Abstract
Objective. Pyelonephritis during pregnancy is associated with a more severe course than in the non-pregnant state. This has been attributed to an increased susceptibility of pregnant women to microbial products. The complement system is part of innate immunity and its alternative pathway is activated mainly by microorganisms. The purpose of this study was to determine if activation of the alternative pathway of the complement system (determined by maternal fragment Bb concentrations) occurs in pregnant women with acute pyelonephritis.
Methods. This cross-sectional study included the following groups: (1) normal pregnant women (n = 62) and (2) pregnant women with pyelonephritis (n = 38). Maternal plasma fragment Bb concentrations were determined by ELISA. Non-parametric statistics were used for analyses.
Results. (1) Pregnant women with pyelonephritis had a higher median plasma concentration of fragment Bb than those with a normal pregnancy (1.3 μg/ml, IQR: 1.1–1.9 vs. 0.8 μg/ml, IQR: 0.7–0.9; p < 0.001); (2) No significant differences were observed in the median maternal plasma concentration of fragment Bb between pregnant women with pyelonephritis who had a positive blood culture and those with a negative blood culture (1.4 μg/ml, IQR: 1.1–3.5 vs. 1.3 μg/ml, IQR: 1.1–1.9; p = 0.2).
Conclusions. Pregnant women with acute pyelonephritis have evidence of activation of the alternative pathway of the complement system, regardless of the presence or absence of a positive blood culture.
Introduction
Pregnancy is a unique immune state characterised by activation of the innate immune response, and suppression of the adaptive immunity [Citation1–3]. These changes are thought to provide protection to the mother against infection [Citation1–3] and promote tolerance to the foetus, who is considered a semi-allograft [Citation4,Citation5]. Nonetheless, pregnant women are more susceptible to the effects of microbial products [Citation6–8]. Indeed, pregnant women with acute pyelonephritis are at risk of developing sepsis and acute respiratory distress syndrome [Citation9–13], complications that are rare in non-pregnant women with pyelonephritis. Although the reasons for this increased susceptibility to complications remains unknown, it has been proposed that it may due either to a Th2 biased immune response [Citation14] or due to the activation of neutrophils and monocytes that occur during normal pregnancy [Citation1,Citation2,Citation15,Citation16].
The complement system, a major component of the innate immune response, plays an important role in host defence against invading pathogens [Citation17,Citation18]. The complement cascade is formed by several plasma proteins with catalytic properties that react in a sequential manner, yielding active biological mediators and lytic components to clear foreign cells [Citation17–19]. In addition, the complement system is involved in the regulation of the adaptive immune response [Citation20]. Complement can be activated by three mechanisms: (1) the classical pathway, triggered by the binding of C1q to antigen-antibody complexes or directly to the surface of a microorganism; (2) the mannan-binding lectin (MBL) pathway, initiated by the binding of MBL to mannose-containing carbohydrates on microorganisms or (3) the alternative pathway, triggered by deposition of spontaneously activated complement components directly on microbial surfaces [Citation18,Citation21]. All three pathways converge at the point of C3-convertase formation [Citation18,Citation22] which cleaves C3 into C3a and C3b. Fragment Bb is a marker for activation of the alternative complement pathway and function as a protease to cleave additional C3 molecules essential for the complement cascade. Moreover, the alternative pathway amplifies the inflammatory effects of the classical pathway [Citation23]. Recently, high maternal plasma concentration of fragment Bb in the midtrimester was associated with obstetrical complications including preeclampsia [Citation24] and preterm labour [Citation25]. Moreover, increased amniotic fluid concentration of fragment Bb was reported in patients with preterm labour and preterm premature rupture of membranes with intra-amniotic infection (IAI)/inflammation [Citation26].
Previous studies have demonstrated that acute pyelonephritis during pregnancy is associated with activation of the complement system as indicated by higher plasma concentrations of complement split product C5a compared to normal pregnancies [Citation27]. The purpose of this study was to determine if activation of the alternative pathway of the complement system (as determined by maternal fragment Bb) occurs in pregnant women with acute pyelonephritis.
Material and methods
Study design
A cross-sectional study was conducted by searching our clinical database and bank of biological samples to compare maternal plasma concentration of complement fragment Bb between normal pregnant women (n = 62) and pregnant patients with acute pyelonephritis (n = 38). Women with multiple pregnancies and/or foetal congenital or chromosomal anomalies were excluded. Patients were considered to have a normal pregnancy if they did not have any obstetrical, medical or surgical complication of pregnancy, and delivered a term neonate of appropriate birth weight for gestational age [Citation28]. Acute pyelonephritis was diagnosed in the presence of fever (temperature ≥38°C), clinical signs and/or symptoms of upper urinary tract infection (e.g. flank pain, costovertebral angle tenderness), and a positive urine culture for microorganisms. Blood cultures were also performed in most patients. The body mass index (BMI) was calculated according to the formula: weight (kg)/height (m2).
Eligible patients were approached at the Detroit Medical Center/Hutzel Hospital in Detroit, Michigan. All women provided written informed consent prior to the collection of blood samples. The collection of samples was approved by the Institutional Review Boards of both the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Wayne State University. Many of these samples have been used in previous studies of the biology of inflammation in normal pregnant women and those with pyelonephritis during pregnancy [Citation27,Citation29–32].
Blood collection
Samples of peripheral blood were collected into tubes containing ethylene diamine tetraacetic acid (EDTA). The samples were centrifuged at 1300g and stored at −70°C until assayed. Maternal plasma concentration of human fragment Bb was determined by sensitive enzyme-linked immunoassays (Quidel Corporation, San Diego, CA). The fragment Bb immunoassay was validated for human plasma in our laboratory prior to the conduction of this study. Immunoassays were carried out according to the manufacturer's recommendations. The calculated inter- and intra-assay coefficients of variation for fragment Bb immunoassays in our laboratory were 3.3% and 2.6%, respectively, and the sensitivity was 0.015 μg/ml.
Statistical analysis
The Kolgomorov-Smirnov test was used to determine whether the data were normally distributed. Mann–Whitney U tests were used to compare the median between normal pregnant women and pregnant patients with acute pyelonephritis. Chi square test was utilised for comparison of proportions. Correlations between continuous variables were assessed by Spearman's rho correlation test. The statistical package used was SPSS 12 (SPSS Inc. Chicago, IL). A probability value <0.05 was considered significant.
Results
Fragment Bb was detected in all plasma samples included in this study. The demographic and clinical characteristics of women in each group are displayed in . There was no difference in the demographic and clinical characteristics between the two study groups.
Table I. Demographic and clinical characteristics of the study population.
The most common microorganism isolated from urine cultures was Escherichia coli [81.5% (31/38)]. Other microorganisms included Gram negative bacilli (n = 1), Klebsiella pneumoniae (n = 1), Staphylococcus aureus (n = 1), Proteus mirabilis (n = 1), Pseudomonas aeruginosa (n = 1), Citrobacter koseri (n = 1) and Streptococcus agalactiae (n = 1). Blood cultures were performed in 86.8% (33/38) of patients with pyelonephritis, and 39.3% (13/33) were positive for microorganisms. Escherichia coli was the most common microorganism isolated from blood cultures [69.2% (9/13)].
Among the normal pregnancy group, the concentrations of fragment Bb in maternal plasma did not correlate with gestational age at venipucture (Spearman's ρ = −0.3, p = 0.8) or with pre-pregnancy BMI (Spearman's ρ = 0.4, p = 0.8).
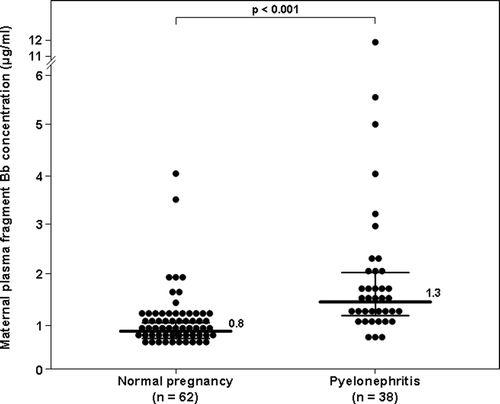
The median maternal plasma concentration of fragment Bb in patients with acute pyelonephritis was significantly higher than that of normal pregnant women (1.3 μg/ml, interquartile range [IQR] 1.1–1.9 vs. 0.8 μg/ml, IQR 0.7–0.9; p < 0.001, ). Among patients with acute pyelonephritis, no significant difference was observed in the median plasma concentration of fragment Bb between those with a positive blood culture and those with a negative blood culture (1.4 μg/ml, IQR 1.1–3.5 vs. 1.3 μg/ml, IQR 1.1–1.9 respectively; p = 0.2).
Figure 1. Plasma fragment Bb concentrations in normal pregnancy and pregnancies complicated with acute pyelonephritis. The median plasma concentration of fragment Bb was significantly higher in patients with acute pyelonephritis than in normal pregnant women (median 1.3 μg/ml, IQR 1.1–1.9 vs. 0.8 μg/m, IQR 0.7–0.9; p < 0.001).
Discussion
Principal findings of this study
Pregnant women with acute pyelonephritis had a higher median plasma concentration of fragment Bb than normal pregnant women. This finding is novel because it demonstrates activation of the alternative complement pathway in acute pyelonephritis during pregnancy.
The alternative complement pathway
The complement system, an effector arm of the innate immune system, plays a pivotal role in the host defence against infection. In addition, the complement system mediates the inflammatory response elicited against infection and participates in activating the adaptive immune system [Citation22,Citation33,Citation34]. Activation of the complement cascade through either of the three complement activation pathways converges at the point of C3 convertase formation [Citation18] which cleaves C3 to C3a and C3b. C3b participates in the formation of the C5 convertase, which cleaves C5 to C5a and C5b. C3a and C5a are also known as anaphylatoxins, which are key inflammatory mediators [Citation22,Citation35]. The alternative pathway is capable of spontaneous activation through a slow rate hydrolysis of C3, generating C3(H2O) [Citation36]. The latter can covalently bind factor B, which is cleaved by factor D to become the active protease, fragment Bb [Citation37]. The active convertase, C3(H2O)Bb complex, can cleave additional native C3 molecules, generating C3b, which in turn can associate with factor B to generate more C3-convertase [Citation22], creating an amplification loop. Thus, production of fragment Bb has been proposed as a marker of activation of the alternative pathway [Citation22].
Since fragment Bb is essential for the alternative pathway and for the cleavage of the C3 molecule of the complement cascade [Citation38] we would have expected to find increased concentration of C3a in patients with acute pyelonephritis. However, we have previously reported that among the complement splits products C3a, C4a and C5a, the latter was the only complement split product increased in the plasma of patients with acute pyelonephritis during pregnancy [Citation27]. A possible explanation for this finding is that another source, different from the conventional complement pathway enzymes, such as neutrophil elastase and myeloperoxidase, as well as macrophage serine proteases, can cleave C5 directly and generate the powerful inflammatory mediator C5a [Citation35,Citation39–42]. Thus, it is possible that leukocyte activation may account for the high concentration of C5a found in the plasma of pregnant patients with acute pyelonephritis.
Alternative pathway and medical conditions
Unregulated activation of the complement system has been implicated in the pathophysiology of numerous disorders [Citation34] such as systemic lupus erythematosus [Citation43], rheumatoid arthritis [Citation44,Citation45] and stroke [Citation46,Citation47]. Moreover, the alternative complement pathway has been specifically suggested to have a role in the pathophysiology of several diseases such as age-related macular degeneration [Citation48–51], rheumatoid arthritis [Citation52,Citation53] and lupus nephritis [Citation54]. More recently, the role of the alternative pathway of the complement system in septic shock, specifically Factor B, (the precursor of fragment Bb) was demonstrated in in vitro studies were peripheral blood mononuclear cells from septic shock patients showed increased Factor B mRNA expression when compared to control patients [Citation55].
Fragment Bb and pregnancy
A significant association between systemic maternal activation of the alternative pathway and pregnancy complications was first suggested in the setting of spontaneous foetal loss [Citation51,Citation56–59]. Subsequently, the alternative pathway has been implicated in other obstetrical complications. Lynch et al. [Citation24] first reported an association between high maternal plasma fragment Bb concentrations (≥90th percentile of their entire cohort) in early pregnancy (<20 weeks of gestation) and an increased risk for development of preeclampsia. A subsequent study by the same group [Citation25] reported an association between high maternal plasma concentrations of fragment Bb (≥75th percentile) in early pregnancy and spontaneous preterm birth. Consistent with the latter finding, we have recently reported an association between increased amniotic fluid concentration of fragment Bb and IAI in patients with spontaneous preterm labour with intact membranes and with preterm prelabour rupture of membranes [Citation26]. We have proposed that these findings represent alternative pathway activation as part of the foetal innate immune response [Citation60,Citation61] to IAI. Moreover, patients with spontaneous preterm labour with intact membranes who had a preterm delivery, either with or without IAI had a higher median maternal plasma fragment Bb concentration than those with an episode of preterm labour who delivered at term [Citation62].
Inflammation and normal pregnancy
Normal pregnancy is considered a pro-inflammatory state. Evidence in support of this view includes: (1) the total white blood cell count in maternal blood increases with advancing gestational age [Citation63]; (2) there is an increased circulating concentration of acute phase proteins, such as fibrinogen and clotting factors [Citation64,Citation65]; (3) the complement system is activated in normal pregnancy [Citation66,Citation67]; (4) leukocytes from normal pregnant women have phenotypic and metabolic changes of monocytes and granulocytes, that are consistent with either priming or activation of the cells [Citation1,Citation2,Citation16]; (5) impaired neutrophil apoptosis; [Citation68] and (6) interleukin (IL)- 12 and IL-1β production by human monocytes obtained during pregnancy is higher compared to non-pregnant state [Citation15,Citation69]. In contrast to the common perception of normal pregnancy characterised by a pro-inflammatory state, the concentrations of fragment Bb in normal pregnancy were not different than those of non-pregnant women.[Citation25,Citation62] Therefore, a bacterial insult is required to activate the alternative pathway and increase the concentration of fragment Bb in pregnancies complicated with pyelonephritis as reported in this study.
In conclusion, acute pyelonephritis during pregnancy is associated with a higher maternal plasma concentration of fragment Bb than normal pregnancy. This finding provides additional evidence that the complement system, specifically the alternative pathway, is activated in pyelonephritis during pregnancy.
Acknowledgements
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–86.
- Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001;185:1118–1123.
- Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today 1999;20:114–118.
- Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J.Reprod.Immunol 2003;60:1–11.
- Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. 1953;7:320–338.
- Kort BA, Cefalo RC, Baker VV. Fatal influenza A pneumonia in pregnancy. Am J Perinatol 1986;3:179–182.
- Rodrigues J, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med 1992;13:679–691.
- Kochar DK, Thanvi I, Joshi A, Shubhakaran, Agarwal N, Jain N. Mortality trends in falciparum malaria – effect of gender difference and pregnancy. J Assoc Physicians India 1999;47:774–778.
- Catanzarite VA, Willms D. Adult respiratory distress syndrome in pregnancy: report of three cases and review of the literature. Obstet Gynecol Surv 1997;52:381–392.
- Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: a clinical review. Obstet Gynecol 1973;42:112–117.
- Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol 1987;156:797–807.
- Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol 1994;8:353–373.
- Pruett K, Faro S. Pyelonephritis associated with respiratory distress. Obstet Gynecol 1987;69:444–446.
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–356.
- Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol 2002;72:874–884.
- Luppi P, Haluszczak C, Trucco M, DeLoia JA. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol 2002;47:72–81.
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol 2004;41:1089–1098.
- Walport MJ. Complement. First of two parts. N Engl J Med 2001;344:1058–1066.
- Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002;296:298–300.
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol 2004;5:981–986.
- Volanakis JE. Overview of the complement system. In: Volanakis JE, Frank MM, editors. The Human Complement System in Health and Disease. New York: Marcel Dekker, Inc.; 1998. pp 9–32.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, editors. Janeway's Immunobiology. New York: Garland Science; 2008. pp 39–103.
- Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 2004;138:439–446.
- Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol 2008;198:385–389.
- Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol 2008;199:354–358.
- Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Pedro KJ, Soto E, Gotsch F, Dong Z, Chaiworapongsa T, Kim SK, et al Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–916.
- Soto E, Richani K, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med 2005;17:247–252.
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Chaiworapongsa T, Romero R, Gotsch F, Kusanovic JP, Mittal P, Kim SK, Erez O, Vaisbuch E, Mazaki-Tovi S, Kim CJ, et al Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. J Matern Fetal Neonatal Med 2010;23:167–178.
- Gotsch F, Romero R, Espinoza J, Kusanovic JP, Mazaki-Tovi S, Erez O, Than NG, Edwin S, Mazor M, Yoon BH, et al Maternal serum concentrations of the chemokine CXCL10/IP-10 are elevated in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med 2007;20:735–744.
- Kusanovic JP, Romero R, Esoinoza J, Gotsch F, Edwin S, Chaiworapongsa T, Mittal P, Soto E, Erez O, Mazaki-Tovi S, et al Maternal serum soluble CD30 is increased in pregnancies complicated with acute pyelonephritis. J Matern Fetal Neonatal Med 2007;20:803–811.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Chaiworapongsa T, Erez O, Mittal P, Kim SK, Gotsch F, Lamont R, Ogge G, et al Low circulating maternal adiponectin in patients with pyelonephritis: adiponectin at the crossroads of pregnancy and infection. J Perinat Med 2010;38:9–17.
- Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol 2003;107:140–151.
- Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol 2006;176:1305–1310.
- Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol 2004;4:133–142.
- Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem 1988;57:321–347.
- Gotze O, Muller-Eberhard HJ. The C3-activator system: an alternate pathway of complement activation. J Exp Med 1971;134:90s–108s.
- Xu Y, Narayana SV, Volanakis JE. Structural biology of the alternative pathway convertase. Immunol Rev 2001;180:123–135.
- Ward PA, Hill JH. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol 1970;104:535–543.
- Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology 1996;195:334–346.
- Vogt W. Cleavage of the fifth component of complement and generation of a functionally active C5b6-like complex by human leukocyte elastase. Immunobiology 2000;201:470–477.
- Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol 2002;161:1849–1859.
- Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res 2002;4(Suppl. 3):S279–S293.
- Nakagawa K, Sakiyama H, Tsuchida T, Yamaguchi K, Toyoguchi T, Masuda R, Moriya H. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann Rheum Dis 1999;58:175–181.
- Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, Judex M, Kullmann F, Mountz JD, Scholmerich J, Gay S, et al Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum 2002;46:934–945.
- del Zoppo GJ. In stroke, complement will get you nowhere. Nat Med 1999;5:995–996.
- Huang J, Kim LJ, Mealey R, Marsh HC Jr., Zhang Y, Tenner AJ, Connolly ES Jr., Pinsky DJ. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science 1999;285:595–599.
- Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308:421–424.
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, et al Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308:419–421.
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, et al Complement factor H polymorphism in age-related macular degeneration. Science 2005;308:385–389.
- Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 2009;50:5818–5827.
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, et al Arthritis critically dependent on innate immune system players. Immunity 2002;16:157–168.
- Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, Holers VM. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol 2006;177:1904–1912.
- Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int 2004;65:129–138.
- Goring K, Huang Y, Mowat C, Leger C, Lim TH, Zaheer R, Mok D, Tibbles LA, Zygun D, Winston BW. Mechanisms of human complement factor B induction in sepsis and inhibition by activated protein C. Am J Physiol Cell Physiol 2009;296:C1140–C1150.
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, et al Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003;112:1644–1654.
- Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, Molina H. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity 2003;19:813–822.
- Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2001;2:64–68.
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 2000;287:498–501.
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202.
- Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–683.
- Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, Dong Z, Chaiworapongsa T, Kim SK, Ogge G, et al Activation of the alternative pathway of complement is a feature of preterm parturition but not of spontaneous labor at term. Am J Reprod Immunol 2010; E-pub Feb 16. PMID: 20163401.
- Efrati P, Presentey B, Margalith M, Rozenszajn L. Leukocytes of normal pregnant women. Obstet Gynecol 1964;23:429–432.
- Comeglio P, Fedi S, Liotta AA, Cellai AP, Chiarantini E, Prisco D, Mecacci F, Parretti E, Mello G, Abbate R. Blood clotting activation during normal pregnancy. Thromb Res 1996;84:199–202.
- Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost 1984;52:176–182.
- Hopkinson ND, Powell RJ. Classical complement activation induced by pregnancy: implications for management of connective tissue diseases. J Clin Pathol 1992;45:66–67.
- Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 2005;17:239–245.
- von Dadelszen P, Watson RW, Noorwali F, Marshall JC, Parodo J, Farine D, Lye SJ, Ritchie JW, Rotstein OD. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol 1999;181:408–414.
- Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol 2003;131:490–497.