Abstract
Twin-twin transfusion syndrome (TTTS) is present in approximately 5–15% of monochorionic-diamniotic twin pregnancies. A chronic blood flow imbalance through placental vascular anastomoses from the donor to the recipient twin is considered the pathophysiologic mechanism responsible for the development of TTTS. Discordant echogenicity between the donor and recipient placenta has been proposed in a previous case report as an additional sonographic sign of TTTS. Here, we present a case of TTTS with discordant placental echogenicity characterized by a hyperechoic and thicker placental side in the donor twin associated with reduced vascular Doppler signals, histologic lesions suggestive of ischemic changes, and overexpression of anti-angiogenic factors.
Case report
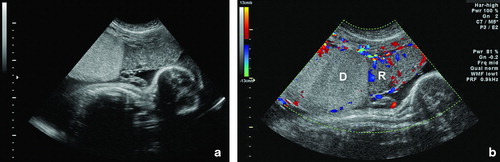
A 20-year-old African-American woman, G2P1001, was referred to our center at 27 4/7 weeks of gestation due to a monochorionic-diamniotic twin pregnancy with suspected twin-twin transfusion syndrome (TTTS) in stage I [Citation1] based on the following findings: (1) single placental mass; (2) same fetal gender (male twins); (3) polyhydramnios in twin A; and (4) oligohydramnios in twin B. Two-dimensional transabdominal ultrasound examination performed upon admission confirmed these findings, as well as the sonographic features of ‘stuck twin’ on twin B. In addition, two different echogenicities within the placenta were observed. Laser therapy was not offered because of the late gestational age at diagnosis; thus, an amnioreduction was performed draining 2600 cc of amniotic fluid from the gestational sac of twin A. An ultrasonographic evaluation performed in our unit 2 days after the procedure revealed that the placental side of the donor was hyperechoic and thicker than that of the recipient's segment ( and Supplemental clip 1). Color ( and Supplemental clip 2) and power Doppler (Supplemental clip 3) interrogation showed reduced vascular Doppler signals within the hyperechoic portion of the placenta. The patient underwent a cesarean section at 28 weeks of gestation because of spontaneous preterm labor. Twin A weighed 922 g (>10th percentile) at delivery, with Apgar scores of 8 and 8 at 1 and 5 min, respectively; and normal umbilical cord blood gases. The hematocrit was 54.8% and hemoglobin 19.5 g/dl. Twin B weighed 595 g (<5th percentile, with an inter-twin birthweight discordancy of 35%), the Apgar scores were 3 and 6 at 1 and 5 min, respectively; and normal cord blood gases were also observed. The hematocrit was 18.6% and hemoglobin 6.1 g/dl. Both neonates had respiratory distress syndrome. The donor twin developed intraventricular hemorrhage grade IV, three episodes of pulmonary hemorrhage, and expired 18 days after delivery.
Figure 1. a) Transabdominal ultrasonographic image at 27 weeks of gestation showing the monochorionic-diamniotic placenta with discordant echogenicity; the hyperechogenic, thicker portion belonged to the donor twin (left), whereas the placental side of the recipient twin had a normal echotexture (right). b) Color Doppler examination showing the presence of blood flow in the depth of the placenta in the recipient (R) side. In contrast, reduced vascular Doppler signals were observed in the donor (D) side.
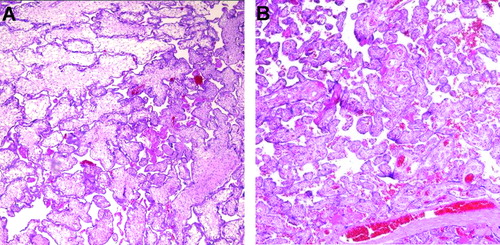
Macroscopic examination of the placenta revealed a 680 g single placental mass with ∼70% of the area belonging to the donor twin. No velamentous insertion of the umbilical cords was observed. The macroscopic appearances of both sides of the placenta (donor and recipient) were very dissimilar on the maternal surface. The placental mass of the donor was pale, whereas that of the recipient side had a congested appearance (). Histologically, the placental segment of the donor showed villous dysmaturity, villous edema, intervillous hemorrhage, and features of ischemic changes [Citation2] such as fibrinoid degeneration of villi, villous stromal karyorrhexis, and early stromal fibrosis, whereas the recipient placental segment was characterized by a more uniform villous development and maturation pattern compatible with that of third trimester (). There was no histologic evidence of placentitis suggesting viral infection.
Figure 2. Maternal surface of the monochorionic-diamniotic placenta showing stark contrast between the donor (D) and the recipient (R) sides. The donor side is more pale and shaggy, while the recipient side is dark red, congested appearance.
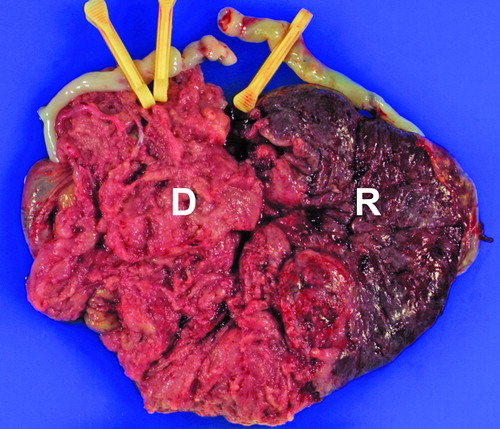
Figure 3. Histologic examination of the placenta with hematoxylin and eosin (H&E): (A) The donor side shows alternating areas of edematous or fibrotic immature intermediate villi; (B) The maturation pattern of chorionic villi of the recipient side is uniform and is compatible with that of a third trimester placenta.
Conclusions
Discordant echogenicity between the donor and recipient placenta has been proposed as an additional sonographic sign of TTTS [Citation3]. However, this sonographic finding was also described in a case of a monochorionic-diamniotic twin pregnancy complicated by ‘stuck twin syndrome’ without TTTS [Citation4]; thus, discordant placental echogenicity may not be limited to pregnancies complicated by TTTS. The twin pregnancy complicated by TTTS presented herein was associated with an unusual sonographic appearance of the placenta, where the hyperechogenic, thicker portion belonged to the donor twin and the sonographically normal placental portion to the recipient twin. Of note, color and power Doppler interrogation of the placenta indicates that the placental segment of the donor twin (hyperechogenic) had substantially reduced vascular Doppler signals than the recipient segment, suggesting reduced perfusion of this placental side. This is consistent with the histological examination of the donor side of the placenta that revealed the presence of lesions proposed to represent ischemic changes.
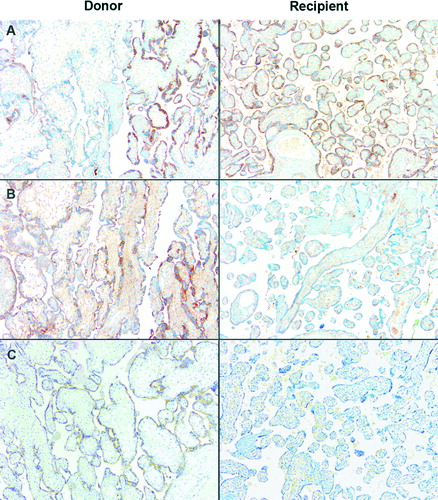
Overexpression of vascular endothelial growth factor receptor-1 (VEGFR-1, an anti-angiogenic factor) mRNA has been reported in the donor villi but not in that of the recipient in some cases of TTTS [Citation5]. In the case presented herein, immunostaining for placental growth factor (PlGF, an angiogenic factor), VEGFR-1, and endoglin (another anti-angiogenic factor) demonstrated variable immunoreactivity of these molecules in the donor side. PlGF was expressed both in the donor and the recipient tissues, but the edematous villi of the donor side were weakly reactive. In contrast, moderate to high distinct immunoreactivity for VEGFR-1 and endoglin was found in the villous trophoblast of the donor side, while the recipient tissue showed decreased or lack of immunoreactivity (). The finding of overexpression of endoglin in the donor segment of the placenta in cases of TTTS is novel. Recently, we have demonstrated that patients with TTTS had a significantly higher median maternal plasma concentration of soluble endoglin and soluble VEGFR-1, and significantly lower maternal plasma concentrations of PlGF than those without TTTS, suggesting that an anti-angiogenic state is present in TTTS and may explain some of the pathophysiology of the syndrome (i.e. mirror syndrome, small for gestational age, etc.) [Citation6].
Figure 4. Protein expression of placental growth factor (PlGF), VEGF receptor-1 (VEGFR-1), and Endoglin in the monochorionic-diamniotic placenta. The donor side is characterized by variable expression of these molecules: (A) PlGF was expressed in both the donor and the recipient tissues, but the immunoreactivity was variable in the donor side, edematous villi being weakly reactive. In contrast, moderate to high distinct immunoreactivity for (B) VEGFR-1 and (C) Endoglin was found in the villous trophoblast of the donor side, while the recipient tissue showed decreased or lack of immunoreactivity.
Collectively, the evidence presented herein indicates that the donor side of the placenta in a case complicated with TTTS and discordant placental echogenicity was associated with: (1) reduced vascularity, as demonstrated by Doppler studies; (2) histologic lesions associated to ischemic changes; and (3) overexpression of the anti-angiogenic factors VEGFR-1 and endoglin.
Acknowledgments
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19:550–555.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26(Suppl A):S114–S117.
- Frisch L, Arava J, David H, Jaschevatzky OE, Ballas S. Severe twin-to-twin transfusion syndrome: a new sonographic feature of the placenta. Ultrasound Obstet Gynecol 1997;10:145–146.
- Lees CC, Schwarzler P, Ville Y, Campbell S. Stuck twin syndrome without signs of twin-to-twin transfusion. Ultrasound Obstet Gynecol 1998;12:211–214.
- Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol 2002;33:1069–1077.
- Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, et al Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol 2008;198:382–388.