Abstract
Objective. Biomarkers for preterm labor (PTL) and delivery can be discovered through the analysis of the transcriptome (transcriptomics) and protein composition (proteomics). Characterization of the global changes in low-molecular weight compounds which constitute the ‘metabolic network’ of cells (metabolome) is now possible by using a ‘metabolomics’ approach. Metabolomic profiling has special advantages over transcriptomics and proteomics since the metabolic network is downstream from gene expression and protein synthesis, and thus more closely reflects cell activity at a functional level. This study was conducted to determine if metabolomic profiling of the amniotic fluid can identify women with spontaneous PTL at risk for preterm delivery, regardless of the presence or absence of intraamniotic infection/inflammation (IAI).
Study Design. Two retrospective cross-sectional studies were conducted, including three groups of pregnant women with spontaneous PTL and intact membranes: (1) PTL who delivered at term; (2) PTL without IAI who delivered preterm; and (3) PTL with IAI who delivered preterm. The first was an exploratory study that included 16, 19, and 20 patients in groups 1, 2, and 3, respectively. The second study included 40, 33, and 40 patients in groups 1, 2, and 3, respectively. Amniotic fluid metabolic profiling was performed by combining chemical separation (with gas and liquid chromatography) and mass spectrometry. Compounds were identified using authentic standards. The data were analyzed using discriminant analysis for the first study and Random Forest for the second.
Results. (1) In the first study, metabolomic profiling of the amniotic fluid was able to identify patients as belonging to the correct clinical group with an overall 96.3% (53/55) accuracy; 15 of 16 patients with PTL who delivered at term were correctly classified; all patients with PTL without IAI who delivered preterm neonates were correctly identified as such (19/19), while 19/20 patients with PTL and IAI were correctly classified. (2) In the second study, metabolomic profiling was able to identify patients as belonging to the correct clinical group with an accuracy of 88.5% (100/113); 39 of 40 patients with PTL who delivered at term were correctly classified; 29 of 33 patients with PTL without IAI who delivered preterm neonates were correctly classified. Among patients with PTL and IAI, 32/40 were correctly classified. The metabolites responsible for the classification of patients in different clinical groups were identified. A preliminary draft of the human amniotic fluid metabolome was generated and found to contain products of the intermediate metabolism of mammalian cells and xenobiotic compounds (e.g. bacterial products and Salicylamide).
Conclusion. Among patients with spontaneous PTL with intact membranes, metabolic profiling of the amniotic fluid can be used to assess the risk of preterm delivery in the presence or absence of infection/inflammation.
Introduction
Preterm parturition is one of the ‘Great Obstetrical Syndromes’. This term was coined to indicate that obstetrical disorders have: (1) multiple etiologies; (2) a long preclinical phase; (3) frequent fetal involvement; (4) be adaptive in nature; and (5) be the result of gene–environment interactions [Citation1–4]. Progress in the understanding of the causes of preterm labor (PTL) has relied on hypothesis-driven research [Citation5–73]. This approach has yielded important information. However, the development of high-dimensional biology promises to provide a comprehensive description of the biological processes in complex diseases such as the preterm parturition syndrome [Citation4,Citation74,Citation75] and identify biomarkers with prognostic and diagnostic implications, which may be more difficult to identify using a reductionist approach.
High-dimensional biology refers to the use of high throughput techniques that allow simultaneous examination of changes in the genome (DNA), transcriptome (mRNA), proteome (proteins), lipidome (lipids), and other biochemical components in a biological sample with the goal of understanding the physiology or mechanisms of disease [Citation76–78]. The term ‘ome’ refers to an abstract entity, group of mass, while ‘omics’ sciences refer to the study of entities in aggregate [Citation79,Citation80]. Such techniques have included genomics [Citation81–85], transcriptomics [Citation86–88], proteomics [Citation89,Citation90], and lipidomics [Citation91–93], and their fundamental principle is that a complex system can be understood more completely by considering its entirety, allowing for a global description of changes in biological samples.
Metabolites, which have small molecular weights, are part of primary and intermediate metabolism and are estimated to be represented by more than 2000 compounds in the human metabolome [Citation94–97], which refers to the catalog of those molecules in a specific organism or compartment, such as human metabolome, plasma metabolome, or amniotic fluid metabolome. Metabolomics is the study of the repertoire of nonproteinaceous, small molecules present in an organ, tissue, or fluid [Citation98]. Like other omics sciences, this discovery-based approach does not require a specific hypothesis and it is presumably unbiased in nature. So far, examination of the cervico-vaginal and amniotic fluid proteome has been employed to identify biomarkers for spontaneous PTL [Citation99–110]. The objective of this study was to determine, for the first time, if amniotic fluid metabolic profiling could be used to identify women with PTL and intact membranes at risk for preterm delivery, regardless of the microbial and inflammatory state of the amniotic cavity.
Materials and methods
Study design and population
We conducted a cross-sectional study by searching our clinical database and bank of biological samples to identify patients admitted to the hospital with the diagnosis of spontaneous PTL with intact membranes, and that underwent amniocentesis for the assessment of the microbial state of the amniotic cavity and/or fetal lung maturity. Inclusion criteria included: (1) singleton gestation; (2) gestational age between 22 and 35 weeks; (3) live fetus; (4) intact membranes; and (5) signed informed consent approved by the Institutional Review Board of the Sotero del Rio Hospital (an affiliate of the Pontificia Universidad Catolica de Chile, Santiago, Chile,) or Wayne State University (Detroit, MI) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Women with multiple pregnancies and those with fetal chromosomal and/or congenital anomalies were excluded.
This study was conducted in two phases and included patients divided in three groups: (1) PTL who subsequently had a delivery at term; (2) PTL without intraamniotic infection/inflammation (IAI) who delivered preterm; and (3) PTL with IAI who delivered preterm. The first study included 16 pregnant women with PTL who delivered at term, 19 women with PTL without IAI who delivered preterm, and 20 patients with PTL and IAI. The second study included 40 pregnant women with PTL who delivered at term, 33 women with PTL without IAI who delivered preterm, and 40 patients with PTL and IAI.
Definitions
Spontaneous PTL was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Preterm delivery was defined as birth before 37 weeks of gestation. Intraamniotic infection was defined as a positive amniotic fluid culture for microorganisms. For the purpose of the first study, intraamniotic inflammation was defined as an amniotic fluid white blood cell (WBC) count >100 cells/mm3 [Citation111,Citation112], while for the second study, intraamniotic inflammation was defined as an amniotic fluid interleukin (IL)- 6 concentration >2.6 ng/ml [Citation113].
Sample collection
Amniotic fluid samples were obtained from transabdominal amniocentesis performed for evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell count, glucose concentration, and Gram-stain were also performed in the amniotic fluid shortly after collection as previously described [Citation112,Citation114,Citation115]. The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged at 1300g for 10 min at 4°C, and the supernatant was aliquoted and stored at –80°C until analysis. Many of these samples have been previously used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Metabolomics methodology
Randomization of samples
Amniotic fluid samples (n = 168) were coded with a unique identifier for subsequent handling, and these identifiers were associated with a random number so that laboratory staff was blinded to the clinical diagnosis (including studies 1 and 2). The samples were processed in ascending order of their random numbers in two sets of samples. All samples were bar-coded throughout and received new barcodes at each step.
Extraction and preparation
The samples were removed from a −80° freezer and placed on ice to thaw. A 550-μl aliquot of pooled amniotic fluid (control sample) was placed on ice as well. A 100-μl aliquot of each sample was placed in individual wells of a 96-well deep-well plate, as were three 100 μl aliquots of the control sample and three 100 μl volumes of water (for blank controls). The sample placement was randomized and controlled by a platform-wide LIMS. Using a method described by Andreoli et al. [Citation116], a 400 μl volume of EtOAc/EtOH (1:1) was added to each sample and mixed on a Hamilton Star liquid-handling robot (Hamilton, Reno, NV). The samples were transferred to a filter plate and a vacuum applied. Subsequent rinse solvents were similarly applied at 200 μl each (MeOH, MeOH/H2O (3:1), and DCM/MeOH (1:1), respectively). The resulting pooled extract was divided into two equal parts for LC/MS and GC/MS, respectively. Samples were concentrated on a Labconco Centrivap Concentrator 7810000, prior to lyophilization in a Labconco Freezone 6 lyophilizer (both Labconco Corp., Kansas City, MO). The remaining samples were returned to a −80° freezer. Samples to be analyzed by liquid chromatography were redissolved in a 40% aqeuous ethanolic solution, while samples to be analyzed using gas chromatography were derivatized according to the procedure described by Gehrke and Leimer [Citation117]. All samples had a final volume of 100 μl.
Mass spectroscopy and data processing
Samples subjected to LC/MS were analyzed on a Thermo-Finnigan LTQ-FT, a hybrid ion trap-Fourier transform mass spectrometer (Thermo Fischer Scientific, Waltham, MA). The ion trap mass spectrometer (ITMS) and Fourier transform mass spectrometry (FTMS) units were run at resolutions of 1500 and 50,000, respectively, and tuned daily for these resolutions. Each sample carried a set of internal standards that were used to validate the performance of the procedures. An ITMS was scanned from 80 to 800 m/z, and FTMS analyzed from 200 to 2000 m/z. A 10-μl aliquot of the redissolved extract was injected into the solvent path of a Perflourophenyl (PFP) column. The nonlinear elution gradient began at water [0.1% formic acid (FA)] to 100% methanol (0.1% FA) at 7.5 min and ultimately to 100% acetone (0.1% FA) at 15 min. The column was reequilibrated for 10 min before the next injection.
The samples were analyzed by GC/MS on a Thermo-Finnigan Mat95 (Thermo Fischer Scientific, Waltham, MA) coupled to an ultra-trace high-resolution gas chromatograph (the Mat95 was tuned to a resolution of 10,000, and mass accuracy checked against the 131 ion of Perfluorokerosene) daily. The chromatographic conditions were nonlinear on a RTX-5-sil column. The temperatures ranged from 60 to 340°C. A 1-μl aliquot of the derivatized extract was injected into a split/splitless injector (kept at 240°C) run in splitless mode. Each sample carried a set of internal standards that were used to validate procedural parameters.
The data analysis was chemocentric; therefore, the resulting data streams were processed to identify as many compounds as could be seen by the Metabolyzer software [Citation118]. All identified peaks had a signal-to-noise ratio of >6, and an area of >50,000 ions. All named compounds were compared to authentic compounds. Compounds seen with regularity, but for which no standard was available, were assigned unique identifiers. Quantitation was relative to internal standards specific to each data stream. Data analyzed included both relative and normalized (Z-score) concentration. The identification of mass spectral peaks for both the LC and GC platforms was accomplished by comparison to authentic standards.
Data mining
Metabolomic profiles in the amniotic fluid were defined as the sets of biochemical compounds and their concentrations that best distinguish patients with PTL in one group from the other two groups. Compounds that are significantly high or low in one group could be discovered by algorithms that have been used to build ‘class predictors’. The class predictors use the concentrations of these informative compounds to distinguish the profiles of patients with PTL in one group from profiles of individuals in the other groups. In general, this is ‘supervised learning’, a type of data mining in which a learning algorithm is presented with known ‘classes’ and then attempts to derive a set of rules (a class predictor) that will predict the class membership of new samples. In investigations of this kind, class predictors are typically trained on a random subset of the data, the ‘training set’. Our study was conducted in two phases. The first set included 16 women with PTL who delivered at term, 19 women with PTL without IAI who delivered preterm, and 20 patients with PTL and IAI. The second set included 40 women with PTL who delivered at term, 33 women with PTL without IAI who delivered preterm, and 40 patients with PTL and IAI. This allowed us to rigorously assess the degree to which the class predictors have captured signatures that truly distinguish the different PTL groups. We used three approaches to class prediction: partial least square discriminate analysis (PLS-DA), relative class association/weighted voting, and scatter analysis.
Statistical analysis
Linear discriminant function analysis, which constructs linear separation boundaries, was used to explore the relationship between the metabolomic profile of amniotic fluid and clinical classification of patients for study No. 1. Random Forest, a classification tree-based algorithm, was used for the analysis of study No. 2. Single classification tree method is useful for selecting from a small collection of predictor variables those that best explain a phenotype. Some of the features of this technique are as follows: (1) ease of interpretation; (2) flexibility for the inclusion of interactions among predictors; and (3) ability to handle heterogeneity among samples. Classification trees use all variables and relevant cases when creating nodes in a single tree that represents the outcome of the learning process. However, single classification tree algorithms may easily over-fit data in the presence of a large number of predictor variables.
Alternatively, Random Forest builds a large number of classification trees, each grown using a subset of a bootstrap sample, while the remaining are left out for prediction. Each bootstrap sample was drawn randomly and of the same size from the original data with replacement. The variables used for splitting nodes in a tree are randomly chosen, much smaller subsamples of the complete set of variables. The introduced double randomness (bootstrap sample and variable selection) and internal validation ultimately leads to an unbiased estimation of misclassification error. Over-fitting in presence of a large number of variables can be therefore avoided. Importantly, Random Forest calculates importance scores which estimate the importance of a variable by looking at how much node impurities or prediction accuracy decreases when data for that variable are permuted while all other data are left unchanged. The Importance Score is a useful tool that organizes the metabolites according to the degree of contribution in classifying the cases into well-defined clinical phenotypes. Importance Scores are used to prioritize the variables in this model, and this information is used to determine the patterns that are informative with respect to the phenotype, creating a classification for each case. The results of classification are compared with actual clinical statuses. As a result of this process, matrices (true-negative, false-positive, true-positive, and false-negative) are created showing the number of patients classified properly. Classification accuracy is calculated as the proportion of total of correct classifications (number of true-negative and true-positive classifications).
In this study, the three classes were PTL and delivery with and without IAI, and PTL delivered at term. Rpackage ‘randomForest’ [Citation119] was applied to classify the three classes based on metabolomic profiling.
Gini index calculation
The variable importance calculation depends slightly upon the specific algorithm used to split nodes in growing a trees in Random Forest. A splitting rule takes all the cases at a particular node in a decision tree and divides those cases so that some go to the left branch and others go to the right branch. There are a very large number of possible splitting rules – one for each explanatory variable and every possible split-point for that variable. At each node in the current tree, Classification and Regression Trees (CART) does a computer-intensive search to find the particular splitting rule that most helps in the classification of the cases. Our analysis used the Gini impurity criterion to determine the best splitting rule at each (nonterminal) node in the tree, because of its fast but generally robust performance in classification problems. Alternative rules include the entropy criterion and the misclassification rate (see Breinan for a comparative study) [Citation120]. The rules are usually consistent with each other. Gini criterion seeks the split such that the cases that go to the left are mostly of one category (or a small set of categories), while the cases that go to the right are mostly of another category (or small set of other categories). One says that the best split maximally reduces the ‘impurity’ at that node. In Random Forest, this splitting process continues until all of the cases in a node have the same classification, and thus the node is ‘pure’.
There are many possible measures of impurity. The most widely used Gini index at a node t is
where there are k different categories among the cases at node t, and Pj is the proportion of cases in the node belonging to category j. CART searches among all the possible splits that divide cases in a parent node t into a left- and right-child nodes to find the one that maximizes
where PL is the proportion of cases assigned to the left-child node, PR is the proportion of cases assigned to the right-child node, and Φ(tL), Φ(tR) are the Gini indices for the left- and right-child nodes, respectively.
Results
and display the demographic and clinical characteristics of patients included in the first and second study, respectively. Microorganisms isolated from the amniotic fluid in the first and second study are presented in .
Table I. Demographic and clinical characteristics of the study population (first study).
Table II. Demographic and clinical characteristics of the study population (second study).
Table III. Microorganisms isolated from the amniotic fluid in the first and second study.
First study (exploratory study)
The purpose of the first study was to examine whether the metabolic profile of amniotic fluid could classify patients according to phenotype. A supervised analysis with the results of the metabolic profiling of amniotic fluid using linear discriminant function analysis was conducted. The results displayed in show that the technique (metabolomics) had promise to classify patients into the three groups. displays the classification of patients according to their metabolomic profile. The accuracy of classification was 96.36% (53/55). Recognizing that this excellent diagnostic performance could have been due to over-fitting, we conducted a second study in an independent and larger set of samples using more robust-to-overfitting method to identify metabolomes separating the three groups.
Figure 1. Supervised analysis for classification of patients according to their metabolic profile of amniotic fluid. In green, patients who presented with PTL but delivered at term; in blue, those with PTL who delivered preterm without IAI and in red, those with IAI (PTL, preterm labor; IAI, intraamniotic infection/inflammation).
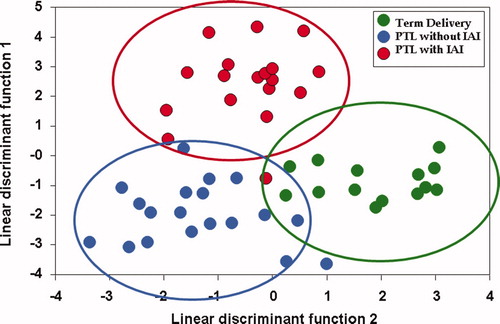
Table IV. Prediction of the clinical class according to the amniotic fluid metabolic profile (first study; supervised).
Second study (validation study)
shows the raw data of the second study in a heatmap, in which every single row represents a metabolic ‘fingerprint’ of a particular patient. A cursory inspection of the graph displays differences among groups. display the differentially expressed metabolites in the amniotic fluid of patients with PTL who delivered at term, PTL without IAI who delivered preterm, and that of those with PTL with IAI, respectively. Random Forest analysis of the metabolic profiling of the amniotic fluid accurately predicted 39 of 40 patients who delivered at term and 29 of 33 patients who delivered preterm without IAI. However, it performed less well in patients with preterm delivery with IAI (32 of 40). The overall accuracy for prediction of clinical classes was 88.5% (100/113) (). To identify the metabolites that contributed to the separation of the groups, we calculated the Gini index ().
Figure 2. Each horizontal line is a fingerprint of the metabolomic profile of amniotic fluid in an individual patient. Each vertical line represents the concentration of a metabolite in amniotic fluid. The color scale provides an index of abundance. Three patient groups are included in the study (see left column), and the metabolic fingerprinting characterizing each group is within a colored box (IAI, intraamniotic infection/inflammation).
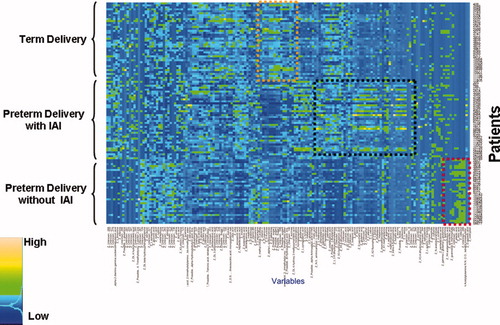
Table V. Differentially regulated metabolites in patients with PTL who delivered at term (second study).
Table VI. Differentially regulated metabolites in patients with PTL without IAI who delivered preterm (second study).
Table VII. Differentially regulated metabolites in patients with PTL with IAI (second study).
Table VIII. Prediction of the clinical class according to the amniotic fluid metabolic profile (second study).
Table IX. Metabolites important in classification determined by Random Forest (Gini index).
A list of the metabolites identified in study No. 1 and study No. 2 is provided as a supplementary table.
Discussion
Principal findings of the study
(1) The metabolome of amniotic fluid can be characterized by using parallel techniques; (2) a group of compounds previously identified as part of the human intermediate metabolism were identified in the amniotic fluid. Notably, we also identified compounds that could be considered xenobiotic, such as salicylamide and bacterial products; (3) the amniotic fluid metabolome can be analyzed for the identification of metabolites differentially expressed in patients with PTL using a combination of liquid chromatography and gas chromatography coupled with mass spectrometry; (4) metabolomic profiling was able to identify patients as belonging to the correct clinical group with a 88.5% precision (100/113); (5) patients who deliver preterm without IAI have a relative decrease in both carbohydrates and amino acids; and (6) in contrast, those with IAI have a more substantial decrease in compounds in the carbohydrate cluster, and a relative increase in amino acids.
The results of the study reported herein indicate that the presence of IAI is associated with an altered amniotic fluid metabolite composition. Specifically, amniotic fluid of women with IAI contains less carbohydrates and more amino acids compared to patients with PTL who delivered at term. Interestingly, while a decrease in carbohydrates was associated with preterm delivery in the presence or absence of IAI, an increase in amino acid metabolites is a unique feature of PTL with IAI (). Indeed, carbohydrates such as mannose, galactose, and fructose were relatively increased in patients with PTL who delivered at term, but decreased in patients with PTL and IAI. The opposite was true with amniotic fluid amino acids. A decrease in alanine, glutamine, and glutamic acid was noted in patients with PTL who delivered at term, while all of these amino acids were increased in the presence of IAI.
Table X. Relative abundance of carbohydrate and amino acids in the study groups.
The cross-sectional nature of our studies does not allow us to infer a causal or a temporal relationship between alterations in amniotic fluid metabolites and IAI. In addition, it is not clear what the specific source of each metabolite is. Fetal urine is a major contributor of amniotic fluid and both amnion cells and amniotic fluid WBC secrete their metabolites into the amniotic cavity. Nevertheless, a possible explanation for the shift from carbohydrate-rich to amino-acid-predominant metabolites could be a fetal catabolic state associated with a fetal systemic response syndrome (FIRS) [Citation121–126]. Catabolic states are associated with an increased demand for carbohydrates and decreased protein synthesis. This, in turn, can affect plasma and urine concentrations of these metabolites. Indeed, early neonatal sepsis (within 48 h from delivery) in preterm neonates born to mothers with chorioamnionitis is characterized by an increased demand for carbohydrates [Citation127]. Likewise, term neonates with infection have a higher consumption rate of carbohydrates than noninfected term newborns that can not be reconciled by changes in caloric intake [Citation128–130]. The increase in amniotic fluid amino acids further supports the hypothesis that fetal infection and/or inflammation induces a catabolic state. Sepsis is associated with changes in metabolism that result in net proteolysis and negative nitrogen balance [Citation131,Citation132]. Of interest, both nonessential (e.g. alanine and glutamate) and essential (e.g. isoleucine) amino acids were included among the differentially expressed metabolites among patients with and without IAI. The latter finding is of special importance since the source of the essential amino acids in amniotic fluid should be the maternal circulation. The increase in the amniotic fluid concentration of amino acids in patients with PTL and IAI may reflect either changes in maternal availability of these amino acids or altered placental-amino acid transport [Citation133].
An additional explanation for the changes in metabolite content of amniotic fluid in patients with PTL and IAI relates to the presence of bacteria in the amniotic cavity. Utilization of carbohydrates as nutrients by bacteria may result in decreased concentrations of these molecules. Several lines of evidence support this view: (1) a low amniotic fluid glucose concentration is a well-established biomarker of IAI [Citation134–137]. Low glucose concentrations are also associated with microbial invasion of other biological fluids including cerebrospinal [Citation138,Citation139] and synovial fluid [Citation140]; (2) fusobacterium species contain a galactose-binding protein, and it is possible that this protein may contribute to the decrease concentrations of galactose in infected amniotic fluid [Citation141]; (3) ureaplasmal lipoglycans are constituted primarily of mannose, glucose, and galactose [Citation142], suggesting that these carbohydrates may be consumed with the replication of this microorganism in the amniotic fluid; and (4) glucose is also required as a fuel by activated neutrophils engaged in the process of microbial phagocytosis and killing [Citation143].
Methyladenine was the most important classifier as determined by Random Forest analysis (Gini Index). N6-methyl-adenine (m6A) is found in the genomes of many fungi, bacteria, and protists [Citation144], as well as in archaeal DNA [Citation145]. N6-methyl-adenine is involved in many fundamental bacterial cell processes including: (1) bacterial defense against bacteriophages and transposons; (2) regulation of chromosome replication, chromosome segregation, and reorganization of the nucleoid after DNA replication; (3) DNA-strand discrimination for mismatch repair; (4) regulation of conjugal transfer of plasmids; (5) packaging of phage DNA into capsids; and (6) transcriptional regulation of fimbrial operons and other virulence genes [Citation146]. This is the first study to report the detection of methyladenine in amniotic fluid. Tavazzi et al. [Citation147] used the highly sensitive, ion-pairing high-performance liquid chromatography (HPLC) method with UV detection to identify several purines, pyrimidines, amino acids, and other molecules in amniotic fluid of 10 normal pregnant women. Despite the high sensitivity of HPLC, methyladenine was not detected. It is possible that the presence of methyladenine in amniotic fluid is due to bacterial destruction. This explanation can account for the importance of this metabolite in the classification of the study groups, as well as for the negative results reported by Tavazzi et al. [Citation147] in normal pregnant women.
The second most important metabolite in the classification of the study groups was heptanedioic acid, also know as pimelic acid [Citation148]. Diamino pimelic acid (DAP) is a component of the bacterial cell wall. Specifically, DAP is a key crosslinking constituent of the peptidoglycan layer. Most bacteria require either lysine or its biosynthetic precursor, DAP, as a component of the peptidoglycan layer of the cell wall [Citation149]. Importantly, mammals do not produce or utilize DAP and require l-lysine as a dietary component. These observations led to the hypothesis that inhibitors of the DAP biosynthetic pathway would not be expected to show mammalian toxicity and made the biosynthesis of l-lysine via DAP as a target for naturally occurring antibiotics [Citation150–152]. Recently, a DAP residue [γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP)] was identified as the minimal structure in cell wall peptidoglycan capable of stimulating nucleotide-binding oligomerization domain protein 1 (NOD1, an important component of the innate immune system) [Citation153]. iE-DAP is known to exist only in particular bacteria including common Gram-negative bacteria, such as Escherichia coli, and several Gram-positive bacteria, such as Listeria monocytogenes [Citation154]. Of note, both Escherichia coli and Listeria monocytogenes were identified in the amniotic fluid of patients in our study. Consistent with this, IL-6 production by folliculostellate cells in the normal pituitary gland is stimulated by diamino pimelic acid. It is tempting to postulate that this metabolite is released to the amniotic cavity following the destruction of bacterial cell wall.
Several metabolites that have been shown to have a prognostic value for the diagnosis of IAI and/or PTL such as prostaglandins [Citation155–163] and leukotrienes [Citation164–166] were not identified as differentially expressed in this study. Low concentrations of these metabolites, their biochemical diversity, and extraction method that are not optimized for these compounds, can account for this limitation. This issue needs to be addressed with an additional analytical platform, such as specifically optimized lipidomics, to cover a broad range of metabolites.
Metabolomics in pregnancy
High-dimensional biology techniques have been used to study normal pregnancy and parturition, as well as pregnancy complications, largely through the application of genomics [Citation167–176], transcriptomics [Citation177–198], and proteomics [Citation99,Citation101,Citation105,Citation107,Citation152,Citation199–232]. Yet, few studies have reported the use of metabolomics in reproduction and pregnancy complications. It has been proposed that metabolomics may play an important role in the management of male infertility [Citation233] and in assisted reproductive technology; indeed, the metabolomic profiling of embryo culture media and follicular fluid has been used to assess oocyte and embryo quality and viability [Citation234–242].
Recently, Heazell et al. [Citation243] used gas-chromatography-mass spectroscopy to examine the metabolic profile of conditioned culture media and tissue lysates of placental villous explants from normal pregnant women cultured at different oxygen tensions. This study demonstrated that detection of the placental metabolic footprint is feasible, and that this metabolic footprint was different among placental samples as a function of the oxygen tension used for culture. Hexadecanoic acid, threitol or erythritol, and 2-deoxyribose were among the most differentially expressed metabolites. The same authors [Citation244] conducted a similar study using ultra performance liquid chromatography–mass spectrometry (LC-MS) in serum-conditioned culture medium of villous trophoblast from placentas of normal pregnant women and patients with preeclampsia. The relative concentration of 154 metabolites was significantly different in culture medium from normal pregnancies between normoxic and hypoxic conditions. Interestingly, 47 metabolites in preeclampsia-derived conditioned media cultured under normoxic conditions had a comparable relative concentration to that of those from normal placentas cultured in hypoxic conditions, suggesting that hypoxia may have a role in preeclampsia. Three metabolic pathways (glutamate and glutamine, tryptophan metabolism, and leukotriene or prostaglandin metabolism) were considered of interest for future studies to determine their role in the pathophysiology of preeclampsia [Citation244].
Nuclear magnetic resonance spectroscopy has been used for more than 10 years to explore the metabolomic profile of the amniotic fluid to determine fetal lung [Citation245,Citation246] and kidney maturation [Citation247], as well as in other pathologic conditions such Down syndrome [Citation247], and open spina bifida [Citation247,Citation248]. Recently, 1H-NMR coupled with LC-NMR/MS has been applied to further characterize the metabolic composition of the amniotic fluid [Citation249,Citation250] and to determine its possible role as a diagnostic method for fetal malformations. The advantage of NMR analysis is that it is nondestructive of the samples. On the other hand, the metabolomics technique described in the present manuscript has a superior sensitivity. Graça et al. [Citation251] studied amniotic fluid from midtrimester genetic amniocenteses of 51 normal pregnant women and 12 pregnancies with fetal anomalies. Maternal age, fetal gender, and maternal age at amniocentesis did not change the metabolic profile of amniotic fluid of normal pregnant women. Using principal component analysis, no differences were observed between the amniotic fluid samples from normal pregnancies and those with fetal malformations. However, orthogonal variant partial least squares-discriminant analysis differentiated the two groups by identifying changes in glucose, succinate, and eight amino acids, as well as changes in the protein fraction and in the ratio of free to protein-interacting lactate. The authors suggest that these changes may represent a shift in energy production towards glycolysis due to hypoxia [Citation251].
In conclusion, this is the first draft of the human amniotic fluid metabolome in patients with spontaneous PTL with intact membranes. A stereotypic pattern of metabolites was identified in clinical groups with different outcomes, and global changes in the metabolic profile of the amniotic fluid allowed classification of patients at risk for preterm delivery (88% accuracy). Characterization of the human amniotic fluid metabolome can serve as the basis for the development of rapid tests to identify the patient at risk for impending preterm birth from the patient who will deliver at term, in the presence or absence of IAI. Further studies with more sensitive tools could increase the number of metabolites identified in amniotic fluid and improve the draft of the amniotic fluid metabolome reported herein. In addition to the study of amniotic fluid, metabolomics characterization of maternal plasma, urine and cervico-vaginal fluid can be of great importance. Insights gained from these studies could help improve the understanding of metabolism of the fetus and fetal membranes in normal and pathologic conditions.
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Romero R. The child is the father of the man. Prenat Neonat Med 1996;8–11.
- Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med 2009; 22:633–635.
- Romero R. Prenatal medicine: the child is the father of the man. J Matern Fetal Neonatal Med 2009;22:636–639.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006;113 (Suppl 3):17–42.
- Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–373.
- Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22.
- Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med 2008;21:663–670.
- Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, et al Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–461.
- Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, et al Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–616.
- Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, Than NG, Mazaki-Tovi S, Chaiworapongsa T, et al The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21:529–547.
- Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, Than NG, Mittal P, Espinoza J, Friel LA, et al Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med 2008;36:217–227.
- Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med 2008;21:902–916.
- Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633–638.
- Lee SE, Romero R, Park IS, Seong HS, Park CW, Yoon BH. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med 2008;21:89–94.
- Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med 2008;36:485–496.
- Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, Espinoza J, Erez O, Nhan-Chang CL, Than NG, et al CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 2008;60:246–257.
- Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, Erez O, Edwin S, Schmidt AM. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008;36:388–398.
- Vaisbuch E, Romero R, Erez O, Kusanovic JP, Gotsch F, Than NG, Mazaki-Tovi S, Mittal P, Edwin S, Hassan SS. Total hemoglobin concentration in amniotic fluid is increased in intraamniotic infection/inflammation. Am J Obstet Gynecol 2008;199:426–427.
- Cruciani L, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Mazaki-Tovi S, Mittal P, Ogge G, Gotsch F, Erez O, et al Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med 2010;38:161–171.
- Erez O, Romero R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Gotsch F, Gomez R, Maymon E, Pacora P, et al Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med 2009;221–12.
- Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Edwin SS, et al Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;19626565:1–16.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, et al Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;19591073:1–11.
- Pacora P, Romero R, Chaiworapongsa T, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Jai KC, Than NG, et al Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med 2009;37:503–511.
- Romero R, Pedro KJ, Munoz H, Gomez R, Lamont RF, Yeo L. Allergy-induced preterm labor after the ingestion of shellfish. J Matern Fetal Neonatal Med 2010;23351–359.
- Romero R, Kusanovic JP, Gomez R, Lamont R, Bytautiene E, Garfield RE, Mittal P, Hassan SS, Yeo L. The clinical significance of eosinophils in the amniotic fluid in preterm labor. J Matern Fetal Neonatal Med 2010;23:320–329.
- Soto E, Romero R, Richani K, Yoon BH, Chaiworapongsa T, Vaisbuch E, Mittal P, Erez O, Gotsch F, Mazor M, et al Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med 2009;22:983–992.
- Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Pedro KJ, Soto E, Gotsch F, Dong Z, Chaiworapongsa T, Kim SK, et al Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2009;22:905–916.
- Vaisbuch E, Kusanovic JP, Erez O, Mazaki-Tovi S, Gotsch F, Kim CJ, Kim JS, Chaiworapongsa T, Edwin S, Than NG, et al Amniotic fluid fetal hemoglobin in normal pregnancies and pregnancies complicated with preterm labor or prelabor rupture of membranes. J Matern Fetal Neonatal Med 2009;22:388–397.
- Kusanovic JP, Romero R, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Vaisbuch E, Erez O, Gotsch F, Gabor TN, Edwin SS, et al Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:34–47.
- Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Nandor GT, Kim SK, Dong Z, Gotsch F, Mittal P, Chaiworapongsa T, et al Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23:111–119.
- Chaiworapongsa T, Romero R, Tarca A, Pedro KJ, Mittal P, Kwon KS, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med 2009;22:1122–1139.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiworapongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al Dysregulation of maternal serum adiponectin in preterm labor. J Matern Fetal Neonatal Med 2009;22:887–904.
- Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Chaiworapongsa T, Mittal P, Kim SK, Pacora P, Gotsch F, Dong Z, et al Maternal plasma visfatin in preterm labor. J Matern Fetal Neonatal Med 2009;22:693–704.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.
- Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, Mittal P, Than NG, Espinoza J, Hassan SS. Recurrent preterm birth. Semin Perinatol 2007;31:142–158.
- Kim JS, Romero R, Cushenberry E, Kim YM, Erez O, Nien JK, Yoon BH, Espinoza J, Kim CJ. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta 2007;28:571–576.
- Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005;18:405–416.
- Nathanielsz PW. A time to be born: implications of animal studies in maternal-fetal medicine. Birth 1994;21:163–169.
- Lappas M, Permezel M, Georgiou HM, Rice GE. Type II phospholipase A2 in preterm human gestational tissues. Placenta 2001;22:64–69.
- Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, Muglia LJ. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol 2003;17:1454–1469.
- Meadows JW, Eis AL, Brockman DE, Myatt L. Expression and localization of prostaglandin E synthase isoforms in human fetal membranes in term and preterm labor. J Clin Endocrinol Metab 2003;88:433–439.
- Mitchell MD, Simpson KL, Keelan JA. Paradoxical proinflammatory actions of interleukin-10 in human amnion: potential roles in term and preterm labour. J Clin Endocrinol Metab 2004;89:4149–4152.
- Maul H, Mackay L, Garfield RE. Cervical ripening: biochemical, molecular, and clinical considerations. Clin Obstet Gynecol 2006;49:551–563.
- Bruni L, Luisi S, Ferretti C, Janneau JL, Quadrifoglio M, Richon S, Dangles-Marie V, Bellet D, Petraglia F. Changes in the maternal serum concentration of proearly placenta insulin-like growth factor peptides in normal vs abnormal pregnancy. Am J Obstet Gynecol 2007;197:606–604.
- Maner WL, Garfield RE. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann Biomed Eng 2007;35:465–473.
- Blank V, Hirsch E, Challis JR, Romero R, Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour – a workshop report. Placenta 2008;29 (Suppl A):S102–S104.
- Casciani V, Premyslova M, Luo D, Marinoni E, Moscarini M, Di Iorio R, Challis JR. Effect of calcium ionophore A23187 on prostaglandin synthase type 2 and 15-hydroxy-prostaglandin dehydrogenase expression in human chorion trophoblast cells. Am J Obstet Gynecol 2008;199:554–558.
- Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 2008;181:1470–1479.
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF III, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206–215.
- Lappas M, Rice GE. Transcriptional regulation of the processes of human labour and delivery. Placenta 2009;30 (Suppl A):S90–S95.
- Stella CL, Bennett MR, Devarajan P, Greis K, Wyder M, Macha S, Rao M, Jodicke C, Moussa H, How HY, et al Preterm labor biomarker discovery in serum using 3 proteomic profiling methodologies. Am J Obstet Gynecol 2009;201:387–413.
- Leguizamon G, Smith J, Younis H, Nelson DM, Sadovsky Y. Enhancement of amniotic cyclooxygenase type 2 activity in women with preterm delivery associated with twins or polyhydramnios. Am J Obstet Gynecol 2001;184:117–122.
- Hirsch E, Goldstein M, Filipovich Y, Wang H. Placental expression of enzymes regulating prostaglandin synthesis and degradation. Am J Obstet Gynecol 2005;192:1836–1842.
- Bastek JA, Sammel MD, Pare E, Srinivas SK, Posencheg MA, Elovitz MA. Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. Am J Obstet Gynecol 2008;199:367–368.
- Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J Matern Fetal Neonatal Med 2008;21:223–230.
- Simhan HN, Krohn MA. Paternal race and preterm birth. Am J Obstet Gynecol 2008;198:644–646.
- Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol 2009;200:93–98.
- Simhan HN, Krohn MA. First-trimester cervical inflammatory milieu and subsequent early preterm birth. Am J Obstet Gynecol 2009;200:377–374.
- Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol 2001;185:1059–1063.
- Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad A, Das A, Miodovnik M, Vandorsten PJ, Caritis SN, Thurnau G, et al The Preterm Prediction Study: toward a multiple-marker test for spontaneous preterm birth. Am J Obstet Gynecol 2001;185:643–651.
- Iams JD, Goldenberg RL, Mercer BM, Moawad AH, Meis PJ, Das AF, Caritis SN, Miodovnik M, Menard MK, Thurnau GR, et al The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 2001;184:652–655.
- Goldenberg RL, Iams JD, Mercer BM, Meis P, Moawad A, Das A, Copper R, Johnson F. What we have learned about the predictors of preterm birth. Semin Perinatol 2003;27:185–193.
- Kalish RB, Vardhana S, Normand NJ, Gupta M, Witkin SS. Association of a maternal CD14 -159 gene polymorphism with preterm premature rupture of membranes and spontaneous preterm birth in multi-fetal pregnancies. J Reprod Immunol 2006;70:109–117.
- Diamond AK, Sweet LM, Oppenheimer KH, Bradley DF, Phillippe M. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod Sci 2007;14:548–559.
- Chaves JH, Babayan A, Bezerra CM, Linhares IM, Witkin SS. Maternal and neonatal interleukin-1 receptor antagonist genotype and pregnancy outcome in a population with a high rate of pre-term birth. Am J Reprod Immunol 2008; 60:312–317.
- Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008;371:164–175.
- Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatr Res 2008;64:581–589.
- Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev 2009; CD007235.
- Carlin A, Norman J, Cole S, Smith R. Tocolytics and preterm labour. BMJ 2009;338:b195.
- Defranco EA, Chang JJ, Macones GA, Muglia LJ. The correlation in birth timing between singleton and twin gestations in the same mother. J Matern Fetal Neonatal Med 2009;22:106–110.
- Scheib S, Visintine JF, Miroshnichenko G, Harvey C, Rychlak K, Berghella V. Is cerclage height associated with the incidence of preterm birth in women with an ultrasound-indicated cerclage? Am J Obstet Gynecol 2009;200:e12–e15.
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab 2009;94:2066–2074.
- Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, Richani K, Gomez R, Hassan S, Mazor M, et al A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol 2006; 195:1025–1030.
- Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, Mazaki-Tovi S, Than NG, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG 2006;113 (Suppl 3):118–135.
- Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet Gynecol 2006;195:360–363.
- Evans GA. Designer science and the “omic” revolution. Nat Biotechnol 2000;18:127.
- Gracey AY, Cossins AR. Application of microarray technology in environmental and comparative physiology. Annu Rev Physiol 2003;65:231–259.
- Mehta T, Tanik M, Allison DB. Towards sound epistemological foundations of statistical methods for high-dimensional biology. Nat Genet 2004;36:943–947.
- Weinstein JN. Fishing expeditions. Science 1998;282:628–629.
- Weinstein JN. ‘Omic’ and hypothesis-driven research in the molecular pharmacology of cancer. Curr Opin Pharmacol 2002;2:361–365.
- Collins FS, Guttmacher AE. Genetics moves into the medical mainstream. JAMA 2001;286:2322–2324.
- Collins FS, Mansoura MK. The Human Genome Project. Revealing the shared inheritance of all humankind. Cancer 2001;91:221–225.
- Ginsburg GS, McCarthy JJ. Personalized medicine: revolutionizing drug discovery and patient care. Trends Biotechnol 2001;19:491–496.
- McKusick VA. The anatomy of the human genome: a neo-Vesalian basis for medicine in the 21st century. JAMA 2001;286:2289–2295.
- Subramanian G, Adams MD, Venter JC, Broder S. Implications of the human genome for understanding human biology and medicine. JAMA 2001;286:2296–2307.
- Hegde PS, White IR, Debouck C. Interplay of transcriptomics and proteomics. Curr Opin Biotechnol 2003;14:647–651.
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995;270:467–470.
- Lipshutz RJ, Morris D, Chee M, Hubbell E, Kozal MJ, Shah N, Shen N, Yang R, Fodor SP. Using oligonucleotide probe arrays to access genetic diversity. Biotechniques 1995;19:442–447.
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, et al Use of proteomic patterns in serum to identify ovarian cancer. Lancet 2002;359:572–577.
- Phizicky E, Bastiaens PI, Zhu H, Snyder M, Fields S. Protein analysis on a proteomic scale. Nature 2003;422:208–215.
- Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res 2003;44:1071–1079.
- Lagarde M, Geloen A, Record M, Vance D, Spener F. Lipidomics is emerging. Biochim Biophys Acta 2003;1634:61.
- Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov 2005;4:594–610.
- Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol 2004;22:245–252.
- Jain S, Jayasimhulu K, Clark JF. Metabolomic analysis of molecular species of phospholipids from normotensive and preeclamptic human placenta electrospray ionization mass spectrometry. Front Biosci 2004;9:3167–3175.
- Nobeli I, Thornton JM. A bioinformatician's view of the metabolome. Bioessays 2006;28:534–545.
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, et al HMDB: the Human Metabolome Database. Nucleic Acids Res 2007;35:D521–D526.
- Rochfort S. Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J Nat Prod 2005;68:1813–1820.
- Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Bienvenut WV, Lemery D, Quadroni M, Dastugue B, et al Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics 2003;3:1521–1525.
- Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, et al Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA 2004;292:462–469.
- Ruetschi U, Rosen A, Karlsson G, Zetterberg H, Rymo L, Hagberg H, Jacobsson B. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J Proteome Res 2005;4:2236–2242.
- Cobo T, Palacio M, Navarro-Sastre A, Ribes A, Bosch J, Filella X, Gratacos E. Predictive value of combined amniotic fluid proteomic biomarkers and interleukin-6 in preterm labor with intact membranes. Am J Obstet Gynecol 2009;200:499–496.
- Shah SJ, Yu KH, Sangar V, Parry SI, Blair IA. Identification and quantification of preterm birth biomarkers in human cervicovaginal fluid by liquid chromatography/tandem mass spectrometry. J Proteome Res 2009;8:2407–2417.
- Horgan RP, Clancy OH, Myers JE, Baker PN. An overview of proteomic and metabolomic technologies and their application to pregnancy research. BJOG 2009;116:173–181.
- Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee KY, et al Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med 2008;21:697–713.
- Klein LL, Jonscher KR, Heerwagen MJ, Gibbs RS, McManaman JL. Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod Sci 2008;15:263–273.
- Cho CK, Shan SJ, Winsor EJ, Diamandis EP. Proteomics analysis of human amniotic fluid. Mol Cell Proteomics 2007;6:1406–1415.
- Dasari S, Pereira L, Reddy AP, Michaels JE, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT Jr, Gravett MG, et al Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res 2007;6:1258–1268.
- Pereira L, Reddy AP, Jacob T, Thomas A, Schneider KA, Dasari S, Lapidus JA, Lu X, Rodland M, Roberts CT Jr, et al Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res 2007;6:1269–1276.
- Di Quinzio MK, Oliva K, Holdsworth SJ, Ayhan M, Walker SP, Rice GE, Georgiou HM, Permezel M. Proteomic analysis and characterisation of human cervico-vaginal fluid proteins. Aust N Z J Obstet Gynaecol 2007;47:9–15.
- Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Espinoza J, Chaiworapongsa T, Gonzalez R, Iams JD, et al Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med 2007;20:167–173.
- Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–830.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–119.
- Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–974.
- Andreoli R, Manini P, Poli D, Bergamaschi E, Mutti A, Niessen WM. Development of a simplified method for the simultaneous determination of retinol, alpha-tocopherol, and beta-carotene in serum by liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization. Anal Bioanal Chem 2004;378:987–994.
- Gehrke CW, Leimer K. Trimethylsilylation of amino acids derivatization and chromatography. J Chromatogr 1971;57:219–238.
- Evans AM, Dehaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–6667.
- Liaw A, Wiener M. Classication and regression by random forest. R News 2002;2:18–22.
- Breiman L. Technical note: some properties of splitting criteria. Mach Learn 1966;24:41–47.
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202.
- Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–193.
- Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–683.
- Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92.
- Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, Park JS. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998;179:1107–1114.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–681.
- Fitzgerald MJ, Goto M, Myers TF, Zeller WP. Early metabolic effects of sepsis in the preterm infant: lactic acidosis and increased glucose requirement. J Pediatr 1992;121:951–955.
- Yeung CY. Hypoglycemia in neonatal sepsis. J Pediatr 1970;77:812–817.
- Yeung CY, Lee VW, Yeung CM. Glucose disappearance rate in neonatal infection. J Pediatr 1973;82:486–489.
- Leake RD, Fiser RH Jr, Oh W. Rapid glucose disappearance in infants with infection. Clin Pediatr (Phila) 1981;20:397–401.
- Long CL, Jeevanandam M, Kim BM, Kinney JM. Whole body protein synthesis and catabolism in septic man. Am J Clin Nutr 1977;30:1340–1344.
- Cerra FB, Siegel JH, Coleman B, Border JR, McMenamy RR. Septic autocannibalism. A failure of exogenous nutritional support. Ann Surg 1980;192:570–580.
- Regnault TR, de Vrijer B, Battaglia FC. Transport and metabolism of amino acids in placenta. Endocrine 2002;19:23–41.
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824.
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–1400.
- Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB, et al A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–851.
- Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–210.
- Wasz-Hockert O, Brander E, Paatela M. Reduced concentration of cerebrospinal fluid glucose in tuberculous meningitis; experimental studies. Ann Paediatr Fenn 1956;2:320–326.
- Levinsky WJ. Hypoglycorrhachia (low cerebrospinal-fluid sugar) in diffuse meningeal neoplasm. N Engl J Med 1963;268:198–199.
- Owen DS. Synovial fluid glucose. JAMA 1978;239:193.
- Murray PA, Kern DG, Winkler JR. Identification of a galactose-binding lectin on Fusobacterium nucleatum FN-2. Infect Immunol 1988;56:1314–1319.
- Smith PF. Detection of lipoglycans in ureaplasmas. J Bacteriol 1985;162:611–614.
- Petty HR, Kindzelskii AL, Chaiworapongsa T, Petty AR, Romero R. Oxidant release is dramatically increased by elevated glucose concentrations in neutrophils from pregnant women. J Matern Fetal Neonatal Med 2005;18:397–404.
- Cheng X. Structure and function of DNA methyltransferases. Annu Rev Biophys Biomol Struct 1995;24:293–318.
- Barbeyron T, Kean K, Forterre P. DNA adenine methylation of GATC sequences appeared recently in the Escherichia coli lineage. J Bacteriol 1984;160:586–590.
- Wion D, Casadesus J. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nat Rev Microbiol 2006;4:183–192.
- Tavazzi B, Lazzarino G, Leone P, Amorini AM, Bellia F, Janson CG, Di P, Ceccarelli L, Donzelli S, Francis JS, et al Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clin Biochem 2005;38:997–1008.
- Gardon M, Pinheiro CB, Chapuis G. Structural phases of hexamethylenetetramine-pimelic acid (1/1): a unified description based on a stacking model. Acta Crystallogr B 2003;59:527–536.
- Hoare DS, Work E. The stereoisomers of alpha epsilon-diaminopimelic acid. II. Their distribution in the bacterial order Actinomycetales and in certain Eubacteriales. Biochem J 1957;65:441–447.
- Scapin G, Blanchard JS. Enzymology of bacterial lysine biosynthesis. Adv Enzymol Relat Areas Mol Biol 1998;72:279–324.
- Cox RJ. The DAP pathway to lysine as a target for antimicrobial agents. Nat Prod Rep 1996;13:29–43.
- Born TL, Blanchard JS. Structure/function studies on enzymes in the diaminopimelate pathway of bacterial cell wall biosynthesis. Curr Opin Chem Biol 1999;3:607–613.
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 2003;4:702–707.
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 1972;36:407–477.
- Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2009;22:880–886.
- Mitchell MD, Chang MC, Chaiworapongsa T, Lan HY, Helliwell RJ, Romero R, Sato TA. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab 2005;90:4244–4248.
- Edwin SS, Romero RJ, Munoz H, Branch DW, Mitchell MD. 5-Hydroxyeicosatetraenoic acid and human parturition. Prostaglandins 1996;51:403–412.
- Romero R, Baumann P, Gomez R, Salafia C, Rittenhouse L, Barberio D, Behnke E, Cotton DB, Mitchell MD. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol 1993;168:1654–1664.
- Romero R, Wu YK, Sirtori M, Oyarzun E, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins 1989;37:149–161.
- Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1988;34:141–145.
- Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid 5-hydroxyeicosatetraenoic acid in preterm labor. Prostaglandins 1988;36:179–189.
- Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1461–1467.
- Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet 1986;1:1380.
- Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454–1460.
- Romero R, Wu YK, Mazor M, Oyarzun E, Hobbins JC, Mitchell MD. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids 1989;36:69–75.
- Romero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol 1987;70:849–851.
- Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, Maymon E, Edwin S. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol 2004;191:1331–1338.
- Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, Foo CH, Xie B, Tsui NB, Lun FM, et al Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci USA 2008;105:20458–20463.
- Breuiller-Fouche M, Charpigny G, Germain G. Functional genomics of the pregnant uterus: from expectations to reality, a compilation of studies in the myometrium. BMC Pregn Childbirth 2007;7 (Suppl 1):S4.
- Dolan S, Biermann J, Damus K. Genomics for health in preconception and prenatal periods. J Nurs Scholarsh 2007;39:4–9.
- Wilson RD. Genomics: new technology for obstetrics. J Obstet Gynaecol Can 2005;27:63–75.
- South ST, Chen Z, Brothman AR. Genomic medicine in prenatal diagnosis. Clin Obstet Gynecol 2008;51:62–73.
- Solomon BD, Jack BW, Feero WG. The clinical content of preconception care: genetics and genomics. Am J Obstet Gynecol 2008;199:S340–S344.
- Kim JS, Romero R, Tarca AL, LaJeunesse C, Han YM, Kim MJ, Suh YL, Draghici S, Mittal P, Gotsch F, et al Gene expression profiling demonstrates a novel role for foetal fibrocytes and the umbilical vessels in human fetoplacental development. J Cell Mol Med 2008;12:1317–1330.
- Knox K, Baker JC. Genomic evolution of the placenta using co-option and duplication and divergence. Genome Res 2008;18:695–705.
- Toft JH, Lian IA, Tarca AL, Erez O, Espinoza J, Eide IP, Bjorge L, Draghici S, Romero R, Austgulen R. Whole-genome microarray and targeted analysis of angiogenesis-regulating gene expression (ENG, FLT1, VEGF, PlGF) in placentas from pre-eclamptic and small-for-gestational-age pregnancies. J Matern Fetal Neonatal Med 2008;21:267–273.
- Buffat C, Mondon F, Rigourd V, Boubred F, Bessieres B, Fayol L, Feuerstein JM, Gamerre M, Jammes H, Rebourcet R, et al A hierarchical analysis of transcriptome alterations in intrauterine growth restriction (IUGR) reveals common pathophysiological pathways in mammals. J Pathol 2007;213:337–346.
- Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, Khalek N, Camacho N, Mittal P, Yoon BH, et al Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2007;197:250–257.
- Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol 2007;179:557–565.
- Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reprod Sci 2007;14:53–62.
- Tsai MS, Hwang SM, Chen KD, Lee YS, Hsu LW, Chang YJ, Wang CN, Peng HH, Chang YL, Chao AS, et al Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells 2007;25:2511–2523.
- Buimer M, Keijser R, Jebbink JM, Wehkamp D, van Kampen AH, Boer K, van der Post JA, Ris-Stalpers C. Seven placental transcripts characterize HELLP-syndrome. Placenta 2008;29:444–453.
- Gielchinsky Y, Bogoch Y, Rechavi G, Jacob-Hirsch J, Amariglio N, Shveiky D, Linial M, Laufer N. Gene expression in women conceiving spontaneously over the age of 45 years. Fertil Steril 2008;89:1641–1650.
- Kang BY, Tsoi S, Zhu S, Su S, Kay HH. Differential gene expression profiling in HELLP syndrome placentas. Reprod Sci 2008;15:285–294.
- Sitras V, Paulssen RH, Gronaas H, Vartun A, Acharya G. Gene expression profile in labouring and non-labouring human placenta near term. Mol Hum Reprod 2008;14:61–65.
- Enquobahrie DA, Williams MA, Qiu C, Meller M, Sorensen TK. Global placental gene expression in gestational diabetes mellitus. Am J Obstet Gynecol 2009;200:206–213.
- Slonim DK, Koide K, Johnson KL, Tantravahi U, Cowan JM, Jarrah Z, Bianchi DW. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci USA 2009;106:9425–9429.
- Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod 2002;8:399–408.
- Reimer T, Koczan D, Gerber B, Richter D, Thiesen HJ, Friese K. Microarray analysis of differentially expressed genes in placental tissue of pre-eclampsia: up-regulation of obesity-related genes. Mol Hum Reprod 2002;8:674–680.
- Gross SJ, Ferreira JC, Morrow B, Dar P, Funke B, Khabele D, Merkatz I. Gene expression profile of trisomy 21 placentas: a potential approach for designing noninvasive techniques of prenatal diagnosis. Am J Obstet Gynecol 2002;187:457–462.
- Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA. Human myometrial gene expression before and during parturition. Biol Reprod 2005;72:707–719.
- Huber A, Hudelist G, Czerwenka K, Husslein P, Kubista E, Singer CF. Gene expression profiling of cervical tissue during physiological cervical effacement. Obstet Gynecol 2005;105:91–98.
- Hansson SR, Chen Y, Brodszki J, Chen M, Hernandez-Andrade E, Inman JM, Kozhich OA, Larsson I, Marsal K, Medstrand P, et al Gene expression profiling of human placentas from preeclamptic and normotensive pregnancies. Mol Hum Reprod 2006;12:169–179.
- Zhou R, Zhu Q, Wang Y, Ren Y, Zhang L, Zhou Y. Genomewide oligonucleotide microarray analysis on placentae of pre-eclamptic pregnancies. Gynecol Obstet Invest 2006;62:108–114.
- Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction 2006;131:837–850.
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195(2):394.e1–24.
- Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–786.
- Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, et al Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology 2007;148:1059–1079.
- Hampton T. Comprehensive “proteomic profile” of amniotic fluid may aid prenatal diagnosis. JAMA 2007;298:1751.
- Hassan MI, Kumar V, Singh TP, Yadav S. Proteomic analysis of human amniotic fluid from Rh(−) pregnancy. Prenat Diagn 2008;28:102–108.
- Kolialexi A, Tsangaris GT, Papantoniou N, Anagnostopoulos AK, Vougas K, Bagiokos V, Antsaklis A, Mavrou A. Application of proteomics for the identification of differentially expressed protein markers for Down syndrome in maternal plasma. Prenat Diagn 2008;28:691–698.
- Kolialexi A, Mavrou A, Spyrou G, Tsangaris GT. Mass spectrometry-based proteomics in reproductive medicine. Mass Spectrom Rev 2008;27:624–634.
- Park JS, Oh KJ, Norwitz ER, Han JS, Choi HJ, Seong HS, Kang YD, Park CW, Kim BJ, Jun JK, et al Identification of proteomic biomarkers of preeclampsia in amniotic fluid using SELDI-TOF mass spectrometry. Reprod Sci 2008;15:457–468.
- Reddy CS, Holzgreve W, Hahn S. Detection of new screening markers for fetal aneuploidies in maternal plasma: a proteomic approach. Methods Mol Biol 2008;444:311–326.
- Zhang Y, Zhang YL, Feng C, Wu YT, Liu AX, Sheng JZ, Cai J, Huang HF. Comparative proteomic analysis of human placenta derived from assisted reproductive technology. Proteomics 2008;8:4344–4356.
- Baker PN, Myers JE. Preeclamptic toxemia: a disease ripe for proteomic discovery. Expert Rev Proteomics 2009;6:107–110.
- Blankley RT, Robinson NJ, Aplin JD, Crocker IP, Gaskell SJ, Whetton AD, Baker PN, Myers JE. A gel-free quantitative proteomics analysis of factors released from hypoxic-conditioned placentae. Reprod Sci 2010;17247–257.
- Blankley RT, Gaskell SJ, Whetton AD, Dive C, Baker PN, Myers JE. A proof-of-principle gel-free proteomics strategy for the identification of predictive biomarkers for the onset of pre-eclampsia. BJOG 2009;116:1473–1480.
- Blumenstein M, McMaster MT, Black MA, Wu S, Prakash R, Cooney J, McCowan LM, Cooper GJ, North RA. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics 2009;9:2929–2945.
- Choolani M, Narasimhan K, Kolla V, Hahn S. Proteomic technologies for prenatal diagnostics: advances and challenges ahead. Expert Rev Proteomics 2009;6:87–101.
- Colquhoun DR, Goldman LR, Cole RN, Gucek M, Mansharamani M, Witter FR, Apelberg BJ, Halden RU. Global screening of human cord blood proteomes for biomarkers of toxic exposure and effect. Environ Health Perspect 2009;117:832–838.
- Kolialexi A, Tsangaris GT, Mavrou A. Proteomics in prenatal diagnosis. Expert Rev Proteomics 2009;6:111–113.
- Kolialexi A, Anagnostopoulos AK, Mavrou A, Tsangaris GT. Application of proteomics for diagnosis of fetal aneuploidies and pregnancy complications. J Proteomics 2009;72:731–739.
- Robinson JM, Vandre DD, Ackerman WE. Placental proteomics: a shortcut to biological insight. Placenta 2009;30 (Suppl A):S83–S89.
- Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, Moser A, Tam S, Leszyk J, Master SR, et al Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010;23261–280.
- Wang TH, Chao AS, Chen JK, Chao A, Chang YL, Cheng PJ, Chang SD, Wang HS. Network analyses of differentially expressed proteins in amniotic fluid supernatant associated with abnormal human karyotypes. Fertil Steril 2009;92:96–107.
- Centlow M, Hansson SR, Welinder C. Differential proteome analysis of the preeclamptic placenta using optimized protein extraction. J Biomed Biotechnol 2010;2010:458748.
- Kolla V, Jeno P, Moes S, Tercanli S, Lapaire O, Choolani M, Hahn S. Quantitative proteomics analysis of maternal plasma in Down syndrome pregnancies using isobaric tagging reagent (iTRAQ). J Biomed Biotechnol 2010;2010:952047.
- Cottingham K. The cervical-vaginal fluid proteome and possible biomarkers of preterm birth. J Proteome Res 2007;6:1241–1242.
- Michaels JE, Dasari S, Pereira L, Reddy AP, Lapidus JA, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT Jr, et al Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational age-dependent changes. J Proteome Res 2007;6:1277–1285.
- Nagalla SR, Canick JA, Jacob T, Schneider KA, Reddy AP, Thomas A, Dasari S, Lu X, Lapidus JA, Lambert-Messerlian GM, et al Proteomic analysis of maternal serum in down syndrome: identification of novel protein biomarkers. J Proteome Res 2007;6:1245–1257.
- Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, Lu X, Rodland M, Pereira L, Sadowsky DW, et al Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res 2007;6:89–96.
- Crawford JT, Pereira L, Buckmaster J, Gravett MG, Tolosa JE. Amniocentesis results and novel proteomic analysis in a case of occult candidal chorioamnionitis. J Matern Fetal Neonatal Med 2006;19:667–670.
- Tsangaris GT, Kolialexi A, Karamessinis PM, Anagnostopoulos AK, Antsaklis A, Fountoulakis M, Mavrou A. The normal human amniotic fluid supernatant proteome. In Vivo 2006;20:479–490.
- Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, Fountoulakis M. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics 2006;6:4410–4419.
- Kim YS, Kim MS, Lee SH, Choi BC, Lim JM, Cha KY, Baek KH. Proteomic analysis of recurrent spontaneous abortion: identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics 2006;6:3445–3454.
- Michel PE, Crettaz D, Morier P, Heller M, Gallot D, Tissot JD, Reymond F, Rossier JS. Proteome analysis of human plasma and amniotic fluid by Off-Gel isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis 2006;27:1169–1181.
- Murphy VE, Johnson RF, Wang YC, Akinsanya K, Gibson PG, Smith R, Clifton VL. Proteomic study of plasma proteins in pregnant women with asthma. Respirology 2006;11:41–48.
- Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, Queloz PA, Schneider P, Tissot JD. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin Chim Acta 2005;360:27–36.
- Gerton GL, Fan XJ, Chittams J, Sammel M, Hummel A, Strauss JF, Barnhart K. A serum proteomics approach to the diagnosis of ectopic pregnancy. Ann N Y Acad Sci 2004;1022:306–316.
- Watanabe H, Hamada H, Yamada N, Sohda S, Yamakawa-Kobayashi K, Yoshikawa H, Arinami T. Proteome analysis reveals elevated serum levels of clusterin in patients with preeclampsia. Proteomics 2004;4:537–543.
- Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab 2002;87:2431–2434.
- Deepinder F, Chowdary HT, Agarwal A. Role of metabolomic analysis of biomarkers in the management of male infertility. Expert Rev Mol Diagn 2007;7:351–358.
- Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril 2007;88:1350–1357.
- Singh R, Sinclair KD. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology 2007;68 (Suppl 1):S56–S62.
- Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod 2008;14:679–690.
- Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol 2008;20:234–241.
- Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril 2008;90:77–83.
- Seli E, Botros L, Sakkas D, Burns DH. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril 2008;90:2183–2189.
- Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online 2009;18:219–225.
- Revelli A, Delle PL, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 2009;7:40.
- Royere D, Feuerstein P, Cadoret V, Puard V, Uzbekova S, bies-Tran R, Teusan R, Houlgatte R, Labas V, Guerif F. [Non invasive assessment of embryo quality: proteomics, metabolomics and oocyte-cumulus dialogue]. Gynecol Obstet Fertil 2009;37:917–920.
- Heazell AE, Brown M, Dunn WB, Worton SA, Crocker IP, Baker PN, Kell DB. Analysis of the metabolic footprint and tissue metabolome of placental villous explants cultured at different oxygen tensions reveals novel redox biomarkers. Placenta 2008;29:691–698.
- Dunn WB, Brown M, Worton SA, Crocker IP, Broadhurst D, Horgan R, Kenny LC, Baker PN, Kell DB, Heazell AE. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta 2009;30:974–980.
- Pearce JM, Krone JT, Pappas AA, Komoroski RA. Analysis of saturated phosphatidylcholine in amniotic fluid by 31P NMR. Magn Reson Med 1993;30:476–484.
- Clifton MS, Joe BN, Zektzer AS, Kurhanewicz J, Vigneron DB, Coakley FV, Nobuhara KK, Swanson MG. Feasibility of magnetic resonance spectroscopy for evaluating fetal lung maturity. J Pediatr Surg 2006;41:768–773.
- Bock JL. Metabolic profiling of amniotic fluid by proton nuclear magnetic resonance spectroscopy: correlation with fetal maturation and other clinical variables. Clin Chem 1994;40:56–61.
- Groenen PM, Engelke UF, Wevers RA, Hendriks JC, Eskes TK, Merkus HM, Steegers-Theunissen RP. High-resolution 1H NMR spectroscopy of amniotic fluids from spina bifida fetuses and controls. Eur J Obstet Gynecol Reprod Biol 2004;112:16–23.
- Graca G, Duarte IF, Goodfellow BJ, Barros AS, Carreira IM, Couceiro AB, Spraul M, Gil AM. Potential of NMR spectroscopy for the study of human amniotic fluid. Anal Chem 2007;79:8367–8375.
- Graca G, Duarte IF, Goodfellow J, Carreira IM, Couceiro AB, Domingues MR, Spraul M, Tseng LH, Gil AM. Metabolite profiling of human amniotic fluid by hyphenated nuclear magnetic resonance spectroscopy. Anal Chem 2008;80:6085–6092.
- Graca G, Duarte IF, Barros AS, Goodfellow BJ, Diaz S, Carreira IM, Couceiro AB, Galhano E, Gil AM. (1)H NMR based metabonomics of human amniotic fluid for the metabolic characterization of fetus malformations. J Proteome Res 2009;8:4144–4150.