Abstract
Objective. Angiogenic factors have been implicated in the pathophysiology of sepsis. In experimental models of sepsis (endotoxemia and/or cecal ligation puncture), there is increased expression of vascular endothelial growth factors (VEGF) and the administration of exogenous soluble VEGF receptor (sVEGFR)-1, an antagonist to VEGF, reduces morbidity and mortality. Moreover, a dramatic elevation in sVEGFR-1 has been demonstrated in human sepsis. Although a balance between angiogenic and anti-angiogenic factors is essential for feto-placental development, the changes of angiogenic factors during pregnancy in the context of infection have never been explored. Angiogenic factors also play crucial roles in the pathophysiology of preeclampsia (PE). This study was conducted to determine if maternal plasma concentrations of placental growth factor (PlGF), sVEGFR-2, and soluble endoglin (sEng) change in pregnancies complicated by acute pyelonephritis (AP) compared with normal pregnancy and PE.
Study design. A case-control study was conducted in patients with AP, normal pregnant (NP) women, and patients with PE (n = 36 for each group) matched for gestational age. AP was diagnosed in the presence of fever (temperature ≥38°C), clinical signs of infection, and a positive urine culture for microorganisms. Plasma concentrations of PlGF, sVEGFR-2, and sEng were determined by ELISA. The results of plasma sVEGFR-1 concentrations have previously been reported, but were included in this study to provide a complete picture of the angiogenic/anti-angiogenic profiles. Serum concentrations of interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, interferon (IFN)-γ, granulocyte macrophage colony stimulating factor, and tumor necrosis factor (TNF) were also determined using high sensitivity multiplexed immunoassays in patients with AP and NP.
Results. AP was associated with a lower median plasma concentration of PlGF and sVEGFR-2 than NP (both p < 0.001). There were no significant differences in the median plasma concentrations of sEng and sVEGFR-1 between AP and NP (p = 0.7 and 0.5, respectively). In contrast, there was a 5-fold decrease of the median plasma concentration of PlGF, and an 8–10-fold increase of the median plasma concentrations of sVEGFR-1 and sEng in PE compared with those in AP (all p < 0.001). No significant difference in the median plasma concentration of sVEGFR-2 was observed between patients with PE and AP (p = 0.5). Pregnant women with AP had median plasma concentrations of IL-6, IL-7, IL-8, IL-10, IFN-γ, and TNF-α significantly higher than those in NP women (all p < 0.001, except IL-7 p = 0.004).
Conclusion. AP is associated with changes in the profile of angiogenic and anti-angiogenic factors. Although some of these changes resemble those in PE (decreased PlGF and sVEGFR-2), the magnitude of the changes of PlGF is much higher in PE. We conclude that despite high plasma inflammatory cytokine concentrations, acute systemic inflammation in pregnancy has a different angiogenic/anti-angiogenic profile than that of PE.
Introduction
Pyelonephritis complicates 1–2% of pregnancies [Citation1] and is one of the most common infectious causes of pregnancy-associated hospitalizations [Citation2]. Despite the favorable outcomes of most patients, pregnant women with pyelonephritis are at risk of developing preterm labor [Citation3], adult respiratory distress syndromes [Citation4,Citation5], sepsis [Citation6], and even death [Citation7]. Indeed, acute pyelonephritis (AP) is the most common cause of septic shock during pregnancy [Citation7–9]. Sepsis is defined as a systemic inflammatory response triggered by bacteria or bacterial products. During an episode of sepsis, there is leukocyte activation, elevation of several pro- and anti-inflammatory cytokines/chemokines/generation of reactive oxygen species, activation of coagulation/complement systems, and endothelial dysfunction leading to multi-organ failure. Recently, angiogenic factors have been implicated in the pathophysiology of sepsis [Citation10–16]. In experimental models of sepsis (endotoxemia and/or cecal ligation puncture) [Citation12–14] and observational studies in septic patients [Citation10,Citation11,Citation15,Citation16], an increase in the plasma concentrations of vascular endothelial growth factors (VEGF) [Citation10–12,Citation15], placental growth factors (PlGF) [Citation14], and soluble VEGF receptor (sVEGFR)-1 [Citation13,Citation16] was observed. The changes in sVEGFR-1 are considered an adaptive response to infection and to have survival value [Citation13,Citation14], as the administration of an adenovirus expressing for sVEGFR-1 [Citation12] or exogenous sVEGFR-1 [Citation13] attenuates the inflammatory response and reduces morbidity/mortality in mice [Citation12,Citation13]. It is unknown if these changes in angiogenic factors in response to infection are also present in pregnant women. Most research on angiogenic factors during pregnancy focus on preeclampsia (PE), a pregnancy specific disorder, which has pathophysiologic changes that overlap with those of sepsis.
PE is characterized by new-onset hypertension and proteinuria in the second half of pregnancy. A central feature in the pathophysiology of PE was originally thought to be endothelial dysfunction [Citation17]. However, Redman et al. proposed that the maternal syndrome of PE is due to an excessive maternal intravascular inflammatory response to pregnancy [Citation18]. The endothelial dysfunction in PE is part of a generalized intra-vascular inflammatory reaction involving circulating leukocytes as well as the coagulation and complement systems. Although the primary insult responsible for these abnormalities remains elusive [Citation19–22], it is postulated that the poor placentation and reduced blood supply (or ischemic-reperfusion injury) [Citation23] in the intervillous space of the placenta in early pregnancy leads to the release of factors [Citation24] into the maternal circulation causing systemic endothelial cell dysfunction [Citation17], intravascular inflammation [Citation25,Citation26], and multiple organ damage. Candidates for these unknown factors include oxidative stress metabolites [Citation23], syncytiotrophoblast microparticles [Citation27], cytokines [Citation28], angiotensin II receptor type 1 antibody [Citation29], and sVEGFR-1, a powerful natural anti-angiogenic factor of VEGF [Citation30,Citation31].
An imbalance between angiogenic (VEGF and PlGF) and anti-angiogenic factors (sVEGFR-1 and the soluble form of endoglin (sEng)) has been implicated in the pathophysiology of PE [Citation31–41]. PlGF is a major member of the VEGF family, which can bind to VEGFR-1 and enhance the angiogenic response of VEGF on endothelial cells especially in pathological conditions, such as limb ischemia or wound healing [Citation42,Citation43]. The soluble form of VEGFR-1 exerts its anti-angiogenic activity by binding to VEGF or PlGF and preventing VEGF from binding to its functional ligand, VEGFR-2 on endothelial cells [Citation42,Citation43]. sEng modulates the actions of transforming growth factors (TGF)-β1 as well as TGF-β3 [Citation44]. Indeed, patients with PE exhibit an angiogenic factor profile consistent with an ‘anti-angiogenic state’ characterized by increased plasma concentrations of sVEGFR-1 and sEng; but decreased plasma concentrations of unbound VEGF and PlGF [Citation31]. These changes have been observed prior to the clinical manifestation of the disease [Citation36,Citation38,Citation40,Citation41]. The role of sVEGFR-2 in human diseases is unclear, and this protein has received less attention because of its less potency of binding to VEGF than sVEGFR-1 [Citation45]. However, plasma sVEGFR-2 concentration might be a surrogate marker of endothelial cell function in the maternal circulation, because VEGF signaling through the membranous isoform of this protein is essential for endothelial cell function and survival [Citation46]. Similarly, a decreased plasma concentration of sVEGFR-2 in PE has been observed prior to the clinical diagnosis of this syndrome [Citation47].
Although a balance between angiogenic and anti-angiogenic factors is essential for feto-placental development, the changes of these factors during pregnancy in the context of infection have not been explored. The objectives of this study were (1) to examine if maternal plasma concentrations of PlGF, sEng, and sVEGFR-2 change in pregnancies complicated by AP; and (2) to determine if these changes differed from that seen in PE.
Patients and methods
Study design
This case–control study was conducted by searching the clinical database and bank of biologic samples of the Perinatology Research Branch. The following groups were included: (1) normal pregnant (NP) women; (2) pregnant women with pyelonephritis; and (3) patients with PE. Women with multiple pregnancies and fetal anomalies were excluded. Patients were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complications of pregnancy, and delivered a term neonate of appropriate birthweight for gestational age without complications. Pyelonephritis was diagnosed in the presence of fever (temperature ≥38°C), clinical signs of an upper urinary tract infection (e.g. flank pain, costovertebral angle tenderness), pyuria, and a positive urine culture for microorganisms. Blood cultures were also obtained in some patients. PE was defined as hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 h to 1 week apart) and proteinuria (≥300 mg in a 24-h urine collection or one dipstick measurement ≥2+) [Citation48]. Patients with chronic hypertension were excluded. Early-onset PE was defined as those who were diagnosed before 34 weeks of gestation [Citation49]. The three studied groups were matched for gestational age at blood sampling (within 3 weeks).
All women provided written informed consent prior to the collection of plasma samples. The collection of samples and their utilization for research purposes was approved by the IRB of the Wayne State University and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples were used previously in studies of intravascular inflammation, angiogenesis and cytokine biology in normal and complicated pregnancies [Citation26,Citation50,Citation51].
Sample collection and angiogenic factors immunoassays
Venipuncture was performed and the blood was collected into tubes containing EDTA. Samples were centrifuged and stored at −70°C. Maternal plasma concentrations of PlGF, sVEGFR-2, sEng, and sVEGFR-1 were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). All four immunoassays utilized the quantitative sandwich enzyme immunoassay technique and their concentrations in maternal plasma were determined by interpolation from the standard curves. The results of plasma sVEGFR-1 concentrations have previously been reported [Citation50], but included in this study for a complete picture of angiogenic profiles. The inter- and intra-assay coefficients of variation (CV) obtained were: PlGF: 6.02% and 4.8%, respectively; sVEGFR-2: 2% and 4%, respectively; sEng: 2.3% and 4.6% respectively; and sVEGFR-1: 1.4% and 3.9%, respectively. The sensitivity of the assays was: PlGF: 9.52 pg/ml; sVEGFR-2: 19.01 pg/ml; sEng: 0.08 ng/ml; and sVEGFR-1: 16.97 pg/ml.
High sensitivity human cytokine multiplexed immunoassays
Concentrations of interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, interferon (IFN)-γ, granulocyte macrophage colony stimulating factor (GM-CSF), and tumor necrosis factor (TNF)-α in maternal serum of NP women and patients with AP were determined simultaneously using microsphere-based high sensitivity human cytokine multiplex immunoassays obtained from Linco Research (St. Charles, MO). The microparticle beads were read using the Luminex100 analyzer (Luminex Corporation, Austin, TX). The instrument settings included measurement of 100 events per microparticle bead type. All standards and samples were assayed in duplicate wells. Five-parameter analysis was performed on the median (100 events) fluorescent units to derive the concentrations of each analyte using StatLIA (Brendan Scientific Corporation, Grosse Pointe Farms, MI). The sensitivity was 0.13 pg/ml. The inter- and intra-assay CV ranged from 7 to 10% and 5 to 9%, respectively.
Statistical analysis
Shapiro–Wilk was used to test the data for normal distribution. Kruskal–Wallis with post-hoc Mann–Whitney U test was utilized to determine the differences of the median among and between groups. Contingency tables, Chi-square, and Fischer's Exact test were employed for comparisons of proportions. Analysis was conducted with SPSS V.15 (SPSS, Chicago, IL). A p value of <0.05 was considered significant.
Results
Clinical characteristics of the study population
This study included 36 patients in each group. The PE group had a higher rate of nulliparous women than the normal pregnancy and AP groups (p = 0.009 and p = 0.02, respectively; ). Among patients with pyelonephritis, 21% (7/34) delivered a small for gestational age neonate. The most common microorganism isolated from urine cultures was Escherichia coli [83% (30/36)]. Other microorganisms included Klebsiella pneumoniae (n = 1), Proteus mirabilis (n = 1), Citrobacter koseri (n = 1), Pseudomona aeruginosa (n = 1), Streptococcus agalactiae (n = 1), and Staphylococcus aureus (n = 1).
Table I. Clinical characteristics of the study population.
Thirty-one (86%) patients had a blood culture for microorganisms performed and 14 of them (45%) had positive blood culture results. Escherichia coli was the most common microorganism isolated from blood cultures [64% (9/14)]. Other microorganisms included Enterobacter aerogenes (n = 1), Coagulase-negative staphylococcus (n = 2), Staphylococcus aureus (n = 1), and Streptococcus agalactiae (n = 1).
Clinical characteristics of patients with PE are displayed in . Among patients with PE, 31 (86%) were diagnosed as severe PE, and 18 (50%) delivered neonates whose birthweights were below the 10th percentile for gestational age. There was no significant difference in the median gestational age at blood sampling among the three study groups (p > 0.05).
Table II. Clinical characteristics of patients with preeclampsia.
All samples had sVEGFR-2, sEng, and sVEGFR-1 concentrations above the detection limits. Although 12 (33%) patients with PE had plasma concentrations of PlGF below the detection limit, only one (2.8%) patient with AP and one (2.8%) NP woman had plasma concentrations of PlGF below the detection limit.
Angiogenic profiles of pregnant women with acute pyelonephritis
Pregnant women with AP had median plasma concentrations of PlGF and sVEGFR-2 lower than those in NP women (PlGF: normal pregnancy: median 896 pg/ml, interquartile range (IQR) 567–1200 pg/ml vs. AP: median 366 pg/ml, IQR 154–547 pg/ml; and sVEGFR-2: normal pregnancy: median 12.9 ng/ml, IQR 10.1–15.7 ng/ml vs. AP: median 8.7 ng/ml, IQR 6.9–10.3 ng/ml; both p < 0.001, and , respectively).
Figure 1. Plasma concentrations of PlGF in normal pregnant women, pregnant women with acute pyelonephritis, and patients with preeclampsia. Pregnant women with acute pyelonephritis and patients with preeclampsia had median plasma concentrations of PlGF lower than normal pregnant women (normal pregnancy: median 896 pg/ml, inter-quartile range (IQR) 567–1200 pg/ml; acute pyelonephritis: median 366 pg/ml, IQR 154–547 pg/ml; preeclampsia: median 67 pg/ml, IQR 0–120.6 pg/ml; both p < 0.001). However, patients with preeclampsia had a median plasma concentration of PlGF lower than those with acute pyelonephritis (p < 0.001). *: p < 0.05.
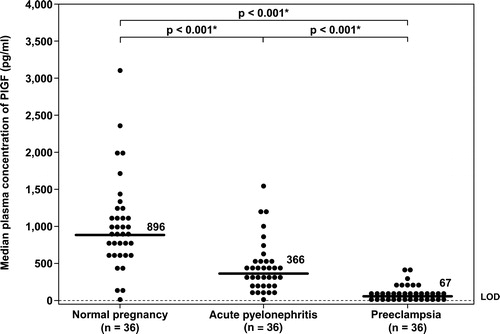
Figure 2. Plasma concentrations of sVEGFR-2 in normal pregnant women, pregnant women with acute pyelonephritis, and patients with preeclampsia. Pregnant women with acute pyelonephritis and patients with preeclampsia had median plasma concentrations of sVEGFR-2 lower than normal pregnant women (normal pregnancy: median 12.9 ng/ml, IQR 10.1–15.7 ng/ml; acute pyelonephritis: median 8.7 ng/ml, IQR 6.9–10.3 ng/ml; preeclampsia: median 7.6 ng/ml, IQR 6.6–10.6 ng/ml; both p < 0.001). There was no significant difference in the median plasma concentrations of sVEGFR-2 between patients with preeclampsia and those with acute pyelonephritis (p = 0.5). *: p < 0.05.
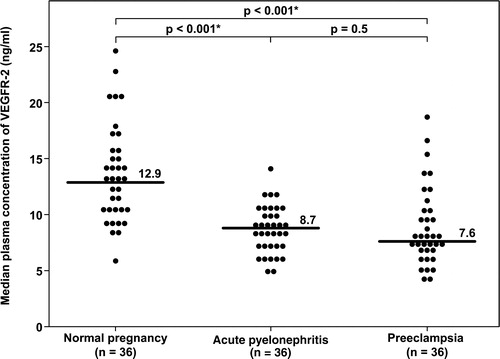
The median plasma concentrations of both sEng and sVEGFR-1 in patients with pyelonephritis were not significantly different from those in NP women (sEng; normal pregnancy: median 6.6 ng/ml, IQR 5.1–8.3 ng/ml vs. AP: median 6.7 ng/ml, IQR 5.2–8.9 ng/ml; p = 0.7 ; and sVEGFR-1; normal pregnancy: median 1052 pg/ml, IQR 644–1539 pg/ml vs. AP: median 903 pg/ml, IQR 540–1355 pg/ml; p = 0.5 ).
Figure 3. Plasma concentrations of sEng in normal pregnant women, pregnant women with acute pyelonephritis, and patients with preeclampsia. Patients with preeclampsia had a median plasma concentration of sEng higher than normal pregnant women and higher than patients with acute pyelonephritis (normal pregnancy: median 6.6 ng/ml, IQR 5.1–8.3 ng/ml; acute pyelonephritis: median 6.7 ng/ml, IQR 5.2–8.9 ng/ml; preeclampsia: median 62.3 ng/ml, IQR 34.6–100.9 ng/ml; both p < 0.001). There was no significant difference in the median plasma concentrations of sEng between patients with acute pyelonephritis and normal pregnant women (p = 0.7). *: p < 0.05.
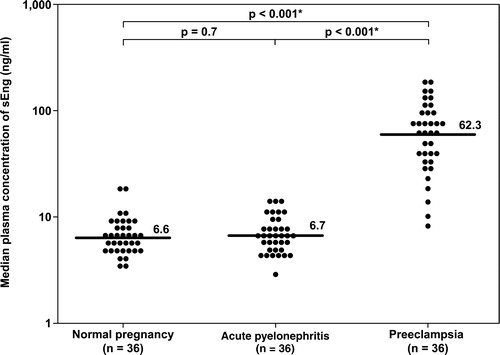
Figure 4. Plasma concentrations of sVEGFR-1 in normal pregnant women, pregnant women with acute pyelonephritis, and patients with preeclampsia. Patients with preeclampsia had a median plasma concentration of sVEGFR-1 higher than normal pregnant women and higher than those with acute pyelonephritis (normal pregnancy: median 1052 pg/ml, IQR 644–1539 pg/ml; acute pyelonephritis: median 903 pg/ml, IQR 540–1355 pg/ml; preeclampsia: median 8247 pg/ml, IQR 4154–10850 pg/ml; both p < 0.001). There was no significant difference in the median plasma concentration of sVEGFR-1 between patients with acute pyelonephritis and normal pregnant women (p = 0.5). *: p < 0.05.
Angiogenic profiles of patients with preeclampsia
The median concentration of PlGF in patients with PE was five-fold lower than that of patients with AP. In contrast, the median plasma concentrations of sVEGFR-1 and sEng was 8–10 fold higher in patients with PE than in those with AP (PlGF: AP: median 366 pg/ml, IQR 154–547 pg/ml vs. PE: median 67 pg/ml, IQR 0–120.6 pg/ml; sEng: AP: median 6.7 ng/ml, IQR 5.2–8.9 ng/ml vs. PE: median 62.3 ng/ml, IQR 34.6–100.9 ng/ml; and sVEGFR-1: AP: median 903 pg/ml, IQR 540–1355 pg/ml vs. PE: median 8247 pg/ml, IQR 4154–10,850 pg/ml; all p < 0.001, ).
There was no significant difference in the median plasma concentration of sVEGFR-2 between patients with PE and those with AP (AP: median 8.7 ng/ml, IQR 6.9–10.3 ng/ml vs. PE: median 7.6 ng/ml, IQR 6.6–10.6 ng/ml; p = 0.5; ).
Similar results were obtained when the comparisons were performed and included only patients with AP who had a positive blood culture result for microorganisms and were matched for gestational age at blood sampling with NP women and patients with PE ().
Table III. Plasma concentrations of PlGF, sVEGFR-2, sEng and sVEGFR-1 in normal pregnant women with acute pyelonephritis who had a positive blood culture result and patients with preeclampsia matched for gestational age at blood sampling.
Cytokine profiles of pregnant women with acute pyelonephritis
To confirm that patients with AP had systemic inflammation, we determined serum concentrations of 12 cytokines in NP women and patients with AP using multiplexed immunoassay. There were 31 cases matched for gestational age between the 2 groups and had serum samples available for cytokine determination. Patients with AP had median serum concentrations of IL-6, IL-7, IL-8, IL-10, IFN-γ, and TNF-α significantly higher than NP women (all p < 0.001, except IL-7 p = 0.004; ).
Table IV. Serum concentrations of 13 cytokines (pg/ml) evaluated by multiplexed immunoassay in normal pregnant women and pregnant women with acute pyelonephritis.
Discussion
Principal findings
(1) The angiogenic profile of pregnant women with AP, regardless of the presence of bacteremia, was characterized by a lower plasma concentration of PlGF and sVEGFR-2, but no changes in plasma concentrations of sVEGFR-1 and sEng compared with that of NP women; (2) PE was associated with lower plasma concentrations of PlGF, but higher plasma concentrations of sVEGFR-1and sEng than AP in pregnancy; and (3) both PE and pregnant women with AP had a similar decrease in plasma sVEGFR-2 concentrations from that of NP women.
Angiogenic profiles in the septic non-pregnant state
In the current study, pregnant women with AP were characterized by lower plasma concentrations of PlGF and sVEGFR-2, but no changes in plasma concentrations of sVEGFR-1 and sEng when compared with NP women. These findings are different from those in the septic non-pregnant state.
After the administration of lipopolysaccharides (LPS) or cecal ligation puncture in mice, there is an increase in plasma concentrations of VEGF, PlGF, and sVEGFR-1 [Citation12–14]. Observational studies in human sepsis also have reported similar results (healthy volunteer vs. sepsis; for PlGF: mean 0.18 pg/ml vs.13 pg/ml; for sVEGFR-1: mean 369 pg/ml vs. 601 pg/ml) [Citation12,Citation16].
Although VEGF is an amplifier of pro-inflammatory cytokine production [Citation12], the increased plasma concentration of sVEGFR-1 is considered to have survival value as the administration of an adenovirus vector expressing sVEGFR-1 [Citation12] or the exogenous administration of sVEGFR-1 [Citation13] inhibits the recruitment of inflammatory cells into the peritoneal cavity and reduces morbidity/mortality in animal models of sepsis [Citation13]. Similarly, the increased plasma PlGF concentrations after sepsis have been proposed to be an adaptive response to infection and have a protective role because PlGF knock-out mice or the administration of neutralizing PlGF antibody is associated with impaired cardiac function, increased vascular permeability and mortality [Citation14]. The high plasma PlGF and VEGF concentrations in sepsis may be a consequence of sepsis-induced inflammatory cytokines because the administration of LPS to triple mutant mice (null for IL-1 receptor, TNF receptor-1 and TNF receptor-2) results in a significantly lower induction of VEGF and PlGF [Citation12]. Conflicting results have been reported regarding the mechanisms of action of sVEGFR-1 in sepsis. Yano et al. demonstrated that the administration of an adenovirus vector for sVEGFR-1 gene before the endotoxin injection was able to block the increase of free VEGF and PIGF, but had no effect on the early increase of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). In contrast, Tsao et al. found that the exogenous administration of sVEGFR-1 attenuated the increased plasma concentrations of TNF-α and IL-1β induced by intraperitoneal injection of endotoxin. They proposed that LPS-induced sVEGFR-1 release occurs in a VEGF- and TNF-α independent manner because both VEGF- and TNF-α neutralizing antibodies failed to inhibit an LPS-induced sVEGFR-1 release [Citation13]. Furthermore, the plasma concentrations of VEGF increased progressively from 2 to 24 h after the administration of endotoxin, whereas plasma concentrations of sVEGFR-1 increased at 2 h and decreased thereafter [Citation13].
The sources of VEGF and sVEGFR-1 in the septic non-pregnant patients are partly from monocytes because LPS could stimulate monocytes to release VEGF and sVEGFR-1 [Citation13], although several organs including the heart, liver, kidney, brain, and spleen also show increased expression of VEGF and PlGF after experimental endotoxemia [Citation12]. No information regarding the expression of VEGFR-1 in organs affected by sepsis has been reported. On endothelial cells, another source of angiogenic factors, there is evidence that TNF-α could stimulate endothelial cells to release sVEGFR-1 and sEng [Citation52]. There was, however, no information regarding plasma concentrations of sEng and sVEGFR-2 in septic patients. Collectively, the major sources of angiogenic factors during sepsis in non-pregnant women are monocytes and endothelial cells.
Angiogenic profiles of normal pregnant women
During pregnancy, the uterine decidua and placenta are rich sources of angiogenic factors. Plasma concentrations of estrogen increase substantially during pregnancy. There is evidence that estrogen upregulates VEGFR-2, but not VEGFR-1, and promotes VEGF-mediated endothelial cell proliferation [Citation53]. The increased plasma concentration of sVEGFR-1, a natural inhibitor of VEGF, during pregnancy is thought to counter-balance the VEGF-induced vasodilatation effect [Citation46]. A recent study examining the concentrations of PlGF and sVEGFR-1 in maternal serum, coelomic fluid and amniotic fluid in pregnant women at 7–9 weeks of gestation suggested that the placenta is the main source of PlGF and sVEGFR-1 in pregnancy [Citation55].
Another important source of angiogenic factors during pregnancy is leukocytes. Pregnancy is considered a unique immunological state characterized by activation of the innate immune system and suppression of the adaptive limb of the immune response in order to promote tolerance to the fetus, as well as protect the mother against infection [Citation56]. In contrast to leukocytes of the non-pregnant women, the granulocytes and monocytes, two principal immune cells of the innate immune system, show evidence of phenotypic and metabolic changes, consistent with increased activation in the circulation of NP women [Citation25,Citation57]. The increased plasma soluble adhesion molecule concentration during pregnancy is consistent with increased activation of leukocytes and platelets [Citation58]. Furthermore, peripheral blood mononuclear cells of pregnant women have been demonstrated to be able to release sVEGFR-1 [Citation59].
As the placenta, in addition to leukocytes and endothelial cells, is a major source of angiogenic factors in the maternal circulation, angiogenic profiles in normal pregnancy are different from that in the non-pregnant state. In non-pregnant women, plasma concentrations of PlGF and sVEGFR-1 are usually below the detection limits of the assays (or detectable at very low concentrations) [Citation12,Citation14,Citation16,Citation35,Citation50] and those of sEng are generally detectable at low concentrations (median 4.5 ng/ml: unpublished data, Chaiworapongsa T). In contrast, during pregnancy, plasma PlGF and sVEGFR-1 concentrations increase substantially (PlGF: median 896 pg/ml; sVEGFR-1: median 1052 pg/ml). Plasma sEng concentrations have a modest increase (median 6.6 ng/ml), whereas no significant change in plasma sVEGFR-2 concentrations has been observed during pregnancy [Citation46].
Angiogenic profiles of pregnant women with acute pyelonephritis
Why were the changes in angiogenic profiles different between pregnant women with AP and septic non-pregnant women? We propose that the differences in angiogenic profiles between pregnant women exposed to bacterial infection and non-pregnant septic patients could be explained, at least in part, by much higher plasma concentrations of pro and anti-angiogenic factors in NP women in the basal state and the different sources of these angiogenic factors between the pregnant and the non-pregnant state.
The increased plasma concentration of PlGF during normal pregnancy is attributable mainly to the growing placenta [Citation35]. Both toll-like receptor (TLR)-4 [Citation60,Citation61], a pattern recognition receptor for Gram-negative bacteria, and TNF receptor type 1 are expressed on trophoblasts [Citation62,Citation63]. There is evidence that either LPS or TNF-α could induce apoptosis (programmed cell death) of both cytotrophoblast and syncytiotrophoblasts [Citation63–65]. Moreover, the administration of LPS or inflammatory cytokines (TNF-α or IL-1β) to first trimester trophoblast cell lines decreased PlGF protein expressions [Citation66]. Consistent with these observations, in the current study, pregnant women exposed to bacterial infection (AP) had a median plasma concentration of PlGF lower than NP women, but higher than patients with PE. If the infection is chronic or repetitive, this could result in a further decrease of plasma PlGF concentrations. These findings may explain, at least in part, the association between urinary tract infection, periodontal disease and PE reported in a recent meta-analysis [Citation67].
There is a paucity of information regarding the sources of sVEGFR-2 during pregnancy. The soluble form of VEGFR-2 can be detected in conditioned media obtained from human endothelial cells [Citation45], suggesting that endothelial cells are one of the sources of plasma sVEGFR-2 [Citation68]. The membranous isoform of VEGFR-2 is expressed mainly on endothelial cells, a fraction of hematopoietic cells (also known as circulating endothelial progenitor cells) and basophils, but not monocytes [Citation45,Citation69]. A low level of VEGFR-2 expression is also observed in neurons, osteoblasts, pancreatic duct cells, and retinal progenitor cells [Citation45]. In term human placentas, VEGFR-2 mRNA and protein expression are localized almost exclusively within vascular endothelial cells, but not trophoblasts [Citation70,Citation71]. These observations suggest that the predominant sources of sVEGFR-2 in pregnancy are vascular endothelium and hematopoietic cells similar to those in the non-pregnant state. The findings that the plasma sVEGFR-2 concentrations decreased in pregnant women with acute infection and in patients with PE would be consistent with the view that both PE and systemic infection are disorders that involved vascular endothelial cell function [Citation46]. It is likely that plasma concentrations of sVEGFR-2 in non-pregnant women with acute infection would also decrease.
Although several lines of evidence indicate that the major source of plasma sVEGFR-1 is the placenta, the source(s) of sEng in maternal circulation is (are) unclear. There is an increased expression of both mRNA and protein of sEng and sVEGFR-1 in the placenta, and the plasma concentrations of these proteins decrease after delivery [Citation31,Citation39,Citation72–75]. Other potential sources include maternal endothelial cells and peripheral blood mononuclear cells [Citation42,Citation44,Citation45].
Amniotic fluid contains several angiogenic factors, however, it is difficult to reconcile how these angiogenic factors gain access into maternal blood. In normal pregnancy, the concentrations of sVEGFR-1 were much higher in amniotic fluid than in maternal plasma both in the mid- and in the third-trimesters of pregnancy (mid-trimester: 10,236 pg/ml vs. 441 pg/ml [Citation76]; third trimester: 33,490 pg/ml vs. 3417 pg/ml) [Citation77]. In contrast, the concentrations of sEng were much higher in maternal circulation than in amniotic fluid (median 15.1 ng/ml vs. 0.6 ng/ml) [Citation78]. These observations suggest that sVEGFR-1 and sEng in maternal plasma might originate from different sources. Plasma concentrations of sVEGFR-1 and sEng are already elevated during pregnancy and our findings indicate that the exposure of trophoblasts, monocytes, and endothelial cells to bacterial products or high concentrations of pro-inflammatory cytokines is not able to stimulate or release more sVEGFR-1 or sEng into the maternal circulation. One possible explanation for these findings could be that peripheral blood mononuclear cells are already activated and the exposure to microbial products or pro-inflammatory cytokines could not further increase the release of sVEGFR-1.
Angiogenic profiles of patients with preeclampsia
The changes in angiogenic/anti-angiogenic factors in PE are more severe than those observed in AP. There was a five-fold decrease in the median plasma concentration of PlGF, whereas the median plasma concentrations of sVEGFR-1 and sEng increased 8–10-fold in PE compared with women with AP. These findings indicate that acute systemic inflammation during pregnancy could not reduce the concentrations of angiogenic factors or increase the concentration of anti-angiogenic factors in maternal plasma to the extent observed in PE, and that PE is a much more severe ‘anti-angiogenic state’ than pregnancy with acute systemic inflammation (AP). There was, however, no significant difference in plasma concentrations of sVEGFR-2, a surrogate marker of endothelial cell function, between patients with PE and pregnant women with AP.
Strength and limitation of the study
This study is the first to examine the changes in plasma concentrations of pro- and anti-angiogenic factors in pregnant women with acute infection. The finding that the changes of angiogenic profiles in pregnant women with AP are different from those observed in the septic non-pregnant patients is novel and deserve further investigation. Moreover, we confirmed that women with AP in this study had high serum cytokine concentrations, which were consistent with systemic inflammation. The limitation of this study is that it did not include pregnant women with more severe clinical manifestations of sepsis (e.g. septic shock or multi-organ failure). However, we have previously reported that most patients (90%) with pyelonephritis fulfilled the criteria of systemic inflammatory response syndrome (SIRS) for non-pregnant subjects at admission [Citation79]. Of note, the criteria for SIRS from the 2001 International Sepsis Definition conference [Citation80] do not consider the physiologic changes of normal pregnancy, and there is no modified definition of SIRS for pregnant women [Citation80].
In conclusion, the changes of angiogenic profiles in pregnant women with AP from NP women are different from those reported in septic non-pregnant patients. Although some of these changes resemble the observations reported in PE (decreased PlGF), the magnitude of the changes is much more severe in PE. These findings lead us to conclude that despite high plasma inflammatory cytokine concentrations, acute systemic inflammation in pregnancy has a different angiogenic profile from that of PE.
Acknowledgement
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Hill JB, Sheffield JS, McIntire DD, Wendel GD, Jr. Acute pyelonephritis in pregnancy. Obstet Gynecol 2005;105:18–23.
- Bacak SJ, Callaghan WM, Dietz PM, Crouse C. Pregnancy-associated hospitalizations in the United States, 1999–2000. Am J Obstet Gynecol 2005;192:592–597.
- Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinat Med 2003;31:41–46.
- Cunningham FG, Leveno KJ, Hankins GD, Whalley PJ. Respiratory insufficiency associated with pyelonephritis during pregnancy. Obstet Gynecol 1984;63:121–125.
- Amstey MS. Frequency of adult respiratory distress syndrome in pregnant women who have pyelonephritis. Clin Infect Dis 1992;14:1260–1261.
- Bubeck RW. Acute pyelonephritis during pregnancy with anuria, septicemia and thrombocytopenia. Del Med J 1968;40:143–147.
- Sheffield JS. Sepsis and septic shock in pregnancy. Crit Care Clin 2004;20:651–660.
- Galvagno SM Jr, Camann W. Sepsis and acute renal failure in pregnancy. Anesth Analg 2009;108:572–575.
- Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstet Gynecol 1997;90:553–561.
- Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 2005;24:508–512.
- van der FM, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock 2005;23:35–38.
- Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 2006;203:1447–1458.
- Tsao PN, Chan FT, Wei SC, Hsieh WS, Chou HC, Su YN, Chen CY, Hsu WM, Hsieh FJ, Hsu SM. Soluble vascular endothelial growth factor receptor-1 protects mice in sepsis. Crit Care Med 2007;35:1955–1960.
- Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, Kang PM, Luscinskas W, Robson SC, Carmeliet P, et al Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med 2008;205:2623–2631.
- Karlsson S, Pettila V, Tenhunen J, Lund V, Hovilehto S, Ruokonen E. Vascular endothelial growth factor in severe sepsis and septic shock. Anesth Analg 2008;106:1820–1826.
- Shapiro NI, Yano K, Okada H, Fischer C, Howell M, Spokes KC, Ngo L, Angus DC, Aird WC. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock 2008;29:452–457.
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989;161:1200–1204.
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506.
- Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol 1988;12:302–323.
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30(Suppl A):S32–S37.
- Sibai BM. Hypertensive disorders of pregnancy: the United States perspective. Curr Opin Obstet Gynecol 2008;20:102–106.
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta 2009;30(Suppl A):S38–S42.
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 2002;90:1274–1281.
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 2002;9:147–160.
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–86.
- Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–797.
- Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von DP. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006;27:56–61.
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 1997;37:240–249.
- Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008;14:855–862.
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993;90:10705–10709.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–1022.
- Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol 2000;183:1554–1557.
- Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001;184:1267–1272.
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–182.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–649.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005;94:209–231.
- Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 2006;9:225–230.
- ten DP, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008;11:79–89.
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39:469–478.
- Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008;21:41–52.
- Chaiworapongsa T, Romero R, Tarca AL, Kusanovic JP, Gotsch F, Mittal P, Erez O, Vaisbuch E, Mazaki-Tovi S, Dong Z, et al A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Reprod Sci 2009.16:264A.
- Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The calcium for preeclampsia prevention (CPEP) study group. Am J Obstet Gynecol 1997;177:1003–1010.
- von DP, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy 2003;22:143–148.
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, Erez O, Bujold E, Goncalves LF, et al Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, et al Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 2007;115:1789–1797.
- Gargett CE, Zaitseva M, Bucak K, Chu S, Fuller PJ, Rogers PA. 17Beta-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: role of estrogen receptor-alpha and -beta. J Clin Endocrinol Metab 2002;87:4341–4349.
- Hastings JM, Licence DR, Burton GJ, Charnock-Jones DS, Smith SK. Soluble vascular endothelial growth factor receptor 1 inhibits edema and epithelial proliferation induced by 17beta-estradiol in the mouse uterus. Endocrinology 2003;144:326–334.
- Makrydimas G, Sotiriadis A, Savvidou MD, Spencer K, Nicolaides KH. Physiological distribution of placental growth factor and soluble Flt-1 in early pregnancy. Prenat Diagn 2008;28:175–179.
- Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today 1999;20:114–118.
- Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001;185:1118–1123.
- Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, Kalache K, Edwin S, Bujold E, Gomez R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002;12:19–27.
- Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005;26:563–573.
- Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology 2002;107:145–151.
- Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, et al Toll-like receptor 4: a potential link between ‘danger signals’, the innate immune system, and preeclampsia?Am J Obstet Gynecol 2005;193:921–927.
- Knofler M, Mosl B, Bauer S, Griesinger G, Husslein P. TNF-alpha/TNFRI in primary and immortalized first trimester cytotrophoblasts. Placenta 2000;21:525–535.
- Yui J, Hemmings D, Garcia-Lloret M, Guilbert LJ. Expression of the human p55 and p75 tumor necrosis factor receptors in primary villous trophoblasts and their role in cytotoxic signal transduction. Biol Reprod 1996;55:400–409.
- Ejima K, Koji T, Tsuruta D, Nanri H, Kashimura M, Ikeda M. Induction of apoptosis in placentas of pregnant mice exposed to lipopolysaccharides: possible involvement of Fas/Fas ligand system. Biol Reprod 2000;62:178–185.
- Sun QH, Peng JP, Xia HF, Yang Y. IFN-gamma promotes apoptosis of the uterus and placenta in pregnant rat and human cytotrophoblast cells. J Interferon Cytokine Res 2007;27:567–578.
- Gotsch F, Kim YM, Kim CJ, Edwin S, Holtra J, Tarca AL, Chaiworapongsa T, Kusanovic JP, Mor G, Redman C, et al A link between inflammation/infection and anti-angiogenic state in preeclampsia: inflammatory mediators mimic effect of hypoxia on trophoblast by increasing sflt-1 and decreasing placental growth factor production. International society for the study of hypertension in pregnancy. Proceedings World Congress XVI. Washington DC 2008, page 46, ABS1-7.
- Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2008;198:7–22.
- Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res 2004;2:315–326.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000;95:952–958.
- Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod 1996;11:1090–1098.
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod 2001;7:205–210.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998;59:1540–1548.
- Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Olovsson M. Early postpartum changes in circulating pro- and anti-angiogenic factors in early-onset and late-onset pre-eclampsia. Acta Obstet Gynecol Scand 2008;87:146–153.
- Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab 2008;93:260–266.
- Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–989.
- Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2005;122:33–39.
- Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol 2007;197:176.
- Kusanovic JP, Romero R, Esoinoza J, Gotsch F, Edwin S, Chaiworapongsa T, Mittal P, Soto E, Erez O, Mazaki-Tovi S, et al Maternal serum soluble CD30 is increased in pregnancies complicated with acute pyelonephritis. J Matern Fetal Neonatal Med 2007;20:803–811.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256.