In particular, lansoprazole has a log P of 1.9, while for the other PPIs log P is between 0.5 and 0.6. A drug's distribution coefficient strongly affects how easily the drug can reach its intended target in the body, how strong an effect it will have once it reaches its target, and how long it will remain in the body in an active formCitation39. log P is one criterion used in medicinal chemistry to assess the drug likeness of a given moleculeCitation40. In the context of pharmacodynamics, the hydrophobic effect is the major driving force for the binding of drugs to their receptor targetsCitation41,Citation42. Another variable that surely might explain the higher efficacy of lansoprazole is the different affinity of PPIs toward V-ATPase. In fact, the possibility to use lansoprazole at lower doses due to its higher chemical performance makes clinical protocols much easier to be implemented: the drug will be attracted by the tumor acidity and there, it will be activated exerting its anti-cancer actionsCitation21,Citation22,Citation24. This will be performed through tumor cell apoptosis that could uncover tumor antigens to the immune system, thus further improving the patient's purging of tumor burdenCitation43. Moreover, the fact that this efficacy is maintained even after drug removal, as shown by the in vitro experiments, will allow clinicians to continue a pulse administration involving a loading phase followed by a maintenance, that is much better tolerated by patientsCitation21,Citation22,Citation24. The problem of drug tolerance is acutely perceived in cancer patients treated with systemic buffering using over saturated sodium bicarbonate solutions that frequently result unpalatable thus causing decreased consumption and reduced efficacyCitation16. This aspect of buffering therapy, while new and more pleasant buffers are developed, makes PPIs the current first choice in tumor alkalizationCitation8,Citation10,Citation16.
The in vitro and in vivo superior efficacy of lansoprazole in the treatment of melanoma is of particular importance, since this histotype is a poor responder to conventional chemotherapy and lansoprazole, beside acting as an anti-tumor agent per se, might act as a chemosensitizer as wellCitation44,Citation45. The anti-proliferative activity of PPIs in osteosarcoma did not come as a surprise, in consideration of the results obtained by our and other groupsCitation22,Citation46. Again, lansoprazole showed high tumoricidal activity starting at low doses, evidencing a particular efficacy for the chondroblastic osteosarcoma subtype that is well known to thrive in a highly hypoxic and acidic environment that frequently results in resistance to limb-sparing neoadjuvant chemotherapyCitation22. Finally, PPIs and especially lansoprazole showed, for the first time, efficacy against glioblastoma cell culture, a well-known chemotherapy refractory tumor. This information, if substantiated by further investigations, could open a new therapeutic avenue for the treatment of these almost always fatal neoplasmsCitation47,Citation48. The possibility of a direct anti-tumor action of PPIs and especially lansoprazole on refractory histotypes is a source of hope for both clinicians and cancer patients, in view of their lack of serious side effects that hamper the use of standard chemotherapy agents. Furthermore, the action of PPIs as chemosensitizers could allow a dose reduction (as opposite to drug escalation, that is the current clinical orientation) making multidrug protocols much better tolerated and appealing to cancer patientsCitation25. It therefore foreseeable that in a near future new drugs will be devised combining PPI with standard chemotherapy agents to act as a chemical delivery system and to neutralize the counter gradient condition that hampers most chemotherapy protocolsCitation8,Citation16,Citation25. Lastly, the results of this study strongly support the use of lansoprazole for the set up of new anti-tumor drugs, also in combination with other inhibitors of proton pump or ion exchangers proven to be very effective against cancerCitation49,Citation50.
Conclusions
All the PPIs have shown different degrees of anti-tumor efficacy. Omeprazole, esomeprazole and lansoprazole evidenced an early and significant effectiveness since the lowest dosages against different tumor histotypes. Among them lansoprazole revealed the highest efficacy both in vitro and in vivo studies, maintaining its in vitro efficacy over time even upon withdrawal confirming the validity of pulse administration in clinical conditions.
Having identified the cross mechanism that allows the neutralization of gastric H+, K+-ATPase, and the neoplastic vacuolar H+ATPase (V-ATPase) by the same agents and the fact that most PPIs are over the counter drugs has been a true serendipity.
Medical investigators have been hoping for such a breakthrough over the past decades and the fact that a drug for the treatment of peptic ulcers has been the key to such a revolution, makes this event almost anti-climactic … . Nevertheless, the recent results of our preclinical and clinical investigations reported a high percentage of responders among our cancer patients, sometimes in individuals affected by rapidly growing and chemotherapy non-responsive histotypes or, among the refractory tumors, particularly resistant subpopulationsCitation21,Citation22,Citation24.
The apparent banality of these discoveries opens a new avenue of easily transferable approaches from bench side to clinics for the first time in the history of oncology and must be aggressively and enthusiastically pursued to speed up the clinical transition.
Declaration of interest
TA was supported by a Grant from the Ministry of Health, Italy.
References
- Wilting RH, Dannenberg JH. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist Updat 2012;15:21–38
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002;53:615–27
- Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract 2005;14:35–48
- Nobili S, Landini I, Giglioni B, Min E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets 2006;7:861–79
- Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy—a quick review. Taiwan J Obstet Gynecol 2009;48:239–44
- Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr 2007;39:267–74
- Gillies R, Raghunand N, Garcia-Martin M, Gateby R. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Magn 2004;23:57–64
- De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol 2005;1:779–86
- Smallbone K, Gatenby RA, Maini P. Mathematical modeling of tumour acidity. J Theor Biol 2008;255:106–12
- Spugnini EP, Sonveaux P, Stock C, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta 2014. [Epub ahead of print]. doi: 10.1016/j.bbamem.2014.10.015
- Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm 2011;8:2032–8
- Shekhar MP. Drug resistance: challenges to effective therapy. Curr Cancer Drug Targets 2011;11:613–23
- Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. Targeting the metabolic microenvironment of tumors. Adv Pharmacol 2012;65:63–107
- Spugnini EP, Citro G, Fais S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res 2010;29:44
- Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther 2006;5:1275–9
- Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Met Rev 2014;33:1095–108
- Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 2013;19:25–35
- Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst 2004;96:1702–13
- De Milito A, Fais S. Proton pump inhibitors may reduce tumour resistance. Expert Opin Pharmacother 2005;6:1049–54
- De Milito A, Iessi E, Logozzi M, et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res 2007;67:5408–17
- Spugnini EP, Baldi A, Buglioni S, et al. Lansoprazole as a rescue agent in chemoresistant tumors: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 2011;9:221
- Ferrari S, Perut F, Fagioli F, et al. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients' bed. J Transl Med 2013;11:268
- Bellone M, Calcinotto A, Filipazzi P, et al. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology 2013;2:e22058
- Spugnini EP, Buglioni S, Carocci F, et al. High dose lansoprazole combined with metronomic chemotherapy: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 2014;12:225
- Azzarito T, Venturi G, Cesolini A, Fais S. Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett 2015;356:697–703
- Hellström PM, Vitols S. The choice of proton pump inhibitor: does it matter? Basic Clin Pharmacol Toxicol 2004;94:106–11
- Kromer W, Krüger U, Huber R, et al. Differences in pH-dependent activation rates of substituted benzimidazoles and biological in vitro correlates. Pharmacology 1998;56:57–70
- Klotz U. Pharmacokinetic considerations in the eradication of Helicobacter pylori. Clin Pharmacokinet 2000;38:243–70
- Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol 2008;64:935–51
- Geran RI, Greenberg NH, Macdonald MM, et al. Protocols for screening chemical agents and natural products against animal tumours and natural other biological systems. Cancer Chemother Rep 1972;3:59–61
- Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet Rec 1985;116:431–6
- De Milito A, Canese R, Marino ML, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer 2010;127:207–19
- Kunkle B, Yoo C, Roy D. Discovering gene–environment interactions in glioblastoma through a comprehensive data integration bioinformatics method. Neurotoxicology 2013;35:1–14
- Tounekti O, Pron G, Belehradek J Jr, Mir LM. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res 1993;53:5462–9
- Pron G, Mahrour N, Orlowski S, et al. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol 1999;57:45–56
- Doshi G, Sonpavde G, Sternberg CN. Clinical and pharmacokinetic evaluation of satraplatin. Expert Opin Drug Metab Toxicol 2012;8:103–11
- Quéreux G, Dréno B. Fotemustine for the treatment of melanoma. Expert Opin Pharmacother 2011;12:2891–904
- Lozupone F, Pende D, Burgio VL, et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res 2004;64:378–85
- Edwards MP, Price DA. Role of physicochemical properties and ligand lipophilicity efficiency in addressing drug safety risks. Ann Rep Med Chem 2010;45:381–91
- Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov 2007;6:881–90
- Eisenberg D, McLachlan AD. Solvation energy in protein folding and binding. Nature 1986;319:199–203
- Miyamoto S, Kollman PA. What determines the strength of noncovalent association of ligands to proteins in aqueous solution? Proc Natl Acad Sci USA 1993;90:8402–6
- Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012;72:2746–56
- Stadler S, Weina K, Gebhardt C, Utikal J. New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci 2014;60:83–8
- Spagnolo F, Ghiorzo P, Orgiano L, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther 2015;8:157–68
- Costa-Rodrigues J, Reis S, Teixeira S, et al. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J 2013;280:5052–64
- Garrido W, Rocha JD, Jaramillo C, et al. Chemoresistance in high-grade gliomas: relevance of adenosine signalling in stem-like cells of glioblastoma multiforme. Curr Drug Targets 2014;15:931–42
- Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 2014;23:1985–96
- Grandane A, Tanc M, Zalubovskis R, Supuran CT. 6-Triazolyl-substituted sulfocoumarins are potent, selective inhibitors of the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem Lett 2014;24:1256–60
- Grandane A, Tanc M, Zalubovskis R, Supuran CT. Synthesis of 6-tetrazolyl-substituted sulfocoumarins acting as highly potent and selective inhibitors of the tumor-associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem 2014;22:1522–8
