Abstract
Carbonic anhydrase (CA, EC 4.2.1.1) inhibitors (CAIs) started to be used in the treatment of peptic ulcers in the 1970s, and for more than two decades, a group led by Ioan Puşcaş used them for this purpose, assuming that by inhibiting the gastric mucosa CA isoforms, hydrochloric acid secretion is decreased. Although acetazolamide and other sulfonamide CAIs are indeed effective in healing ulcers, the inhibition of CA isoforms in other organs than the stomach led to a number of serious side effects which made this treatment obsolete when the histamine H2 receptor antagonists and the proton pump inhibitors became available. Decades later, in 2002, it has been discovered that Helicobacter pylori, the bacterial pathogen responsible for gastric ulcers and cancers, encodes for two CAs, one belonging to the α-class and the other one to the β-class of these enzymes. These enzymes are crucial for the life cycle of the bacterium and its acclimation within the highly acidic environment of the stomach. Inhibition of the two bacterial CAs with sulfonamides such as acetazolamide, a low-nanomolar H. pylori CAI, is lethal for the pathogen, which explains why these compounds were clinically efficient as anti-ulcer drugs. Thus, the approach promoted by Ioan Puşcaş for treating this disease was a good one although the rationale behind it was wrong. In this review, we present a historical overview of the sulfonamide CAIs as anti-ulcer agents, in memoriam of the scientist who was in the first line of this research trend.
Introduction
Carbonic anhydrase (CA) is a ubiquitous metalloenzyme catalysing the reversible conversion of carbon dioxide to bicarbonate and has various biological functions in bacterial metabolism, respiration, photosynthesis, renal acidification, gastric acid and pancreatic bicarbonate secretion as well as bone metabolism, to mention only the most important ones. After a historical overview of CA research, this article will summarize the contribution of Romanian researcher Ioan Puşcaş to the multi-decade CA story, focusing on the relationship between gastric CAs and peptic ulcer disease, followed by a presentation of main achievements of bacterial CAs.
History of CA
Physiologists and chemists from the early decades of the 20th century were interested to find out how carbon dioxide (CO2) is transported in bloodCitation9,Citation10. The timeline of the experiments is presented in . Many important personalities of modern physiology were involved and the research culminated in 1933, when Norman Urquhart Meldrum and Francis John Worsley Roughton first prepared the enzyme from erythrocytes of goats, oxen, rabbits, whales and men, thus differentiating its effect from that of hemoglobinCitation11,Citation13. The term CA was coined by their co-worker, Philip Eggleton, but his name is not mentioned in the articlesCitation1. In 1961, after development of the enzyme nomenclature, CA was listed in Enyzme Commission Class 4 (EC 4.2.1.1). Until 1976, isoenzymes I and II were only known, but since then, a total of 18 isoenzymes have been described which belong to distinct genetic families (alpha-, beta- and gamma-CAs). Their functional differences have been described in detail and the structure was elucidated by Roentgen diffraction.
Table 1. Chronology of carbonic anhydrase researchCitation1–12.
The CA theory of acid secretion
The formation of hydrochloric acid secretion by the stomach was an interesting topic in gastric physiology for decades. In 1934, Franklin Hollander (1899–1966) of Mount Sinai Hospital, New York, formulated the two components theory. Accordingly, gastric juice comprises a parietal cell-secreted acid solution and an alkaline one produced by non-parietal cellsCitation14. The retrodiffusion theory belongs to Torsten Teorell (1905–1992) (Karolinska Institute, Stockholm). Hydrogen ions are primarily secreted by the parietal cells and are exchanged with NaCl from plasma through the mucosaCitation15. In 1932, the so-called alkaline tide was observed (i.e. increase of blood bicarbonate level during stimulation of HCl secretion) and based on this, Davenport presumed the existence of gastric CA, which was subsequently demonstrated in 1939. In 1940, the author formulated the CA theory in which by catalysing the hydration of CO2 and further dissociation to H+ and HCO3, the enzyme represents the main source of hydrogen ionsCitation12. And, for the first time, he also demonstrated the inhibition of CA and that of gastric acid secretion by thiocyanateCitation16. His theory did not explain the driving force which moves H+ ions against a huge concentration gradient between the gastric lumen and parietal cells. Thus, in 1946, the author himself withdrew his theoryCitation1.
Ioan Puşcaş also envisaged a central role for CA in the parietal cells. In a series of original experiments, using Wilbur–Anderson’s electrometric and colorimetric method (1948) and Maren’s simplified micromethod (1960), he showed that mediators of gastric acid secretion (histamine, pentagastrin, acetylcholine and calcium ions) activate gastric mucosa CA depending on the doseCitation17,Citation18. The effect of histamine on CA was, however, described in 1966 by Romanian pharmacologist Dumitru Dobrescu (1927) on ratsCitation19 and confirmed in 1972 by Russian authorsCitation20. In 1984, the validity of this phenomenon was verified on isolated guinea pig oxyntic cells. The activity of all the secretagogues was inhibited by the new AB Hassle product H149/94, suggesting a functional link between CA and the proton pumpCitation21. In 1999, Ioan Puşcaş et al.Citation22 showed that omeprazole inhibits both gastric mucosa H+/K+-ATP-ase and CA. Finally, in 2011, Supuran et al.Citation23 demonstrated the activation of CA I and II by mono- and dihalogenated histamine derivatives at subnanomolar concentrations. Ioan Puşcaş also showed that H2 histamine receptor blockers (cimetidine and ranitidine), muscarinic antagonist pirenzepine, Zn ions and prostaglandins inhibit gastric mucosa CA. However, the importance of these phenomena in the parietal cell physiology was never shown.
Treatment of gastric and duodenal ulcers by CA inhibitors
Shortly after the discovery of acetazolamide, Henry D. Janowitz (1922–2008) (Mount Sinai Hospital, New York) administered it in dogsCitation24 and humansCitation25. Some years later, it was shown that higher diuretic doses of acetazolamide are needed for several days to ensure the efficient inhibition of acid secretionCitation26. Work done in 1956 at the Chemical Defence Experimental Establishment, Liverpool, showed that acetazolamide in usual doses did not abolish HCl secretionCitation27, but, in 1956, Rodolfo Cheli (1928–1997) reported some inhibitory effects in normal subjects and chronic gastritis patientsCitation28.
In 1960, acetazolamide was introduced to the therapy of peptic ulcers in a military hospital from Illinois, USACitation29. Acetazolamide was given in a dose of 250 mg q.i.d. to 125 patients for 7–20 days and along with a decrease of acid secretion, the typical ulcer symptoms were relieved promptly. However, no radiographic control was performed and the drug produced reversible metabolic acidosis. In 1963, a German physician working in GhanaCitation30 administered 3 × 2 tablets of 250 mg acetazolamide/day to 11 patients for 9 days and also noted a 70–92% decrease in histamine-stimulated acid secretion. In the same time, CA inhibitors were used in several other conditions ().
Table 2. Timeline of clinical uses of acetazolamide (AAZ).
In 1968, Ioan Puşcaş began to use acetazolamide in Şimleu Silvaniei Hospital and he rapidly gained experience with a large number of patients. The results of his studies were largely published in the national and international medical press. In several consecutive studies, he showed that acetazolamide, given in dose of 250–500 mg/t.i.d. for 10–21 days, healed gastric and duodenal ulcer in high percentages, approaching 100%. These results were unparalleled with any other treatment in those daysCitation31,Citation32. To compensate the electrolytic losses stemming from the diuretic effect of the drug, a mixture of sodium, potassium and magnesium salts was added – without any antacid effect – and the whole composition was marketed as Ulcosilvanil (ulcer + Transylvania). These high-healing percentages were documented radiologically and later by fibre-optic or videoendoscopy. Similar results were obtained with ethoxzolamideCitation33. The drug was also efficient as maintenance treatment for preventing peptic ulcer relapses when administered for 10 days/monthCitation34. However, the high efficacy was overshadowed by a wide spectrum of side effects (fatigue, paraesthesias, renal colic, loss of appetite, vision disturbances, decreased libido and headache) in an era of rapid development of clinical pharmacology, when the “safety” of any drug was more important than its “efficacy”. Although these side-effects were recognized from the beginning of acetazolamide use, they were often masked or only partially reported in the published studies. Thus, Ulcosilvanil, although marketed countrywide, remained a miracle drug given in its parent town, Şimleu Silvaniei, for more than two decades. The advent of the safer histamine H2 receptor blockers, proton pump inhibitors and the discovery of Helicobacter pylori seriously curtailed the use of Ulcosilvanil and because of a drop in sales, its production was terminated before the end of the last century. Ioan Puşcaş was aware of the discovery of H. pylori, but he never recognized the importance of the bacterium in peptic ulcer disease (see Appendix and Supplementary Table).
Helicobacter pylori CAs and their inhibition: the rationale of treating peptic ulcers with CA inhibitors
As other bacteria, H. pylori encodes for two CAs, an α-class and a β-class enzyme, called HpαCA and HpβCA, respectivelyCitation35,Citation36. The two enzymes possess a significant CO2 hydrase activity, as determined by several groups, which is probably due to their crucial physiological roles for the bacteriumCitation37,Citation38–44.
HpαCA is composed of 247 amino acid residues and shows 27–36% similarity with other α-class bacterial CAs, including Klebsiella pneumonia, Neisseria gonorrhoeae, Enterococcus faecalis, Anabaena PCC7120 and Synechococcus PCC7942Citation37,Citation38,Citation45. Surprisingly, the HpαCA amino acid sequence shows relatively high similarity (23–36%) with the 15 types of human CAs (hCA), although there is a large evolutionary distance between the two species, indicating thus that CAs possess a fundamental biological function which has been conserved during the evolution of life on earth. Most amino acid residues which are known to be involved in the catalytic mechanism or to form a network of hydrogen bonds are conserved between hCAs and HpαCA, including the three zinc-liganded His residues (at position 94, 96 and 119, hCA I numbering system) and several important residues for the binding of the substrates, inhibitors and activators, such as Tyr7, His64 and Leu198Citation45–48.
HpβCA is composed of 221 amino acid residues and shows 25–34% similarity with other β-class bacterial CAs, including the two enzymes from Escherichia coli, the one from Synechococcus elongatus, Brucella suis and Haemophilus influenzaeCitation35,Citation36,Citation49,Citation50. It has been reported that most of the bacterial β-CAs are composed of three sequential components: (i) an N-terminal arm including two α-helixes (H1 and H2), (ii) a zinc-binding core including three β-sheets (S1–S3) and two α-helixes (H3 and H4) and (iii) a C-terminal subdomain including two β-sheets (S4–S5) and five α-helixes (H5–H9)Citation51,Citation52. The amino acid sequence of the zinc-binding core of HpβCA is highly conserved among other bacterial β-CAs sequenced to date. These residues include the Zn(II)-coordinating amino acids Cys42, Asp44, His98 and Cys101 (residue numbering are based on the E. coli CynT2 numbering system)Citation51,Citation52. The principal difference between α- and β-CAs consists in the fact that generally β-CAs are oligomers usually consisting of two to six monomers of molecular weight of 25–30 kDaCitation51,Citation52.
The catalytic activity of recombinant, purified HpαCA and HpβCA for the physiologic reaction (CO2 hydration), in comparison with that of several α-CAs of human origin, such as hCA I–III (cytosolic isozymes), hCA VA and VB (mitochondrial isoforms) and hCA XII and XIV (transmembrane isozymes), are shown in . It may be observed that HpαCA and HpβCA are catalytically efficient CAs, possessing a high enzymatic activity (the β-class enzyme is 3.2 times more active than the α-CA from this bacterium)Citation38–41. Furthermore, this activity is almost identical (as kcat/Km value) to that of hCA I, whereas the Km value of the bacterial enzyme is closer to that of hCA II than that of hCA I. In fact, HpβCA is a medium-efficiency CA, possessing a catalytic activity higher than that of hCA III, hCA VA, hCA XII and hCA XIV among others. Only hCA VB and especially hCA II, one of the best catalysts known in nature, show a better activity than HpβCACitation38–41. Also, it may be observed that the activity of all these enzymes is inhibited by the CA inhibitor (CAI) par excellence, the sulfonamide drug, acetazolamide AAZCitation53–57, which may explain why Ulcosilvanil showed a range of severe side effects, due to inhibition of CA isoforms in other organs than the stomach, or better to say, due to hCAs and not Hpα/βCA inhibition ().
Table 3. Kinetic parameters for CO2 hydration reaction catalysed by some human α-CA isozymes at 20 °C and pH 7.5, and HpCA isozymes belonging to the α- and β-CA class, and their inhibition data with acetazolamide AAZ (5-acetamido-1,3,4-thiadiazole-2-sulfonamide), a clinically used sulfonamideCitation39–41.
Chirica et al.Citation37 studied the subcellular localization of HpαCA and HpβCA by using electron microscopy with immunonegative staining and SDS-digested freeze-fracture immunogold labelling and then showed that hpαCA was attached to the outer membrane and that hpβCA was localized in the cytosol, on the cytosolic side of the inner membrane, and on the outer membrane facing the periplasmic spaceCitation37.
What are the biological roles of periplasmic HpαCA and cytosolic HpβCA in H. pylori? Stähler et al.Citation58 reported that HpαCA and HhpβCA deletion mutants as well as the double mutant displayed a significant decrease in urease activity, indicating a metabolic link of these two enzyme types, urease and CAs. They also showed that only the HpβCA deletion mutant showed a clearly reduced growth at pH 6.0–6.25 for 48 h in culture, compared to the parental strain, the HpαCA deletion mutant and the double mutant, suggesting thus that the cytosolic HpβCA is only important for acid resistance when the periplasmic HpαCA is functionalCitation58. In another study by Marcus et al.,Citation59 the HpαCA deletion mutant showed an ∼3 log10 decrease in survival after 30 min of exposure to pH 2.0 when compared with the wild-type strain. These discrepant findings are probably due to the different culture conditions employed in the two studies. In the presence of the CAI acetazolamide (AAZ), acting against both hpαCA (KI: 21 nM) and hpβCA (KI: 40 nM) (), another H. pylori strain also (ATCC 43504) showed an ∼3 log10 decrease in surviveCitation59. These findings indicate that both hpαCA and HhpβCA might be essential for the bacterial survival.
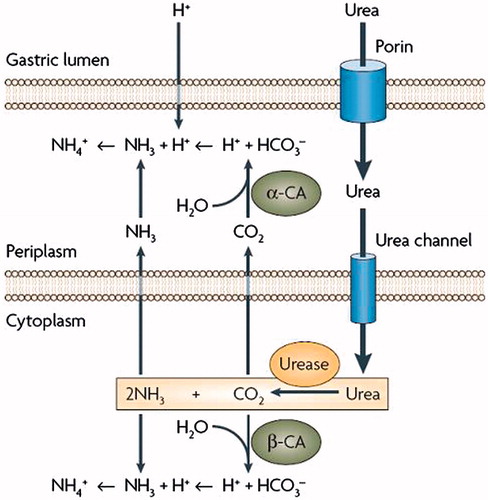
Marcus et al.Citation59 has proved that HpαCA is essential for the acid acclimation and survival of the pathogen and then proposed a model for the role of urease and hpαCA in maintenance of the periplasmic pH in acidic environmentCitation58,Citation59. Marcus et al.Citation59 employed a CA deletion mutant of H. pylori and showed that the generation of NH3 by urease and bicarbonate by hpαCA has a major role in the regulation of the periplasmic pH 6.1 and inner membrane potential −101 mV under acidic conditionsCitation59. Thus, buffering of the periplasm to a pH consistent with viability depends not only on the ammonia efflux from the cytoplasm but also on the conversion of CO2 (produced by urease) to bicarbonate by the periplasmic hpαCA (). In fact, the pKa of the carbonic acid/bicarbonate couple of ∼6.1 is very appropriate for such a task, unlike the ammonium/ammonia buffer, which having a pKa of 9.2, is less useful for buffering the periplasm to pH values close to neutrality. The similar reactions to the one mentioned above for the periplasm will occur in the cytoplasm, i.e. hydration of CO2 by cytoplasmic hpβCA producing bicarbonate to buffer the cytoplasmic pH at ∼8.0 ()Citation59,Citation60. Another product of the hpβCA-catalysed reaction, i.e. the protons, are neutralized by NH3, the product of urease. NH3, can also neutralize entering protons, which may occur in the highly acidic condition present in the stomach. Accordingly, in cooperation with cytoplasmic urease, hpαCA and HpβCA work for acid acclimation of H. pylori in its periplasm and cytoplasm, respectively, suggesting that these CAs may be attractive drug targets for developing anti-H. pylori agents.
Figure 1. Helicobacter pylori CAs and urease and their involvement in the acid acclimation of the pathogen within the gastric mucosaCitation46.
Preliminary in vitro studies, with the potent and clinically used CAI, AAZ (KI: 21 nM for HpαCA and 40 nM for HpβCA)Citation41 from Lindskog’s groupCitation37 did not show any inhibition of bacterial growth at the concentration of 5 µM. However, in the presence of 1 mM of AAZ, there was an ∼3 log10 decrease in acid survival of H. pyloriCitation37. Nishimori’s groupCitation41 also published data showing that another CAI, methazolamide (MZA), displayed growth inhibition of certain strains of H. pylori in vitro. MZA possesses weaker inhibitory activity than AAZ but it is however a strong inhibitor of both HpαCA and HpβCA. Interestingly, the growth of the bacterial strain SS1 was totally inhibited at 100 µg/ml of MZA, but there was no inhibitory effect of the drug at this concentration on the growth of another strainCitation41, 11 637. However, at a concentration of 500 µg/ml of MZA, the growth of strain 11 637 was also inhibited. These findings indicate that CAIs suppress the growth of H. pylori, but the susceptibility to the drug greatly varies in each strainCitation41.
Conclusions
The two CAs present in H. pylori together with the urease play a central role for acid acclimation and survival in the highly acid condition of the stomach of this pathogen. Several sulfonamide CAIs have been shown to possess inhibitory effect on the growth of H. pylori in vitro, as well as inhibition of acid secretion from the parietal cells, as shown by Ioan PuşcaşCitation32. These findings strongly recommend the clinical use of CAIs as novel agents for the eradication therapy of H. pylori. This may include, of course, combination therapy of a CAI with various antibiotics. Fortunately, no serious complication of AAZ were observed so far in previous clinical studies, apart for the appearance of mild paraesthesias in the limb and around the mouth, which were frequently reportedCitation32,Citation33,Citation61. For the development of alternative eradication therapies that possess different pharmacological mechanism from those of the previously used drugs, clinical trials with a carefully designed protocol using a panel of CAIs should be warranted. Furthermore, the recent reportCitation62 of the X-ray crystal structure of HpαCA complexed with acetazolamide and methazolamide might lead to the development of bacterial CA-selective inhibitors with less affinity for the human enzymes, which might lead to anti-ulcer agents with reduced side effects when compared with AAZ. In conclusion, although the starting hypothesis for treating peptic ulcers with sulfonamide CAIs was wrong, the treatment was efficacious and this was due to inhibition of H. pylori CAs, which are involved in crucial physiologic processes in the bacterium. Finding CAIs selective for the bacterial over the human enzymes may lead to efficient treatment approaches for this disease, which was in a way pioneered, but not thoroughly explored by Ioan Puşcaş and his collaborators.
IENZ_A_1051042-supp.pdf
Download PDF (36.4 KB)Acknowledgements
G.M.B. thanks Miss Anna Szilágyi (Semmelweis University, Department of Physiology, Budapest) for collecting the Web of Science data, Mrs Jolán Józan for typesetting and secretarial assistance, and Mr Douglas Arnott (EDMF Language Services, Budapest, Hungary) for lecturing the English text.
Declaration of interest
The authors declare no financial interest.
The authors were working with Ioan Puşcaş between 1977 and 1990 (G.M.B.) and1987 and 1994 (C.T.S.), respectively.
References
- Davenport HW. In memoriam: the carbonic anhydrase theory of gastric acid secretion. Gastroenterology 1946;7:374
- Davenport HW. The early days of carbonic anhydrase. Ann NY Acad Sci 1984;429:3–4
- Sebastian A. A dictionary of the history of medicine. New York-London: The Parthenon Publishing Group; 1999: 172,368,608
- Keilin D, Mann T. Carbonic anhydrase. Nature 1939;144:442–3
- Sneader W. Drug discovery. A history. Chichester: John Wiley and Sons Ltd; 2005
- Roblin RO, Clapp JW. The preparation of heterocyclic sulfonamides. J Am Chem Soc 1950;11:4890–2
- Maren TH. Carbonic anhydrase: chemistry, physiology and inhibition. Physiol Rev 1967;47:595–781
- Swensson ER. A comparative approach to carbonic anhydrase: the work of Thomas H. Maren. Comp Biochem Physiol A Mol Integr Physiol 2003;1236:229–41
- Forster RE. Remarks on the discovery of carbonic anhydrase. In: Chegwidden WR, Carter NF, Edwards YH, eds. The carbonic anhydrases. New Horizons. Basel: Birkhauser Verlag; 2000:1–11
- Van Slyke DD, Hawkins JA. Studies on gas and electrolyte equilibrium in blood. XVI. The evolution of carbon dioxide from blood and buffer solutions. J Biol Chem 1930;87:265–79
- Meldrum NU, Roughton FJW. Carbonic anhydrase. Its preparation and properties. J Physiol (London) 1933;80:113–42
- Davenport HW, Fisher RB. The mechanism of the secretion of acid and chloride by the gastric mucosa. Am J Physiol 1940;131:165–75
- Davenport HW. A history of gastric secretion and digestion, experimental studies to 1975. New York-Oxford: Oxford University Press; 1992
- Hollander F. The composition of pure gastric juice. Am J Dig Dis 1934;1:319–29
- Teorell T. On the primary acidity of the gastric juice. J Physiol (London) 1940;97:308–15
- Davenport HW. The inhibition of carbonic anhydrase and of gastric acid secretion by thiocyanate. Am J Physiol 1940;129:505–14
- Puşcaş I, Chiu A, Buzás G, et al. Elucidation of the mechanism of gastric acid secretion. Rev Med Interne 1980;32:233–47
- Puşcaş I. Histamine and calcium interaction in the process of gastric acid secretion in humans. Rev Med Interne 1979;312:549–54
- Dobrescu D. A propos de l’action de l’histamine sur l’anhydrase carbonique. CR Seances Soc Biol Fil 1966;160:220–2
- Salganik RI, Arqutinskaya SV, Bersimbaev RI. The stimulating action of gastric pentapeptide, histamine and cyclic adenosine 3′,5′-monophosphate on carbonic anhydrase in rat stomach. Experientia 1972;28:1191–1
- Vinik AI, Heldsinger AA. Cytochemical quantification of physiologic regulation of oxyntic cell carbonic anhydrase. Ann NY Acad Sci 1984;429:592–6
- Puşcaş I, Coltău M, Baican M, Domuţa G. Omeprazole has a dual mechanism of action: it inhibits both H+K+ATPase and gastric mucosa carbonic anhydrase enzyme in humans (in vitro and in vivo experiments). J Pharmacol Exp Ther 1999;290:530–4
- Saada MC, Vulloo D, Montero JL, et al. Carbonic anhydrase I and II activation with mono- and dihalogenated histamine. Bioorg Med Chem Lett 2011;21:4884–7
- Janowitz HD, Colcher H, Hollander F. Inhibition of gastric secretion in dogs by carbonic anhydrase inhibitor 2-acetyl-amino-1,3,4-thiadiazole-5-sulfonamide. Am J Physiol 1952;171:325–30
- Janowitz HD, Dreiling DA, Roblin HL, Hollander F. Inhibition of the formation of hydrochloric acid in the human stomach by diamox: the role of carbonic anhydrase in gastric acid secretion. Gastroenterology 1957;33:378–81
- Lindner AE, Cohen N, Berkowitz J, Janowitz HD. A note on the oral dose of acetazolamide required to inhibit acid secretion in man. Gastroenterology 1964;46:273–5
- Poller L. The effect of acetazolamide, an inhibitor of carbonic anhydrase, on gastric secretion. Br J Pharmacol 1956;11:263–5
- Cheli R, Dodero M. The effect of acetazolamide on gastric secretion in normal subjects and in patients of chronic gastritis confirmed by biopsy. Minerva Gastroenterol 1956;2:120–2
- Gailitis RJ, Schreiber W. Carbonic anhydrase inhibition in the management of symptomatic peptic ulcers. Clinical studies with diamox on 125 patients. Am J Dig Dis 1960;5:473–8
- Gothe KM. Senkung der Magensaftaziditat durch gezielte Schadigung der saurebildenden Zellen.Behandlungsversuche bei peptischen Magen- und Darmerkrankungen. Med Welt 1963;46:2363–7
- Puşcaş I. Inhibition of gastric acid secretion by acetazolamide. Doctoral Thesis, Medical and Pharmaceutical Institute, Timişoara; 1971
- Puşcaş I. Treatment of gastroduodenal ulcers with carbonic anhydrase inhibitors. Ann NY Acad Sci 1984;423:587–91
- Puşcaş I, Buzás G. Treatment of duodenal ulcers with ethoxzolamide, an inhibitor of gastric mucosa carbonic anhydrase. Int J Pharmacol Ther Toxicol 1986;24:97–899
- Puşcaş I. Carbonic anhydrase inhibitors in the treatment of gastric and duodenal ulcers. In: Szabó S, Mózsik Gy, eds. New pharmacology of ulcer disease. New York: Elsevier Publishing Co.;1987: 164–79
- Supuran CT. Bacterial carbonic anhydrases as drug targets: towards novel antibiotics? Front Pharmacol 2011;2:34
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32
- Chirica LC, Petersson C, Huirtig M, et al. Expression nand localization of alpha- and beta-carbonic anhydrase in Helicobacter pylori. Biochim Biophys Acta 2002;1601:192–9
- Nishimori I, Minakuchi T, Morimoto K, et al. Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. J Med Chem 2006;49:2117–26
- Nishimori I, Vullo D, Minakuchi T, et al. Carbonic anhydrase inhibitors: cloning and sulfonamide inhibition studies of a carboxyterminal truncated alpha-carbonic anhydrase from Helicobacter pylori. Bioorg Med Chem Lett 2006;16:2182–8
- Nishimori I, Minakuchi T, Kohsaki T, et al. Carbonic anhydrase inhibitors: the beta-carbonic anhydrase from Helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. Bioorg Med Chem Lett 2007;17:3585–94
- Nishimori I, Onishi S, Takeuchi H, Supuran CT. The α- and β-classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr Pharm Des 2008;14:622–30
- Supuran CT. Novel targets against Helicobacter pylori: a bioinformatic approach. Future Microbiol 2007;2:111–14
- Maresca A, Vullo D, Scozzafava A, Supuran CT. Inhibition of the alpha- and beta-carbonic anhydrases from the gastric pathogen Helicobacter pylori with anions. J Enzyme Inhib Med Chem 2013;28:388–91
- Nishimori I, Takeuchi H, Supuran CT. Inhibition of beta- and alpha-CAs from Helicobacter pylori as potential novel gastric drugs. In: Supuran CT, Winum JY, eds. Drug design of zinc-enzyme inhibitors: functional, structural, and disease applications. Hoboken: Wiley; 2009:359–74
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnologic use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30
- Smith KS, Jakubzick C, Whittam TS, Ferry JG. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc Natl Acad Sci USA 1999;96:15184–9
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Bilginer S, Unluer E, Gul HI, et al. Carbonic anhydrase inhibitors. Phenols incorporating 2- or 3-pyridyl-ethenylcarbonyl and tertiary amine moieties strongly inhibit Saccharomyces cerevisiae β-carbonic anhydrase. J Enzyme Inhib Med Chem 2014;29:495–9
- Singh S, Supuran CT. 3D-QSAR CoMFA studies on sulfonamide inhibitors of the Rv3588c β-carbonic anhydrase from Mycobacterium tuberculosis and design of not yet synthesized new molecules. J Enzyme Inhib Med Chem 2014;29:449–55
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs-antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87
- Carta F, Scozzafava A, Supuran CT. Sulfonamides (RSO2NH2): a patent review 2008–2012. Expert Opin Ther Pat 2012;22:747–58
- Supuran CT, Maresca A, Gregáň F, Remko M. Three new aromatic sulfonamide inhibitors of carbonic anhydrases I, II, IV and XII. J Enzyme Inhib Med Chem 2013;28:289–93
- Stähler FN, Ganter L, Lederer K, et al. Mutational analysis of the Helicobacter pylori carbonic anhydrases. FEMS Immunol Med Microbiol 2005;44:183–9
- Marcus EA, Moshfegh AP, Sachs G, Scott DR. The periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol 2005;187:729–38
- Sachs G, Weeks DL, Wen Y, et al. Acid acclimation by Helicobacter pylori. Physiology (Bethesda) 2005;20:429–38
- Shahidzadeh R, Opekun A, Shiotani A, Graham DY. Effect of the carbonic anhydrase inhibitor, acetazolamide, on Helicobacter pylori infection in vivo: a pilot study. Helicobacter 2005;10:136–8
- Modak JK, Liu YC, Machuca MA, et al. Structural basis for the inhibition of Helicobacter pylori α-carbonic anhydrase by sulfonamides. PLoS One 2015;10:e0127149
Appendix
Ioan Puşcaş was born on 10 July 1932 in a humble mountain village in northern Transylvania, Romania. He completed his medical studies in the multi-ethnic town of Timişoara between 1952 and 1958. For a short period, he worked as GP in his native county, before moving to Oradea hospital from where was transferred upon request to the Department of Internal Medicine of Şimleu Silvaniei Hospital in 1962. Between 1962 and 1966, he worked beside István Mártonfy (1893–1966), one of the last medical polymaths of his time. In 1968, Ioan Puşcaş observed that in chronic obstructive pulmonary disease patients treated with acetazolamide as a diuretic, peptic ulcer pain rapidly ceased and the ulcers healed radiologically within 2–3 weeks. He started to study this problem in detail and over a few some years, he developed a self-standing department of internal medicine and gastroenterology, dedicated almost entirely to the diagnosis and treatment of peptic ulcer disease with CAIs. Şimleu Silvaniei became a kind of Mecca for ulcer patients from all over the country: during its peak periods, some 5000 patients were treated yearly. In the meantime, much experimental and clinical research was conducted and he obtained his PhD degree in 1971. He patented the drug Ulcosilvanil, containing acetazolamide and an electrolyte mixture, produced and marketed by the Drug Factory Bucureşti. Attempts were made to register the drug abroad, especially in Hungary and the Soviet Union, but without success. Foreign endoscopy training was received at the Bichat Hospital in Paris and Leiden University Clinic. He was especially interested for improving diagnostic possibilities, so his department was one of first to be equipped with fibrescopes (1974), abdominal ultrasound (1983), optical camera endoscope (1980) and videoendoscope (1986) in Romania. Although he never pursued any political activity, this bigger-than-life personality had an innate ability to communicate with the authorities of his time (party, police, army and court of justice) at local, county and even national level. He was able to attend most of the World Congresses of Gastroenterology between 1972 and 1998 (Mexico City, Buenos Aires, Madrid, Sao Paolo, Stockholm and Vienna), where he used to take some carefully selected co-workers with him, presenting 6–12 lectures/posters at one event: he never learned English and continuous translation was necessary. This was a great achievement in a period of dictatorship and severe curtailments in Western trips. At a time when scientific information was restricted, subscriptions for some Western gastroenterology journals and the weekly Current Content were all available. Another important achievement was his support for the construction of a new, 400-bed county hospital with departments of internal medicine, gastroenterology, surgery and paediatrics, also realized in a period of marked economic decay. Journalists claim he authored more than 600 publications: most of them, however, are congress abstracts, as presented in . He and his co-workers wrote some books on peptic ulcer and CA, published in Romania and book chapters published in Hungary, USA and Brazil. In 1985, he organized an international symposium of “Progress in the Pathophysiology and Treatment of Gastric and Duodenal Ulcers”, attended by leading specialists from all over the world. In 1987, he was appointed director of the new hospital and in 1990, leader of the Centre for Healthcare and Research. He was also professor of gastroenterology at the Faculty of Medicine of the newly established University Vasile Goldiş in Oradea, teaching doctoral fellows. He had 42 patents (drug compositions and diagnostic procedures), but apart from Ulcosilvanil, no one was realized and marketed as product. Doctor Honoris Causa and a member of the United States Academy of Sciences, the hospital Şimleu Silvaniei took his name in his lifetime. In 1988, he was successfully operated on in Vienna for malignant prostate tumour. He died on 4 April 2015 in his hospital, after a short course of renal failure. He married twice and had two children. His daughter, Carmen, continues his work and one of his four nephews has recently started his medical studies.
Supplementary material available online. Supplementary Table.
