Abstract
Objective. Increased amniotic fluid concentrations of anti-microbial peptides, components of the innate immune system, have been reported in patients with preterm labor (PTL) with intact membranes and intra-amniotic infection and/or inflammation (IAI), as well as in patients with preterm prelabor rupture of the membranes (PPROM). This study was designed to confirm these results using a targeted approach, detecting DEFA1, DEFB1, GNLY, and S100A9 gene expression in the choriamniotic membranes in pregnancies complicated with PTL and intact membranes or PPROM, with and without histologic chorioamnionitis.
Study design. Human fetal membranes were obtained from patients in the following groups: (1) PTL with intact membranes (n = 15); (2) PTL with intact membranes with histologic chorioamnionitis (n = 12); (3) PPROM (n = 17); and (4) PPROM with histologic chorioamnionitis (n = 21). The mRNA expression of α-defensin-1, β-defensin-1, calgranulin B and granulysin in the fetal membranes was determined by qRT-PCR.
Results. (1) The expression of α-defensin-1 mRNA in the fetal membranes was higher in patients with PTL and intact membranes with histologic chorioamnionitis, than those without chorioamnionitis (19.4-fold, p < 0.001); (2) Among patients with histologic chorioamnionitis, patients with PTL and intact membranes had a higher α-defensin-1 mRNA expression than those with PPROM (5.5-fold, p = 0.003); (3) Histologic chorioamnionitis was associated with a higher calgranulin B mRNA expression in the chorioamniotic membranes of patients with both PTL and intact membranes (7.9-fold, p = 0.03) and PPROM (7.6-fold, p < 0.0001); (4) The expression of calgranulin B mRNA in the fetal membranes was higher in patients with PTL and intact membranes without histologic chorioamnionitis than in those with PPROM without histologic chorioamnionitis (2.7-fold, p = 0.03); (5) There were no differences in the expression of β-defensin-1 and granulysin in the chorioamniotic membranes between the study groups even in the presence of histologic chorioamnioniotis.
Conclusions. (1) Among patients with histologic chorioamnionitis, the mRNA expression of α-defensin-1 and calgranulin B in the fetal membranes of patients with PTL and intact membranes as well as that of calgranulin B in the fetal membranes of patients with PPROM is higher than in the membranes of those without histologic chorioamnionitis; (2) histologic chorioamnionitis is associated with differences in the pattern of α-defensin-1 mRNA expression in the fetal membranes in patients with PTL and intact membranes and those with PPROM.
Introduction
The traditional view is that the amniotic cavity in normal pregnancy is sterile and does not contain viable bacteria [Citation1] despite the presence of a large number of microorganisms in the lower genital tract (vagina and ectocervix). The sterile status of the amniotic cavity is presumably accomplished by the participation of the innate immune system, including the cervical mucous plug [Citation2–5], chorioamniotic membranes [Citation6–8] and cellular components of the decidua, amnion, and chorion, including neutrophils, macrophages, natural killer (NK) cells, and trophoblasts [Citation6,Citation9,Citation10].
Natural anti-microbial peptides have been identified in plants, insects, and vertebrates [Citation11] as part of the innate limb of the immune system that provides protection against bacteria, yeast, and viruses [Citation11–13]. In humans, anti-microbial peptides have been detected in white blood cells and [Citation14,Citation15] epithelial cells [Citation11,Citation16–18], as well as in the placenta [Citation9,Citation19], decidua, fetal membranes [Citation16,Citation20] and amniotic fluid [Citation1,Citation21]. The latter contains defensins, bactericidal/permeability-increasing (BPI) protein, and S100B [Citation22] as well as other proteins, such as lactoferrin and calprotectin (MRP8/14) [Citation21].
Defensins are anti-microbial peptides classified into three major groups: alpha (α), beta (β), and theta (θ) [Citation23]. α-defensins have a broad anti-microbial activity against Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses [Citation14,Citation23–25]. These anti-microbial peptides interact with the cell membrane of invading organisms, causing a disruption of ion-fluxes and eventually leading to cell lysis [Citation14,Citation23–25]. The group of α-defensins consists of six distinct peptides, of which α-defensins-1, -2, and -3 share many similarities as their primary structure differs by only one amino acid [Citation12,Citation26–28]. Bone marrow precursors of neutrophils synthesize and store these anti-microbial peptides intracellularly in azurophil granules [Citation12,Citation28–32]. Thus, α-defensins are often referred to as human neutrophil peptides (HNP)-1, -2, and -3 [Citation14,Citation33]. In addition to their anti-microbial activity, α-defensins are capable of stimulating a systemic inflammatory response as well as to chemo-attract T-cells and induce histamine release from mast cells [Citation34–37]. β-defensins are mainly effective against Gram-negative bacteria and yeast, while some have also microbial activity against Gram-positive bacteria [Citation38–40]. Human β-defensin-1 has anti-microbial properties against Gram-positive and Gram-negative bacteria [Citation39–42], as well as adenovirus [Citation43]. Calgranulin B (MRP14, S100A9) is an additional anti-microbial peptide that forms calprotectin (MRP8/14) heterodimer with calgranulin A (MRP8, S100A8) [Citation44]. Calgranulin B can be detected in neutrophils, monocytes and activated macrophages, as well as in endothelial and epithelial cells [Citation44–51]. Calprotectin regulates the adhesion of myeloid cells to the vascular endothelium and to the extracellular matrix, controlling the activation of these effector cells and their direct anti-bacterial effect by zinc-capturing [Citation44].
Granulysin, a 9 kD protein [Citation52] secreted from cytolytic granules of cytotoxic T-lymphocytes and NK cells [Citation53–56], is effective against Gram-positive and Gram-negative bacteria, as well as fungi [Citation55] and mycobacteria [Citation55]. Its anti-microbial activity is mediated through the induction of an increase in intracellular calcium and the efflux of intracellular potassium into the pathogen, leading to the activation of sphingomyelinase and the ceramide pathway, as well as mitochondrial damage by the activation of caspases and, consequently, apoptosis [Citation57–59].
Term parturition is associated with both an inflammatory response and the activation of the three clinically manifested components of the common pathway of parturition, including uterine contraction, cervical dilatation, and decidual/membranes activation [Citation60–62]. Our group demonstrated that each of these components has a distinct transcriptome during labor at term [Citation20,Citation63,Citation64]. Moreover, microarray experiments have revealed that human term labor is characterized by an acute inflammation gene expression signature in the extraplacental membranes, which includes the differential expression of multiple genes encoding for cytokines and chemokines known to orchestrate acute inflammatory response [Citation65].
Intrauterine infection and/or inflammation (IAI) can activate the common pathway of parturition, and is a major cause of preterm labor (PTL) and delivery [Citation66–69]. Microbial invasion of the amniotic cavity (MIAC), spontaneous PTL and preterm prelabor rupture of the membranes (PPROM) are associated with increased intra-amniotic concentrations of α-defensins, BPI, calprotectin, β-defensin-2 [Citation1,Citation21], and S100B [Citation22]. African-American women with elevated HNP-1–3 concentrations in vaginal fluid at mid pregnancy (15–27 weeks of gestation) had an increased risk for spontaneous preterm birth at 32–36 weeks (OR 2.4, 95% CI 1.2–4.7) after adjustment for maternal age, gestational age at enrollment, and bacterial vaginosis [Citation70]. Calgranulin B (S100A9), was differentially expressed in the transcriptome of chorioamniotic membranes of women with preterm deliveries. Thus, this study was designed to determine by RT-PCR changes in the chorioamniotic expression of the mRNA for S100A9 and additional genes encoding for the following anti-microbial peptides α-defensin-1 (DEFA1), β-defensin-1 (DEFB1), and granulysin (GNLY), of patients with PTL with intact membranes and PPROM with and without histologic chorioamnionitis.
Materials and methods
Study design and population
The basis for the current study are the results of a previous microarray study that was completed during March–April 2001, in which calgranulin B (S100A9) was differentially expressed in the transcriptome of chorioamniotic membranes of women with histologic chorioamnionitis who had either PTL with intact membranes or PPROM. This confirmatory RT-PCR cross-sectional study was designed to investigate the differential expression of the DEFA1, DEFB1, GNLY, and S100A9 genes in the fetal membranes of patients in the following groups: (1) PTL with intact membranes without histologic chorioamnionitis (n = 15); (2) PTL with intact membranes with histologic chorioamnionitis (n = 12); (3) PPROM without histologic chorioamnionitis (n = 17); and (4) PPROM with histologic chorioamnionitis (n = 21). Patients presenting with medical complications, multiple pregnancies, and fetal chromosomal or congenital abnormalities were excluded. All patients provided written informed consent prior to the collection of samples. The collection and utilization of samples for research purposes was approved by the Institutional Review Boards of both the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS) and Wayne State University. Many of these samples have been employed to study the biology of PTL and the inflammation of the fetal membranes.
Definitions
Spontaneous PTL with intact membranes was defined as the presence of regular uterine contractions that occurred at a frequency of at least 2 in every 10 minutes and associated with cervical changes which led to spontaneous preterm delivery (<37 weeks of gestation) [Citation60,Citation62]. PPROM was defined as the prelabor rupture of membranes occurring <37 weeks of gestation, diagnosed by speculum examination of vaginal pooling, nitrazine, and ferning tests [Citation71]. Amniocentesis was performed at the discretion of the treating physician. Amniotic fluid was analyzed for the assessment of the microbial state of the amniotic cavity. Amniotic fluid was cultured for aerobic and anaerobic bacteria, as well as for genital mycoplasmas.
Placental histopathologic examinations
Chorioamniotic membranes containing attached maternal decidua were obtained from placentas delivered by spontaneous labor or cesarean section at the Hutzel Women's Hospital (Wayne State University, Detroit, MI). Fetal membranes were fixed in 10% neutral buffered formalin overnight and embedded in paraffin. Five micrometer paraffin sections were stained with hematoxylin and eosin then examined using bright-field light microscopy. Histopathologic examinations were performed by pathologists blinded to the clinical information based on the diagnostic criteria previously described [Citation72]. Histologic chorioamnionitis was diagnosed in the presence of acute inflammation using previously described criteria [Citation73,Citation74].
Total RNA extraction
The membranes were dissected from the placentas, rinsed thoroughly with a sterile ice-cold phosphate buffered saline solution (Sigma Chemical Company, St Louis, MO), cut into small pieces, placed in RNA later solution (Ambion, Austin, Texas), and stored at +4°C for no longer than 2 weeks. Total RNA was isolated with a modification of the standard guanidinium isothiocyanate-cesium chloride method [Citation20]. Briefly, tissues were homogenized with a PRO200 rotor-stator homogenizer (Pro Scientific, Monroe, CT) in the presence of 4 mol/l guanidinium isothiocyanate, 0.1 mol/l mercaptoethanol, 0.5% sarkosyl, and 5 mmol/l sodium citrate (pH 7); solid CsCl was added to the sample (final concentration, 0.25 g/ml), and the samples were pelleted by ultracentrifugation, according to the protocol. RNA pellets were resuspended and extracted with chloroform:isoamylalcohol, and the RNA was precipitated with ethanol and glycogen (Roche Molecular Biochemicals, Indianapolis, IN) as a carrier. Before the first use, the RNA was pelleted and resuspended in water that contained RNasin (Promega Corp, Madison, WI).
Quantitative real-time reverse transcription-polymerase chain reaction
Total RNA (2.5 μg) from each sample and a positive control sample was reverse transcribed using Superscript II reverse transcriptase, random hexamer primers, and oligo(dT) primers (Invitrogen Life Technologies, Rockville, MD). The standard curve was run with the DEFA1, DEFB1, GNLY, and S100A9 genes and the 18S ribosomal RNA housekeeping gene to determine the quantity of cDNA needed for an approximate cycle threshold (Ct) of 25. Subsequently, cDNA derived from an equivalent of 75 ng RNA from each sample were run in triplicate on 96-well plates to obtain technical replicates for the target and reference assays. A calibrator sample was run in triplicate in all plates to account for plate effects. In addition, a negative control containing no RNA and 12.5 ng of human genomic DNA were also tested in duplicates. Samples from the study groups were randomly allocated on the plates; the DEFA1, DEFB1, GNLY, S100A9, and 18S rRNA assays were run with the same allocation on the parallel plates. The qPCR reactions were assembled based on the TaqMan Universal PCR Master Mix protocol (Applied Biosystems) using the 18S rRNA TaqMan gene expression assay (Hs99999901_s1; Applied Biosystems, Foster City, CA) for the quantification of the housekeeping gene and self-designed primers and probe for the target genes (DEFA1, forward primer: 5′-CCCAGAAGTGGTTGTTTCCCT-3′; reverse primer: 5′-TTTTCCTTGAGCCTGGATGCT-3′; probe: 5′-TGGAGCCAAGCTTTCGTCCCATG-3′; DEFB1, forward primer: 5′-ATTGCGTCAGCAGTGGAGG-3′; reverse primer: 5′-AACAGGTGCCTTGAATTTTGGT-3′; probe: 5′-CAATGTCTCTATTCTGCCTGCCCGATCTT-3′; GNLY, forward primer: 5′-AGCAACCTCTGCCGGCT-3′; reverse primer: 5′-GACAGCAGAGGGAGTCAGGG-3′; probe: 5′-CTTCCTCGATCCAGAATCCACTCTCCAGTCT-3′; S100A9, forward primer: 5′-CAGCTGAGCTTCGAGGAGTTC-3′; reverse primer: 5′-GCATCTTCTCGTGGGAGGC-3′; probe: 5′-CAGGTTAGCCTCGCCATCAGCATGA-3′). Data were collected by the ABI Prism 7700 Sequence Detection System (Applied Biosystems).
Statistical analysis
Demographic and clinical characteristics of the study groups were compared using the Pearson's chi-square test and the Fisher's exact test for proportions, and the Mann–Whitney U test for non-normally distributed continuous variables using SPSS version 12.0 (SPSS, Chicago, IL). Quantitative RT-PCR data were analyzed using the R statistical software.
Gene expression levels were profiled in multiple sample groups (term not in labor, term in labor, PPROM without histologic chorioamniotis, PPROM with histologic chorioamnionitis, PTL without histologic chorioamniotis, PTL with histologic chorioamniotis) by qRT-PCR experiments, using between 6 and 29 samples per group. The RT reactions were run on 96-well plates. Samples from the study groups were randomly allocated on the plates, and only one target gene and the 18S reference assay were run in parallel on each given plate. Each reaction was repeated either two or three times to obtain technical replicates for both the target assay and the reference assay. A calibrator patient sample was placed on all plates to account for eventual plate effects. Briefly, the δ-δ method [Citation75,Citation76] was used to generate an outcome variable, Y, which is a surrogate of the log2 concentration of the target gene in each patient sample, corrected already for potential plate effects. A linear model was employed in which Y values were fitted using the Group variable and the gestational age as predictors without including the interaction term between these two variables. The coefficients of the two predictors in the linear model were estimated together with their significant p-values.The outcome variable, Y, included also a positive constant to render the Y values positive so that larger values correspond to higher expression. A False Discovery Rate adjustment [Citation76] of resulting p-values was performed to account for all parallel tests. For each pair-wise comparison, the Group effect was considered significant, if the adjusted p-values were <0.05 and the magnitude of change was at least 2-fold (one Ct unit difference). For the gestational age effect, adjusted p-values < 0.05 were considered significant.
Results
Demographic, clinical and histopathological data
Demographic and clinical characteristics of the study groups are displayed in . The diagnosis of histologic chorioamnionitis was based on the presence of maternal and/or fetal inflammatory response in the placenta and fetal membranes. Among the 12 patients with PTL intact membranes and histologic chorioamnionitis, maternal inflammatory response was diagnosed in one case, whereas 11 patients had both a maternal and a fetal inflammatory response. Among the 21 patients with PPROM and histologic chorioamnionitis, 7 had a maternal inflammatory response, 2 had a fetal inflammatory response, and 12 had both. Amniocentesis was performed in 10 patients with PTL intact membranes and 14 patients with PPROM. A positive amniotic fluid culture was detected in 30% (3/10) of patients with PTL intact membranes and in 46.2% (6/14) of patients with PPROM (p = 0.4). The microorganisms found in amniotic fluid cultures are presented in . Within the study groups, there was no correlation between the chorioamniotic expression of the DEFA1, DEFB1, GNLY, and S100A9 genes and gestational age at delivery, in which these samples were collected (data not shown).
Table I. Demographic and clinical characteristics of the study groups.
Table II. Microorganisms detected in positive amniotic fluid cultures.
Changes in the fetal membranes mRNA expression of anti-microbial peptides
α-defensin (Human neutrophil peptide)-1
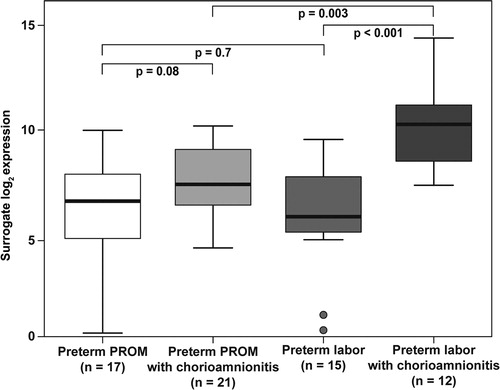
Among patients with PTL and intact membranes, histologic chorioamnionitis was associated with a higher α-defensin-1mRNA expression in the chorioamniotic membranes (19.4-fold, p < 0.001). This difference did not reach statistical significance in those with PPROM (2.7-fold, p = 0.08) (). Among women with histologic chorioamnionitis, patients with PTL with intact membranes had a higher amount of α-defensin-1 mRNA expression in the fetal membranes than those with PPROM (5.5-fold, p = 0.003) ().
Figure 1. α-defensin 1 mRNA expression in the fetal membranes of patients with spontaneous preterm labor (PTL) or preterm prelabor rupture of membranes (PPROM). In the presence of histologic chorioamnionitis, there was an increased expression among patients with preterm labor with intact membranes (19.4-fold, p < 0.001) or PPROM (2.7-fold, p = 0.08). The amount of α-defensin 1 mRNA was higher in the fetal membranes of patients presenting with preterm labor with intact membranes and histologic chorioamnionitis than in patients with PPROM and histologic chorioamnionitis (5.5-fold, p = 0.003).
β-defensin-1
Among patients with PTL with intact membranes and those with PPROM, histologic chorioamnionitis was not associated with a higher β-defensin-1 mRNA expression in the chorioamniotic membranes (p = 0.2 for both comparisons). Moreover, the expression of β-defensin-1 mRNA in the fetal membranes did not differ between patients presenting with PTL with intact membranes and those presenting with PPROM, regardless of the presence of histologic chorioamnionitis (without chorioamnionitis: p = 0.2; with chorioamnionitis: p = 0.2).
Granulysin
There was no correlation between granulysin mRNA expression level and histologic chorioamnionitis in membranes from either patients with PTL and intact membranes or from patients with PPROM (p = 0.2 for both comparisons). Moreover, the expression of granulysin mRNA in the fetal membranes did not differ between patients presenting with PTL and intact membranes and those with PPROM, irrespective of the presence of histologic chorioamnionitis (no chorioamnionitis: p = 0.2; chorioamnionitis: p = 0.2).
Calgranulin B
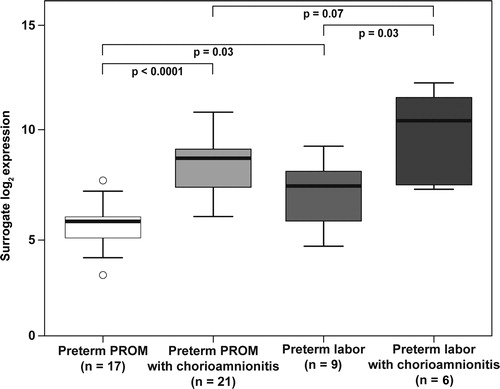
The expression of calgranulin B mRNA was significantly higher in the fetal membranes of patients with histologic chorioamnionitis, regardless of whether the patients had PTL with intact membranes or PPROM (PTL 7.9-fold, p = 0.03 and PPROM 7.6-fold, p < 0.0001) patients (). The expression of calgranulin B mRNA was higher in the fetal membranes of patients presenting with PTL intact membranes without chorioamnionitis than in those with PPROM in the absence of histologic chorioamnionitis (2.7-fold, p = 0.03). This difference was not significant in the presence of histologic chorioamnionitis (p = 0.07).
Figure 2. Calgranulin B mRNA expression in the fetal membranes of patients with spontaneous preterm labor intact membranes or preterm prelabor rupture of membranes (PPROM). The expression was increased in patients with preterm labor intact membranes (7.9-fold, p = 0.03) or in those with PPROM (7.6-fold, p < 0.0001) when histologic chorioamnionitis was present. The amount of calgranulin B mRNA was higher in the fetal membranes of patients presenting with preterm labor with intact membranes without histologic chorioamnionitis than in those with PPROM without histologic chorioamnionitis (2.7-fold, p = 0.03). There was no difference in calgranulin B mRNA expression in the fetal membranes between patients with preterm labor with intact membranes or PPROM when histologic chorioamnionitis was present (p = 0.07).
Discussion
Principal findings of this study
(1) The expression of DEFA1 in the chorioamniotic membranes was higher in patients with histologic chorioamnionitis than in those without chorioamnionitis. Moreover, among patients with histologic chorioamnionitis, those with PTL with intact membranes had a higher DEFA1 expression than those with PPROM; (2) the expression of S100A9 was higher in patients with histologic chorioamnionitis than in those without histologic chorioamnionitis, regardless of membrane status; (3) unlike DEFA1, the expression of S100A9 in the fetal membranes was higher in women with PTL with intact membranes than in those with PPROM only in patients without histologic chorioamnionitis; and (4) there are no differences in the expression of DEFB1 and GNLY in the chorioamniotic membranes between the study groups regardless of the presence of chorioamnioniotis.
High-dimensional biology in the study of pregnancy complications
The current study confirmed, with real-time PCR, the observations made by microarray studies by our group and presented differences in the expression pattern of S100A9, as well as other anti-microbial peptides among patients with PTL with intact membranes and those with PPROM. The use of high-dimensional biology is a promising and evolving field in obsterics [Citation77,Citation78]. Indeed, our group previously demonstrated a distinctive difference in the transcriptome of the chorioamniotic membranes of patients with PTL with intact membranes and PPROM, with and without intra-amniotic infection/inflammation [Citation20]. However, the major concern of the omics studies is the report of false positive results. The large number of mRNAs tested upon a single chip can currently reach 50,000 transcripts resulting in a high number of comparisons that increases the risk for type I error [Citation79,Citation80]. Therefore, these genomics studies can be considered as hypothesis-generating with less stringent p-values and the results of the microarray analysis need to be validated in a targeted approach such as by quantitative real-time RT-PCR.
What are anti-microbial peptides?
The innate component of the immune system applies ancient and highly conserved mechanisms of defense against foreign antigens [Citation81–85]. This innate system provides immediate protection for the host against microbial challenge by recognizing the presence of microorganisms and preventing their tissue invasion, thus limiting microbial proliferation and inflammation [Citation81–85]. The innate immune system recognizes microbes through cellular elements (such as neutrophils and macrophages) and pattern-recognition molecules (such as the Toll-like receptors). Anti-microbial peptides may serve as a line of defense [Citation81–85]. Defensins are a family of anti-microbial peptides classified into three major groups: alpha (α), beta (β), and theta (θ) [Citation23,Citation86,Citation87] and the genes encoding for α- and β-defensins are located in a tight cluster on chromosome 8p23 [Citation88]. These peptides have a broad anti-microbial activity against Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses [Citation14,Citation23–25]. The group of α–defensins (HNP) consists of six distinct peptides, of which α-defensins-1, -2, and -3 share many similarities, as their primary structure differs by only one amino acid [Citation12,Citation26–28]. Bone marrow precursors of neutrophils synthesized and stored these anti-microbial peptides intracellularly in azurophil granules [Citation12,Citation28–32]. Because of their origin, α-defensins are often referred to as HNP-1, -2 and -3 [Citation14,Citation33]. In addition to their anti-microbial activity, α-defensins are capable of stimulating a systemic inflammatory response as well as to chemo-attract T-cells and induce histamine release from mast cells [Citation34–37]. Human β-defensins are slightly longer peptides than α-defensins. They are mainly effective against Gram-negative bacteria and yeast, though some are also effective against Gram-positive bacteria [Citation38–40,Citation89]. Human β-defensin-1, first discovered in 1995 [Citation90], is expressed by the epithelium of the urinary and respiratory tracts [Citation39,Citation41,Citation42]. Moreover, its expression is modulated by inflammation [Citation91–95] and can be induced by lipopolysaccharide (LPS), heat inactivated Pseudomonas aeruginosa, as well as interferon gamma (IFNγ) [Citation91–93,Citation95,Citation96]. Human β-defensin-1 displays anti-microbial activity against Gram-negative bacteria and fungi, but is relatively less potent against Gram-positive bacteria [Citation38–40]. In addition to its anti-microbial properties, human β-defensin-1 can recruit immature dentritic cells and memory T-cells [Citation97], thus facilitating foreign antigen presentation and generation of specific immune response [Citation98–100]. Moreover, human β-defensin-1 can induce the production of pro-inflammatory cytokines (e.g. interleukin (IL)-8, IL-18, and IL-20) [Citation98–100].
Calgranulin B is part of the S100 family of calcium binding proteins [Citation44]. It is an additional anti-microbial peptide that composes the calprotectin heterodimer together with calgranulin A, and can be detected in neutrophils, monocytes and activated macrophages, as well as in endothelial and epithelial cells [Citation44–51]. Calgranulin B has a candidastatic effect in a zinc-rich medium, an effect abrogated by calgranulin A [Citation101]. In contrast, a recent report suggested that calgranulin A is the active component of calprotectin while calgranulin B seems to regulate calgranulin A function [Citation102]. Moreover, deletion of the mouse calgranulin A gene results in an embryonically lethal phenotype [Citation103], while mice homozygous for the targeted deletion of S100a9 (S100a9−/−) gene protects mice model against LPS-induced shock [Citation102], but the authors attributed this effect to the fact that calgranulin A is highly dependent of calgranulin B and is almost undetectable in the plasma of S100a9−/− mice despite normal mRNA levels of S100a8 [Citation102]. Thus, the calprotectin heterodimer of calgranulin A and calgranulin B is regarded as the active form [Citation104]. This heterodimer regulates both the adhesion of myeloid cells to the endothelium and extracellular matrix, and the activation of effector cells and their direct anti-bacterial effect by capturing zinc. Calprotectin concentrations of 50–250 μg/ml can inhibit the growth of Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis; however, concentrations as low as 4–32 μg/ml can already inhibit the growth of Candida albicans [Citation101]. In addition, the expression of calprotectin assists the cells against the invasion of Listeria monocytogenes, Salmonella enterica serovar typhimurium [Citation105], suggesting that this polypeptide complex may serve as a defense mechanism against microbial invasion. This is relevant to our study because the fetal membranes serve as barriers against invading microorganisms from the maternal decidua and lower genital tract, and calprotectin may play an important role in this mechanism.
The fourth anti-microbial peptide explored in this study is granulysin. This protein is secreted in cytolytic granules from cytotoxic T-lymphocytes and NK cells [Citation53–56]. It is a 9 kD protein with four α-helices connected by a short loop [Citation52]. Recombinant human granulysin is effective against Gram-positive and Gram-negative bacteria, as well as fungi, Mycobacterium tuberculosis, Mycobacterium leprae, Cryptococcus neoformans, and Plasmodium falciparum [Citation55]. In addition, granulysin induces apoptosis in varicella infected cells and blocks viral replication in vitro [Citation55,Citation106–110]. The anti-microbial activity of the positively charged granulysin is mediated through its attachment to the negatively charged phospholipids of the invading pathogens and the subsequent induction of a coupled increase in intracellular calcium and efflux of intracellular potassium, leading to the activation of sphingomyelinase, generation of ceramide, and mitochondrial damage by the activation of caspases, leading to apoptosis [Citation57–59].
Innate immune and anti-microbial peptides in the reproductive system in the non-pregnant and pregnant state
Anti-microbial peptides are prevalent in the female reproductive tract, and their concentrations change in the different stages of the menstural cycle [Citation111,Citation112]. Indeed, the highest endometrial mRNA expression of β-defensin-1 is during the mid-secretory phase, of granulysin in the late secretory phase, and of β-defensin-2 during menstruation [Citation111,Citation112]. Treatment with hormonal contraceptives was associated with lower endometrial expression of these anti-microbial peptides [Citation113], suggesting that the fine-tuning of the innate immune response in the female reproductive tract may be under hormonal regulation.
The need to protect the uppper genital tract from ascending infection is crucial during pregnancy. Indeed, multiple mechanisms of defense against infection are thought to protect pregnancy. Normally, epithelia represent more than a physical barrier against microorganisms as most epithelia produce natural anti-microbial peptides (e.g. defensins, surfactant proteins) which can kill bacteria by damaging their cell membrane or facilitating phagocytosis [Citation14,Citation23–25]. However, evidence suggests that bacteria can gain access to the amniotic cavity by penetrating intact chorioamniotic membranes [Citation114,Citation115], or by transplacental passage in cases of hematogenous dissemination (bacteremia in the context of periodontal disease [Citation116–120] or other distant infections [Citation121]). Thus, the control of microbial proliferation and the destruction of such microorganisms are required to maximize the likelihood of a normal pregnancy outcome. Indeed, anti-microbial peptides are present in the chorioamniotic membranes, decidua, and placenta [Citation9,Citation19], and the mRNA expression of human β-defensin-2 and elafin (an inhibitor of neutrophil elastase with anti-microbial activity) was shown to be up-regulated by IL-1β in primary trophoblast cell culture [Citation19]. Multiple anti-microbial peptides are also present in the amniotic fluid: lactoferrin [Citation122–124], lysozyme [Citation124–128], BPI [Citation21], calprotectin (MRP8/14) [Citation21], LL-37 [Citation125], and α-defensins-1–3 [Citation21,Citation124,Citation125,Citation129]. We have previously reported that human β-defensin-2 is a physiological constituent of amniotic fluid, and its amniotic fluid concentration increases in patients with preterm delivery and MIAC (with either intact or ruptured membranes), as well as in those with PTL and intra-amniotic inflammation [Citation1]. The combination of several anti-microbial peptides enhances microbial killing. For example, there is evidence that human β-defensin-2 can act synergistically with LL-37 to kill Group B Streptococci (GBS) [Citation130]. While LL-37 alone has a minimal bactericidal concentration (MBC) of 16 μM and human β-defensin-2 alone has an MBC of 8 μM against GBS, the combination of these two peptides effectively reduces their MBC; and at a concentration of 4 μM each, they kill 100% of GBS [Citation130]. These studies were conducted in hypotonic media, which maximizes the anti-microbial action of both anti-microbial peptides [Citation130]. This observation is relevant since LL-37 is present in amniotic fluid [Citation125], Moreover, the minimal inhibitory concentration of human β-defensin-2 against E. coli, P. aeruginosa, and E. faecalis is reduced in the presence of lactoferrin or lysozyme [Citation89]. Collectively, this evidence indicates that the apparent redundancy in anti-microbial peptides and proteins in the amniotic fluid is aimed to maximize anti-microbial activity.
The chorioamniotic membrane is an important barrier against microbial invasion of the amniotic fluid and fetal infection. These membranes isolate the sterile intra-amniotic environment from the contaminated uterine and extra-uterine environment [Citation131]. Indeed, increased mRNA expression of human β-defensins-1–3, secretory leukocyte protease inhibitor, and elafin (also PI3 or SKALP) were reported in primary cultures of amnion cells [Citation131]. Moreover, the administration of IL-1β stimulated the expression of human β-defensins-2 by these cells [Citation131], supporting the anti-microbial role of these peptides in the fetal membranes.
The anti-microbial peptide profile of the fetal membranes of patients with preterm labor and prelabor rupture of the membranes
The findings of the current study demonstrate a distinctive pattern of anti-microbial peptide expression in the fetal membranes of patients with PTL intact membranes and those with PPROM, especially in the presence of histologic chorioamnionitis. Among patients with PTL intact membranes, but not among those with PPROM, the expression of the DEFA1 gene was higher in the presence of histologic chorioamnionitis. Moreover, among patients with histologic chorioamnionitis, DEFA1 gene expression was higher in women with PTL intact membranes than in those with PPROM. Of interest, the expression of the S100A9 gene was higher in the presence of histologic chorioamnionitis in both study groups. Nevertheless, in the absence of histologic chorioamnionitis, patients with PTL intact membranes had a higher S100A9 mRNA expression than those with PPROM. These findings also suggest that the patients with PTL intact membranes have a different pattern of anti-microbial peptide expression in the fetal membrane than those with PPROM, and this pattern changes according to the presence of histologic chorioamnionitis. Collectively, the results presented in our study raise several questions. For example, could the profile of anti-microbial peptide expression by the chorioamniotic membranes be associated with an increased risk for PPROM? Also, is a higher DEFA1 expression in the fetal membranes during chorioamnionitis or an increased S100A9 gene expression in the abscence of histologic chorioamnionitis nescessary to maintain the integrity of the chorioamniotic membranes? Previous studies reported that intra-amniotic infection was associated with a significant increase in amniotic fluid concentrations of immunoreactive α-defensins-1–3 [Citation21,Citation129], BPI [Citation21], calprotectin [Citation21,Citation132] and S100B, both in women with PTL with intact membranes and in women with PPROM [Citation21,Citation22]. Parturition at term was associated with a significant increase in amniotic fluid concentrations of immunoreactive α-defensins-1–3 [Citation21]. Among patients with PTL and intact membranes, the elevation of amniotic fluid concentrations of α-defensins-1–3, BPI, calprotectin [Citation21,Citation132] and S100B [Citation22] was associated with intra-amniotic inflammation, histologic chorioamnionitis, and a shorter diagnosis-to-delivery interval. In addition, proteomic analysis of amniotic fluid from Rhesus monkeys [Citation133] and humans [Citation133,Citation134] with IAI identified calgranulin B [Citation133,Citation134] and a fragment of insulin-like growth factor binding protein 1 [Citation133] were differentially expressed. Thus, the present study supports the role of α-defensin-1 and calgranulin B in the host defense in PTL with intact membranes and of calgranulin B in PPROM during IAI through their increased expression in the fetal membranes and higher intra-amniotic concentrations.
Further attention has to be given to the role of α-defensins in the fetal response to infection and perhaps to the integrity of the fetal membranes. Evidence in support of this view are: (1) among patients with IAI, those with PTL intact membranes had a higher median intra-amniotic α-defensins concentration than those with PPROM [Citation21]; and (2) among patients with histologic chorioamnionitis, those with PTL intact membranes had a higher mRNA expression of α-defensin 1 in the fetal membranes than those with PPROM. In contrast to α-defensin 1, patients with PTL had a higher calgranulin B mRNA expression in the fetal membranes than those with PPROM, only among women without histologic chorioamnionitis. These suggest that the increased calgranulin B expression in the fetal membranes and its higher intra-amniotic concentrations during infection/inflammation may be associated with the inflammatory response of the fetal membranes and regardless to the clinical presentation (PTL intact membranes or PPROM). Moreover, in contrast to the higher concentrations of calprotectin in the amniotic fluid of patients with PPROM than in those with PTL intact membranes [Citation21], there was no difference in calgranulin B mRNA expression in the fetal membranes of patients with histologic chorioamnionitis, either with PTL intact membranes or PPROM. Thus, further study is needed in order to elucidate the implication of the differences in the expression of calgranulin B mRNA in the fetal membranes, among patients with PTL intact membranes without histologic chorioamnionitis.
Conclusions
(1) Among patients with histologic chorioamnionitis, the mRNA expression of a-defensin-1 and calgranulin B in the fetal membranes of patients with PTL and intact membranes as well as that of calgranulin B in the fetal membranes of patients with PPROM is higher than in the membranes of those without histologic chorioamnionitis; (2) histologic chorioamnionitis is associated with differences in the pattern of α-defensin-1 mRNA expression in the fetal membranes in patients with PTL and intact membranes and those with PPROM.
The authors thank Lark Technologies, Inc. (Houston, TX) for performing the qRT-PCR experiments. They wish to acknowledge the invaluable contributions made to this manuscript by Sandy Field, Gerardo Rodriguez, and the nursing staff of the Perinatology Research Branch and the Detroit Medical Center.
References
- Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human β-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22.
- Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol 1993;168:312.
- Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod 2000;15:778–784.
- Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol 2001;185:586–592.
- Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 2002;187:137–144.
- King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007;28:161–169.
- Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta 1991;12:285–288.
- Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol 2001;94:224–229.
- Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol 1997;38:252–255.
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med 2000;6:589–593.
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–395.
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:710–720.
- Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol 2005;116:241–249.
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985;76:1427–1435.
- Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem 1978;253:2664–2672.
- Zhao C, Wang I, Lehrer RI. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996;396:319–322.
- Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992;267:23216–23225.
- Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res 2000;1:141–150.
- King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during pregnancy. Placenta 2007;28:1099–1106.
- Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, Maymon E, Edwin S. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol 2004;191:1331–1338.
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, et al Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003;13:2–21.
- Friel L, Kuivaniemi H, Gomez R, Goddard K, Nien JK, Tromp G, Lu Q, Xu Z, Behnke E, Solari M, et al Genetic predisposition for preterm PROM: results of a large candidate-gene association study of mothers and their offspring. Am J Obstet Gynecol 2005;193:S17.
- Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med 2005;83:587–595.
- Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 1993;11:105–128.
- Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol 1986;60:1068–1074.
- Raj PA, Dentino AR. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett 2002;206:9–18.
- Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol 2006;6:447–456.
- Joseph G, Tarnow L, Astrup AS, Hansen TK, Parving HH, Flyvbjerg A, Frystyk J. Plasma α-defensin is associated with cardiovascular morbidity and mortality in type 1 diabetic patients. J Clin Endocrinol Metab 2008;93:1470–1475.
- Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 1989;84:553–561.
- Lindemann RA, Lala A, Miyasaki KT. The in vitro effect of human polymorphonuclear leukocyte azurophil granule components on natural killer cell cytotoxicity. Oral Microbiol Immunol 1994;9:186–192.
- Oren A, Taylor JM. The subcellular localization of defensins and myeloperoxidase in human neutrophils: immunocytochemical evidence for azurophil granule heterogeneity. J Lab Clin Med 1995;125:340–347.
- Faurschou M, Sorensen OE, Johnsen AH, Askaa J, Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim Biophys Acta 2002;1591:29–35.
- Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest 1985;76:1436–1439.
- Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 1996;271:2935–2940.
- Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol 1999;163:947–953.
- Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky AS. Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion. Am J Physiol Lung Cell Mol Physiol 2001;280:L947–L954.
- Vaschetto R, Grinstein J, Del SL, Khine AA, Voglis S, Tullis E, Slutsky AS, Zhang H. Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes. J Leukoc Biol 2007;81:1022–1031.
- Schulz A, Kluver E, Schulz-Maronde S, Adermann K. Engineering disulfide bonds of the novel human β-defensins hBD-27 and hBD-28: differences in disulfide formation and biological activity among human β-defensins. Biopolymers 2005;80:34–49.
- Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997;88:553–560.
- Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature 1997;387:861.
- Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 1998;101:1633–1642.
- McCray PB Jr, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol 1997;16:343–349.
- Gropp R, Frye M, Wagner TO, Bargon J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther 1999;10:957–964.
- Striz I, Trebichavsky I. Calprotectin – a pleiotropic molecule in acute and chronic inflammation. Physiol Res 2004;53:245–253.
- Roth J, Goebeler M, Sorg C. S100A8 and S100A9 in inflammatory diseases. Lancet 2001;357:1041.
- Pillay SN, Asplin JR, Coe FL. Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol 1998;275:F255–F261.
- Helbert MJ, Dauwe SE, De Broe ME. Flow cytometric immunodissection of the human distal tubule and cortical collecting duct system. Kidney Int 2001;59:554–564.
- Doussiere J, Bouzidi F, Vignais PV. The S100A8/A9 protein as a partner for the cytosolic factors of NADPH oxidase activation in neutrophils. Eur J Biochem 2002;269:3246–3255.
- Brandtzaeg P, Dale I, Fagerhol MK. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. II. Normal and aberrant occurrence in various epithelia. Am J Clin Pathol 1987;87:700–707.
- Bhardwaj RS, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol 1992;22:1891–1897.
- Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol 1993;53:197–204.
- Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, Eisenberg D. Granulysin crystal structure and a structure-derived lytic mechanism. J Mol Biol 2003;325:355–365.
- Krensky AM, Clayberger C. Granulysin: a novel host defense molecule. Am J Transplant 2005;5:1789–1792.
- Clayberger C, Krensky AM. Granulysin. Curr Opin Immunol 2003;15:560–565.
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998;282:121–125.
- Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol 1997;9:117–125.
- Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol 1998;161:1758–1764.
- Li Q, Dong C, Deng A, Katsumata M, Nakadai A, Kawada T, Okada S, Clayberger C, Krensky AM. Hemolysis of erythrocytes by granulysin-derived peptides but not by granulysin. Antimicrob Agents Chemother 2005;49:388–397.
- Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, Hanson DA, Kluck RM, Hitoshi Y, Johnson DE, et al A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol 2001;167:350–356.
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–429.
- Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–584.
- Romero R, Espinoza J, Kusanovic J, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113(Suppl 3):17–42.
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394.e1–394.e24.
- Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–786.
- Haddad R, Gould BR, Romero R, Tromp G, Farookhi R, Edwin SS, Kim MR, Zingg HH. Uterine transcriptomes of bacteria-induced and ovariectomy-induced preterm labor in mice are characterized by differential expression of arachidonate metabolism genes. Am J Obstet Gynecol 2006;195:822–828.
- Slattery MM, Morrison JJ. Preterm delivery. Lancet 2002;360:1489–1497.
- Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. 2004;First:28–60.
- Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period: linked birth/infant death data set. Natl Vital Stat Rep 2004;53:1–29.
- Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004;329:675–678.
- Xu J, Holzman CB, Arvidson CG, Chung H, Goepfert AR. Midpregnancy vaginal fluid defensins, bacterial vaginosis, and risk of preterm delivery. Obstet Gynecol 2008;112:524–531.
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:368–373.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report 140. Placenta 2005;26 (Suppl A):S114–S117.
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25.
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301.
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 1999;270:41–49.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–408.
- Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet Gynecol 2006;195:360–363.
- Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, Mazaki-Tovi S, Than NG, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006;113 (Suppl 3):118–135.
- Tarca AL, Romero R, Draghici S. Analysis of microarray experiments of gene expression profiling. Am J Obstet Gynecol 2006;195:373–388.
- Romero R, Tarca AL, Tromp G. Insights into the physiology of childbirth using transcriptomics. PLoS Med 2006;3:e276.
- Janeway CA Jr, Travers P, Walport M. Innate immunity. 2001;5th:35–42.
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216.
- Borghesi L, Milcarek C. Innate versus adaptive immunity: a paradigm past its prime. Cancer Res 2007;67:3989–3993.
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007;449:819–826.
- Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest 2008;118:413–420.
- Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 1999;286:498–502.
- Selsted ME, Tang YQ, Morris WL, McGuire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS. Purification, primary structures, and antibacterial activities of β-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem 1993;268:6641–6648.
- Linzmeier R, Ho CH, Hoang BV, Ganz T. A 450-kb contig of defensin genes on human chromosome 8p23. Gene 1999;233:205–211.
- Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest 1998;102:874–880.
- Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel β-defensin from human plasma. FEBS Lett 1995;368:331–335.
- Zhu BD, Feng Y, Huang N, Wu Q, Wang BY. Mycobacterium bovis bacille Calmette-Guerin (BCG) enhances human β-defensin-1 gene transcription in human pulmonary gland epithelial cells. Acta Pharmacol Sin 2003;24:907–912.
- Sorensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, Ganz T. Differential regulation of β-defensin expression in human skin by microbial stimuli. J Immunol 2005;174:4870–4879.
- Joly S, Organ CC, Johnson GK, McCray PB Jr, Guthmiller JM. Correlation between β-defensin expression and induction profiles in gingival keratinocytes. Mol Immunol 2005;42:1073–1084.
- Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human β-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res 2005;84:445–450.
- Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of β-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 2002;106:517–525.
- Fang XM, Shu Q, Chen QX, Book M, Sahl HG, Hoeft A, Stuber F. Differential expression of α- and β-defensins in human peripheral blood. Eur J Clin Invest 2003;33:82–87.
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, et al β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–528.
- Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, Jia HP, Tack BF, McCray PB. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol Immunol 2003;18:95–99.
- Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol 2004;22:181–215.
- Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cell Mol Life Sci 2006;63:1294–1313.
- Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol 1993;151:6291–6301.
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der PT, Sorg C, et al Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007;13:1042–1049.
- Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol 1999;163:2209–2216.
- Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech 2003;60:569–580.
- Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun 2001;69:3692–3696.
- Mackewicz CE, Ridha S, Levy JA. HIV virions and HIV replication are unaffected by granulysin. AIDS 2000;14:328–330.
- Ochoa MT, Stenger S, Sieling PA, Thoma-Uszynski S, Sabet S, Cho S, Krensky AM, Rollinghoff M, Nunes SE, Burdick AE, et al T-cell release of granulysin contributes to host defense in leprosy. Nat Med 2001;7:174–179.
- Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M. Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol 2004;34:2248–2256.
- Ma LL, Spurrell JC, Wang JF, Neely GG, Epelman S, Krensky AM, Mody CH. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J Immunol 2002;169:5787–5795.
- Hata A, Zerboni L, Sommer M, Kaspar AA, Clayberger C, Krensky AM, Arvin AM. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol 2001;14:125–133.
- King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol 2003;1:116.
- King AE, Critchley HO, Sallenave JM, Kelly RW. Elafin in human endometrium: an antiprotease and antimicrobial molecule expressed during menstruation. J Clin Endocrinol Metab 2003;88:4426–4431.
- Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril 2003;79:856–863.
- Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest 1983;15:230–241.
- Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 1984;148:915–928.
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996;67:1103–1113.
- Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc 2001;132:875–880.
- Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL Jr, Beck JD, Offenbacher S. Maternal periodontitis and prematurity, Part II: maternal infection and fetal exposure. Ann Periodontol 2001;6:175–182.
- Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG 2002;109:527–533.
- Dortbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol 2005;32:45–52.
- Boggess KA, Madianos PN, Preisser JS, Moise KJ Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol 2005;192:554–557.
- Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, Yanaihara T. Amniotic fluid lactoferrin in intrauterine infection. Placenta 1999;20:175–179.
- Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, Romero R. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol 2000;183:904–910.
- Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol 2004;191:2090–2096.
- Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, Agerberth B. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res 2003;53:211–216.
- Cherry SH, Filler M, Harvey H. Lysozyme content of amniotic fluid. Am J Obstet Gynecol 1973;116:639–642.
- Ford LC, DeLange RJ, Lebherz TB. Identification of a bactericidal factor (B-lysin) in amnionic fluid at 14 and 40 weeks’ gestation. Am J Obstet Gynecol 1977;127:788–792.
- Hisanaga S, Umezu T, Shimokawa H, Maesato S. Amniotic fluid lysozyme content in normal and abnormal pregnancy. Nippon Sanka Fujinka Gakkai Zasshi 1982;34:541–544.
- Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis 1998;27:513–518.
- Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res 2003;53:566–572.
- Stock SJ, Kelly RW, Riley SC, Calder AA. Natural antimicrobial production by the amnion. Am J Obstet Gynecol 2007;196:255–256.
- Ruetschi U, Rosen A, Karlsson G, Zetterberg H, Rymo L, Hagberg H, Jacobsson B. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J Proteome Res 2005;4:2236–2242.
- Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, et al Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA 2004;292:462–469.
- Buhimschi IA, Christner R, Buhimschi CS, Chaiworapongsa T, Romero R. Proteomic analysis of preterm parturition: a novel method of identifying the patients at risk of impending preterm delivery. Am J Obstet Gynecol 2002;187:S55.