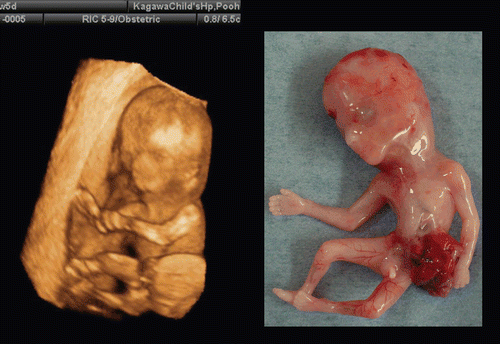
Abstract
The introduction of 3D/4D sonography with high frequency transvaginal transducer has resulted in remarkable progress in ultrasonographic visualization of early embryos and fetuses and development of new fields of 3D sonoembryology. With the proper use of this new diagnostic modality and with experienced examiner, both structural and functional development in the first trimester of gestation can be assessed more objectively and reliable. Indeed new technology moved embryology from postmortem studies to the in vivo environment. Furthermore, there are good reasons to believe that 3D/4D sonography moved prenatal diagnosis of fetal abnormalities from the second to the first trimester of pregnancy. We will try to illustrate it with the number of convincing slides.
Introduction
The introduction of 3D/4D sonography with high frequency transvaginal transducer has resulted in remarkable progress in ultrasonographic visualization of early embryos and fetuses and development of new fields of 3D sonoembryology. With the proper use of this new diagnostic modality and with experienced examiner, both structural and functional development in the first trimester of gestation can be assessed more objectively and reliable.
Indeed new technology moved embryology from postmortem studies to the in vivo environment. Furthermore, there are good reasons to believe that 3D/4D sonography moved prenatal diagnosis of fetal abnormalities from the second to the first trimester of pregnancy. We will try to illustrate it with the number of convincing figures.
Visualization of normal embryo
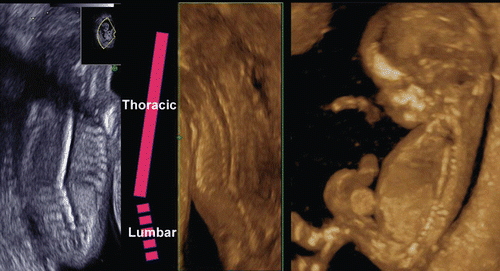
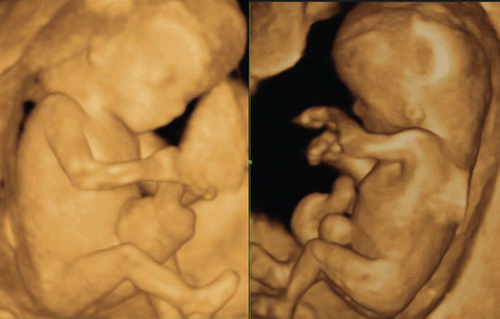
Recent introduction of three-dimensional and four-dimensional ultrasounds combined with the transvaginal approach has produced more objective and accurate information on embryonal and early fetal development [Citation1–4]. shows a gestational sac at four weeks of gestation and the yolk sac visualization at five weeks of gestation. Demonstration of small embryo less than 10 mm of greatest length has been difficult and not visualized in detail. and show 5.5 mm long embryo with yolk sac at 6 weeks of gestation and 18.3 mm CRL-embryo at 8 weeks of gestation respectively, depicted by using the most up-dated 3D equipment with high-frequency transvaginal transducer (Voluson® E8 with 12 MHz/256 element vaginal probe, GE Healthcare, Milwaukee, USA). Early neural tube and premature spinal cord are successfully demonstrated in a small embryo. is macrographic images of an aborted specimen taken just after abortion. Premature spinal cord is visualized on the back, which is compatible with 3D image on . shows the development of spinal cord in embryonal size of 5.5 mm, 18.3 mm and 26.3 mm [Citation7]. Thereafter, the vertebral bony structure can be visualized from 11 weeks of gestation and gradual closure of bilateral laminae caudally from the cervical region ().
Figure 1. Development of gestational sac (4 weeks and 5 weeks of gestation) (left) 4 weeks (2 weeks after conception) of gestation. Three orthogonal view and 3D constructed image demonstrated early gestational sac. (right) The beginning of 5 week, yolk sac is detectable inside gestational sac.
Figure 2. 3D reconstructed image of the yolk sac and 5.5 mm-CRL-embryo (6 weeks of gestation). Normal 6-week-embryo (CRL 5.5 mm) and yolk sac. Occipital view shows the neural tube on the embryonal back.
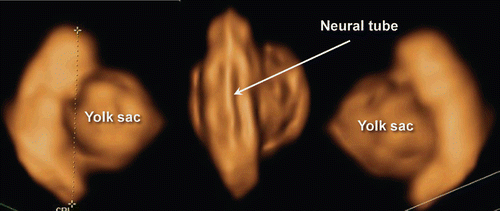
Figure 3. 3D reconstructed image of the embryo (8 weeks of gestation). Normal 8-week-embryo. Occipital view shows the premature spinal cord which is seen in the (right).
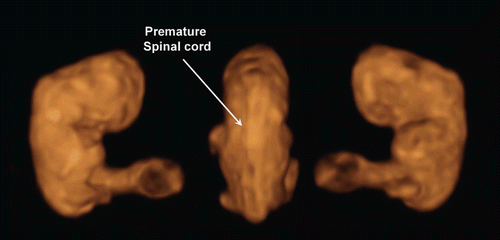
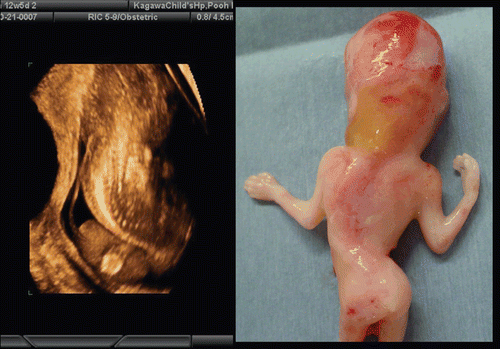
Figure 4. Lateral and back view of aborted embryo at 8 weeks of gestation. (left) lateral view. Physiological umbilical hernia is seen. (right) backside view. Premature spinal cord is seen. This photo was taken just after abortion before preserving in formalin.
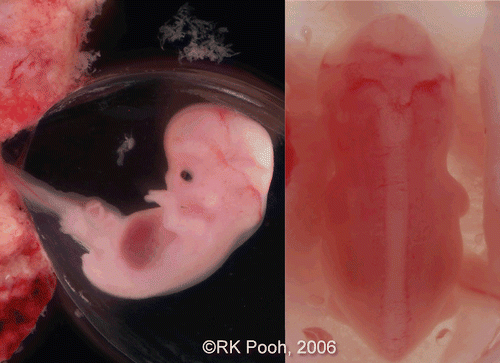
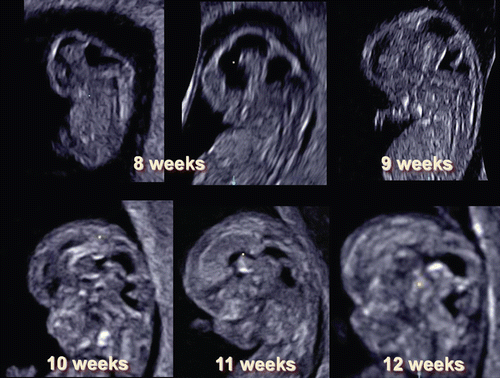
Figure 5. 3D reconstructed image of the embryo (6–9 weeks of gestation). Embryonal sizes are 5.5 mm, 18.3 mm and 26.3 mm. At 9 weeks of gestation, the spinal cord is demonstrated as a thin line, compared with that at 8 weeks of gestation.
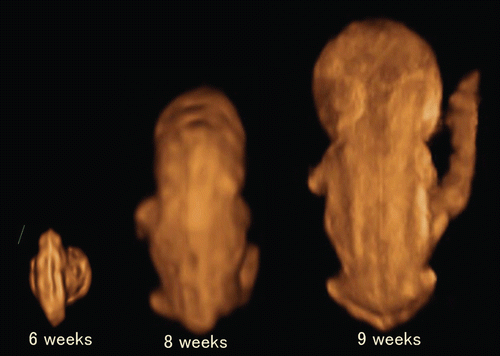
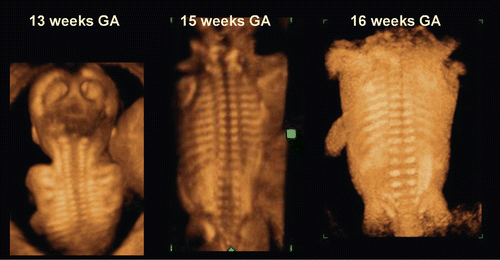
Figure 6. 3D reconstructed image of fetal vertebral development between 13 and 16 weeks of gestation.
The development of the embryonic circulation became visible by 3D power Doppler imaging technology [Citation5]. illustrates the vascular network of an embryo at 7 weeks of gestation. In 1993 and 1994, color Doppler detection and assessment of brain vessels in the early fetus using a transvaginal approach was reported [Citation6,Citation7]. Clear visualization by transvaginal power Doppler of the common carotid arteries, internal and external carotid arteries, and middle cerebral arteries at 12 weeks of gestation was reported in 1996[Citation8]. By using 3D power Doppler technology, the vascular anatomy can now be imaged clearly by identification of the common carotid arteries, internal carotid arteries, circle of Willis and middle cerebral arteries ().
Figure 7. Early vascular system at 7 weeks of gestation. (left) 3D gray mode. (right) 3D power Doppler with surface appearance.
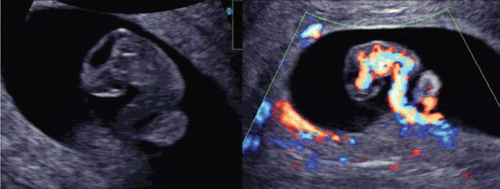
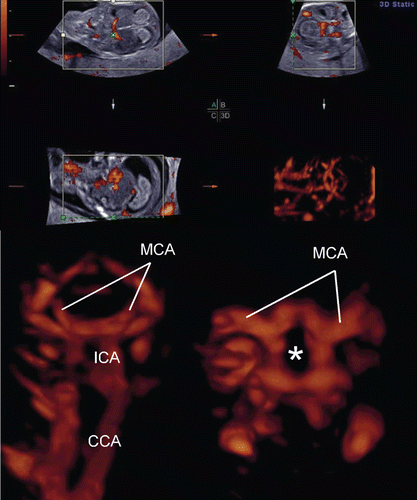
Figure 8. Premature vascularity of normal 12 week brain. (upper) Three orthogonal view of power Doppler image and 3D reconstructed image (front-back view of A plane). (lower left) Front-back image of common carotid arteries (CCA), internal carotid arteries (ICA) and brain basilar arteries. MCA; middle cerebral artery. (lower right) 3D.
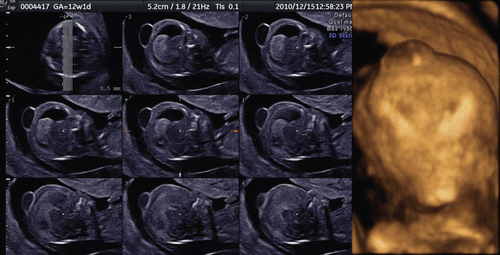
The utilization of post-processing algorithms such as maximum mode can be used to demonstrate the fetal skeleton. Chaoui et al. [Citation9] reported clear 3D images for the identification of an abnormally wide metopic suture in the second trimester of pregnancy. However, rapid ossification of the craniofacial bones occurs during the first trimester of pregnancy. We demonstrate in this paper the identification of the craniofacial skeleton from 10 weeks of gestation onwards. and show early fetal craniofacial bony structures at 11 and 14 weeks, respectively, using the maximum mode algorithm. The difference of frontal bone morphology between 11 and 14 weeks demonstrates membranous ossification of the cranium at this stage. demonstrates the structure of the skull in the occipital region at 13 weeks of gestation.
Figure 9. 3D maximum mode image of normal craniofacial structure at 11 weeks of gestation. Premature bony structure of frontal bone (F), parietal bone (P), nasal bone (NB), maxilla and mandible are recognizable.
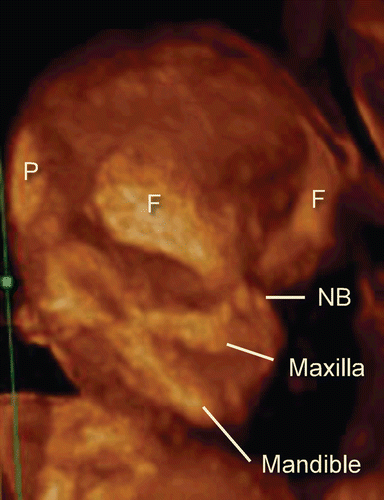
Figure 10. 3D maximum mode image of normal craniofacial structure at 14 weeks of gestation. (left) Oblique view. (right) Frontal view. Anterior fontanelle (AF), sphenoidal fontanelle (SF), frontal suture (FS), coronal suture (CS), nasal bone (NB), maxilla and mandible are gradually formed according to cranial bony development.
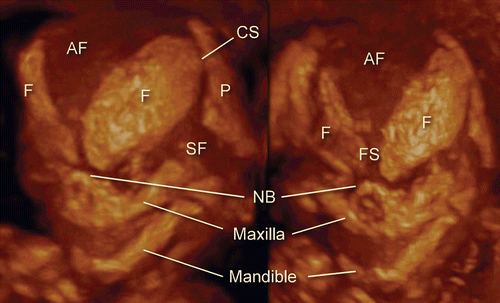
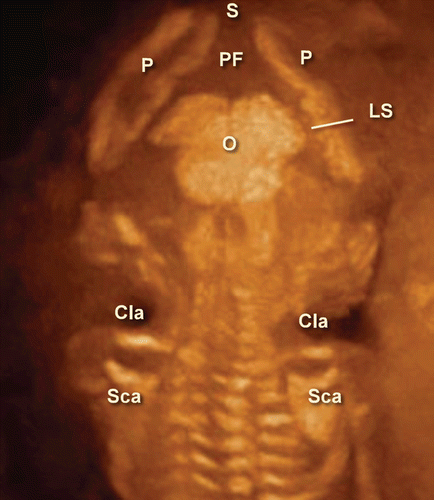
Figure 11. 3D maximum mode image of occipital view at 13 weeks of gestation. Note the premature occipital bone appearance. Midline crack is demonstrated. S; Sagittal suture, P; Parietal bone, PF; Posterior fontanelle, O; Occipital bone, Cla; Clavicular, Sca; Scapula, LS; Lambdoid suture.
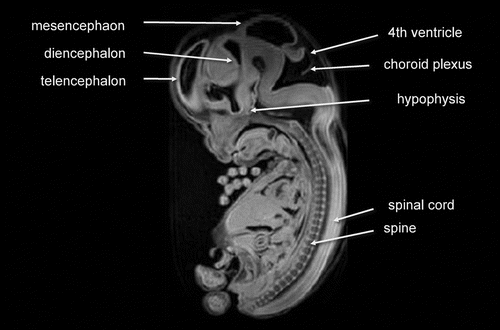
During the early embryonic period, the central nervous system anatomy rapidly changes in appearance. MR images shows remarkable change of CNS appearance in a short period between Carnegie stage 18 and 23. Detailed cerebrospinal structure by MRI at Carnegie stage 23 is visualized in .
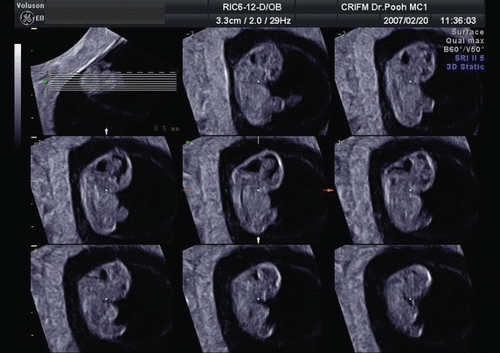
3D sonography using transvaginal sonography with high-resolution probes allows imaging of early structures in the embryonic brain. through 15 demonstrate fetal brain detailed morphology between the beginning of 8th and 10th week and this can be accomplished through the use of three orthogonal planes, and “tomographic ultrasound imaging”. Serial examinations allow obtaining similar sections of the fetal brain at different stages of development. Therefore, it is possible to document the changes in CNS development between 8th and 12th week as demonstrated in . Recent reports published on embryonal ventricular development from 6th or 7th week by use of 3D inversion-rendering mode, have made sonoembryology more sophisticated and objective [Citation10,Citation11]. It is possible that by developing 3D neurosonoembryology imaging in-utero, current fetal staging (which uses gestational age based on last menstrual period or crown-rump length measurement) may change into a ‘morphological staging system,’ such as the Carnegie staging system, which has been central to embryology [Citation4].
Figure 14. Three orthogonal image of normal brain at the end of 8 weeks of gestation. The development of premature ventricular system is seen. Note the different appearance from the beginning of 8 weeks of gestation ().
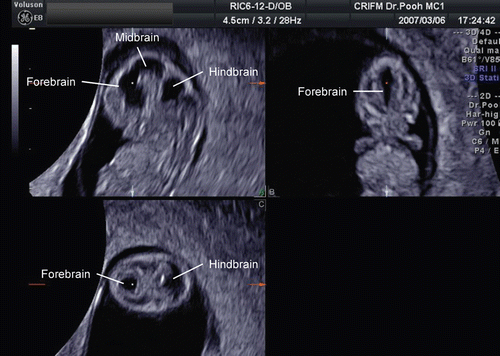
Figure 15. Sagittal section and coronal sections of 10-week-fetus CP; Choroid plexus of the lateral ventricles.
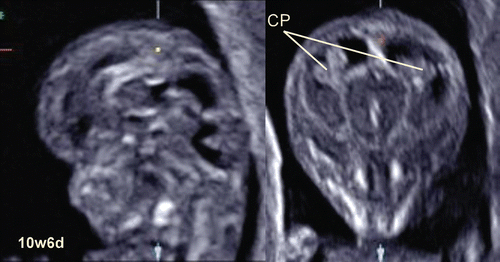
Figure 16. Normal brain development by mid-sagittal 3D US section between 8 and 12 weeks of gestation).
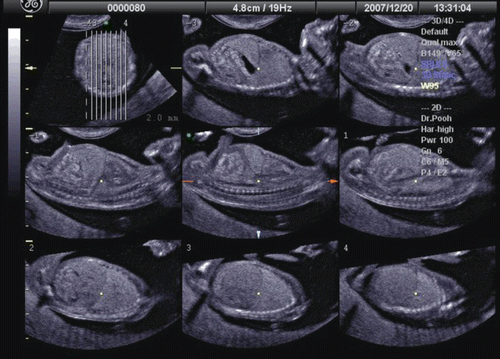
Thoraco-abdominal structures can also be imaged in the first trimester. For example, shows tomographic ultrasound imaging of fetal chest and abdomen at 13 weeks of gestation. Clear visualization of the lung-liver interface can be of value in the early diagnosis of thoraco-abdominal abnormalities, such as a diaphragmatic hernia.
Fetal abnormalities in the first trimester
Yolk sac
The yolk sac plays an important role in embryonal hemopoiesis and fetomaternal transportion of nutritive properties before establishment of fetoplacental circulation. The primary yolk sac forms at around 3rd week of the menstrual age, then following the formation of the extraembryonic coelom, and the secondary yolk sac is formed. From the 6th week of gestation, it appears as a spherical and cystic structure covered by numerous superficial small vessels merging at the basis of the vitelline duct. This connects the yolk sac to the ventral part of the embryo, the gut and main blood circulation. During the 10th week of gestation, the yolk sac begins to degenerate and rapidly ceases to function [Citation12].
It has been reported that abnormal size and/or shape of yolk sac may be associated with ominous pregnant outcome [Citation13]. Large yolk sac is defined more than two standard deviations above the mean, which indicates over 5.6 mm of diameter at less than 10 weeks menstrual age [Citation13,Citation14]. Large yolk sac is associated with chromosomal aberration of autosomal trisomy (, ). Echogenic yolk sac () is also related to adverse pregnant outcome with autosomal trisomy.
Figure 18. Tomographic imaging of large yolk sac at 6 weeks of gestation. Large yolk sac with normal fetal heart beat was observed. Fetal demise was confirmed at 8 weeks. Villous chromosome exam resulted in 47, XY, +22.
Figure 19. Large yolk sac (arrowheads) at 8 weeks of gestation. (left) 2D sagittal section of the fetus. Normal appearance of 8-week-fetus and amniotic membrane are visible, but large yolk sac with 12 mm of diameter is demonstrated. (middle) regular fetal heart rate of 174 bpm is confirmed. (right) 3D image intrauterine fetal demise was confirmed 30 hours later. Villous chromosome exam resulted in doubled trisomy of 48, XY, +15, +21.
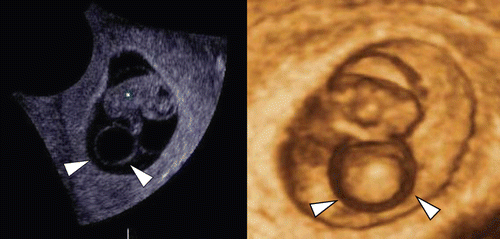
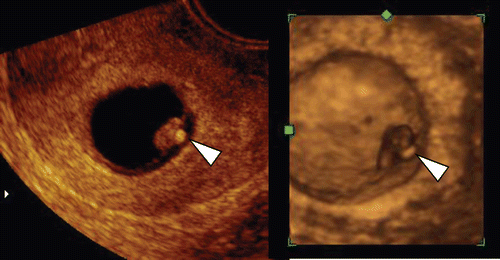
Figure 20. High echogenic yolk sac (left) 2D ultrasond image of yolk sac (arrowhead) at 9 weeks and 1 day. Small embryo compatible with 7-week-embryo was visible with regular heart beats in the abnormally large amniotic sac. (right) 3D ultrasound image. intrauterine fetal demise was confirmed three days later and villous chromosome was trisomy 15.
Diagnosis of congenital anomalies in the first trimester
The prenatal diagnosis of congenital anomalies with ultrasound is based upon identification of a substantial departure of normal anatomy. This has been possible in the second and third trimester of pregnancy, and this achievement has made the diagnosis of congenital anomalies one of the objectives of modern prenatal care. The definition of the “normal anatomy” of the human embryo provides the basis for the identification of congenital anomalies at the earliest stages of human development. This goes beyond the mere identification of nuchal translucency, because it is now possible to identify anomalies even in the absence of an abnormal nuchal translucency. Therefore, the scope of prenatal diagnosis during embryonic life has been widened by sonoembryology with three-dimensional ultrasound [Citation4].
Conjoined twin
Conjoined twins are defined as monochorionic-monoamniotic twins fused at any portion of their body as a result of an incomplete division of the embryonic disk after the 13th day of conception. Pathogenesis of the condition is considered as the result from failure of complete separation. Careful observation in the first trimester can reveal this condition from the early pregnancy. demonstrates conjoined twin with limb body-wall complex at 9 weeks of gestation. 3D ultrasound can objectively depict this rare abnormality.
Central nervous system (cranium, brain, vertebra and spinal cord) anomalies
Acrania, frequently found by early ultrasound scan, is characterized by a partial or complete absence of the cranium. The cranial shape and appearance vary, according to the extent of brain destruction. shows acrania complicated with cervical spina bifida, which is demonstrated by 3D reconstructed imaging. Holoprosencephaly can be detectable by careful observation of the midline and choroid plexus appearance (). Occasionally, cephalocele is detectable as shown in .
Figure 22. Acrania with cervical spina bifida (craniorachischisis) at 11 weeks of gestation. Left upper; 2D image. Note the amniotic fluid is more turbid than chorio-amniotic cavity due to floating nerve tissue from destroyed brain. Left lower; Three orthogonal view and reconstructed image. Right; 3D reconstructed image of cerebrospinal region. This fetus is complicated with acrania and cervical spina bifida, so-called craniorachischisis.
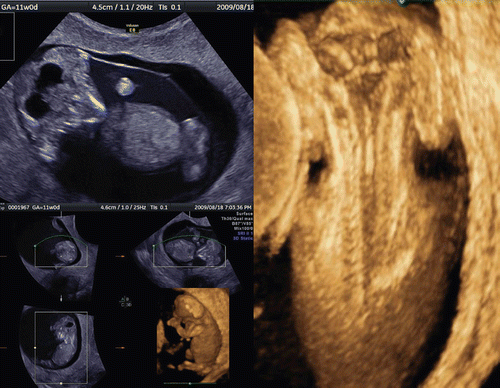
Figure 23. Holoprosencephaly at 12 weeks of gestation. Tomographic ultrasound imaging shows a single ventricule due to non-separated hemispheres.
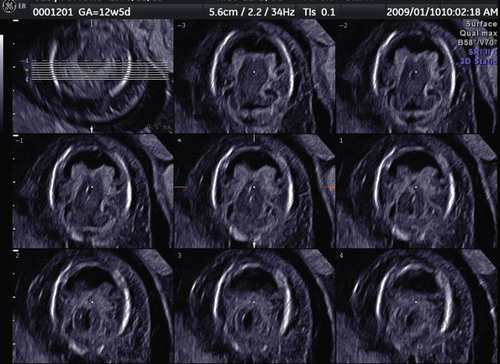
Figure 24. Cephalocele at 12 weeks of gestation Cephalocele containing translucent fluid is seen on the parietal right side of the head. This cyst disappeared 2 weeks later.
Spina bifida is the most common congenital spinal cord anomaly. It is often detected during the second and third trimesters. However, the fundamental basis for this anomaly is a failure of the neural tube to close during early embryonic age. Most reports of the diagnosis of spina bifida in utero have occurred after 12 weeks of gestation. Blaas et al. [Citation15] reported an early diagnosis using 2D and 3D ultrasound before 10 weeks of gestation. shows the early diagnosis of spina bifida in a case of OEIS complex (omphalocele, bladder exstrophy, imperforate anus, spina bifida) at 9 weeks of gestation. Iniencephaly is a rare neural tube defect that combines extreme retroflexion (backward bending) of the head with severe defects of the spine, associated with acrania, anencephaly and encephalocele. The prognosis for those with iniencephaly is extremely poor. Early detection of Iniencephaly is possible (). Scoliosis is often associated with Limb body-wall complex () and rarely scoliosis is associated with early vertebral fusion as shown in . Extreme hyper-dorsiflexion (backward bending) is a rare finding with unknown clinical significance. demonstrates extreme hyper-dorsiflexion seen in a case of trisomy 21.
Figure 25. Spina bifida at 9 weeks of gestation. Left; 2D sagittal image. Cystic formation was seen (white circle) at lumber part. Right; 3D image of neural tube. Clear dilatation of the neural tube is demonstrated (arrows).
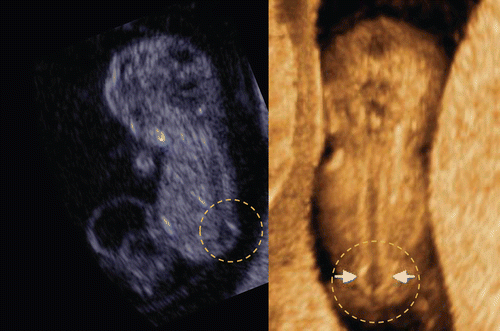
Figure 26. Iniencephaly at 12 weeks of gestation. Left; 3D reconstructed image. Acrania, short body with dorsoflexion are well demonstrated. Right; This fetus had reverse flows of descending aorta (right upper) and umbilical artery (right lower) and intrauterine fetal demise was confirmed one week later.
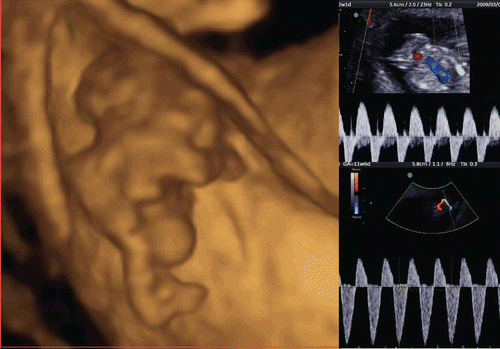
Figure 27. Severe scoliosis at 12 weeks of gestation. Left; 3D image of fetal back. Severe scoliosis is demonstrated. Right; Back view of aborted fetus. Fetal karyotype was normal.
Facial abnormalities
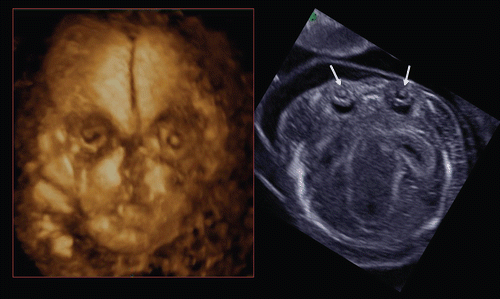
A facial anomaly can be associated with a central nervous system anomaly (e.g. holoprosencephaly as shown in and ), be an isolated finding, or part of a syndrome. Detection of the presence or absence of a nasal bone has been found to be of value in the assessment of aneuploidy in the first trimester of pregnancy (risk assessment for trisomy 21 [Citation16–20]). Cicero and her colleagues reported that the nasal bone was absent in 113 (0.6%) of the 20,165 chromosomally or phenotypically normal fetuses and in 87 (62.1%) of the 140 fetuses with trisomy 21 [Citation20]. 3D sonography allows a mid-sagittal section of the fetal face to be obtained by utilizing the three orthogonal planes, and avoids the pitfall of obtaining a para-sagittal view, which could lead to false negative results. Tomographic ultrasound imaging also allows demonstration of facial midline structures in detail, by examining close parallel sections (). It is important to be aware that the quality of the orthogonal planes or tomographic imaging is strongly dependent upon the original plane of acquisition. Rembouskos et al. [Citation21] described that routine application of 3D scanning for the nasal bone in screening for trisomy 21 is likely to be associated with a very high false-positive rate due to the possible limits of 3D technology. Benoit and Chaoui [Citation22] described the diagnosis of bilateral or unilateral absence or hypoplasia of nasal bones in second trimester screening for Down syndrome by using 3D sonography with maximal mode rendering. shows the midsagittal section of fetal face and craniofacial bony reconstructed image of normal fetus and trisomy 21 fetuses in the first trimester.
Figure 30. Cleft palate associated with holoprosencephaly. 3D maximum mode image demonstrates midline large cleft of maxilla.
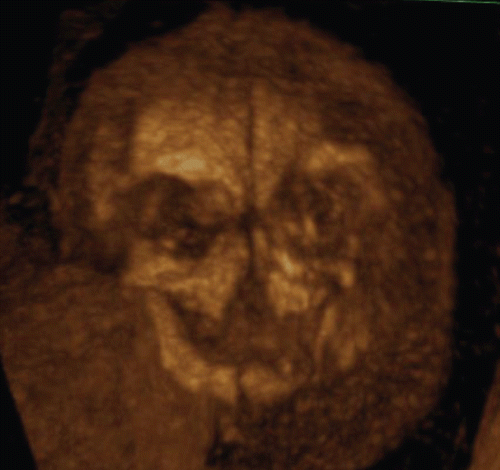
Figure 31. Proboscis associated with holoprosencephaly at 12 weeks of gestation. Upper; Tomographic facial sagittal imaging of holoprosencephalic fetus. Lower left; 3D reconstructed image of fetal face, lateral view. Lower right; Macroscopic picture after termination of pregnancy.
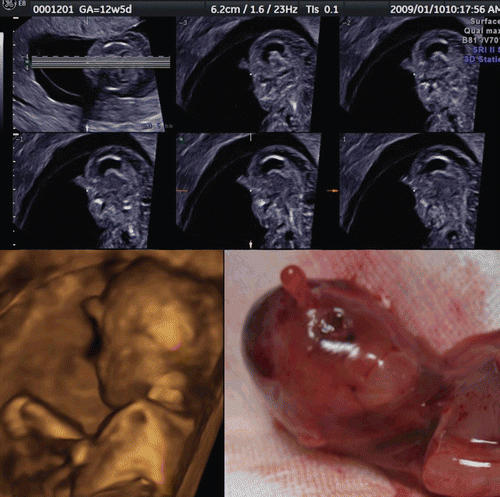
Figure 32. Tomographic ultrasound image of fetal profile with hypoplastic nasal bone at the end of 13 weeks of gestation. Thinly sliced parallel cutting section of mid-sagittal plane shows fetal profile in detail and hypoplastic nasal bone. Trisomy 21 was confirmed by CVS.
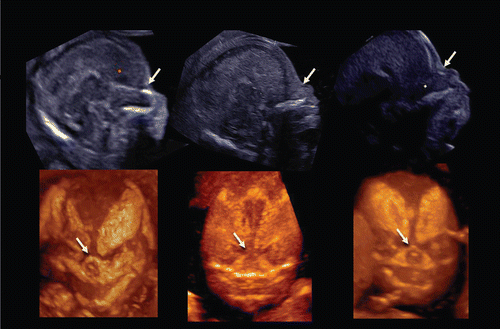
Figure 33. Midsagittal 2D image of fetal profile and craniofacial bony reconstructed image of normal fetus (left) and trisomy 21 fetuses(middle and right) at 13–14 weeks of gestation. (left) Normal fetus. Nasal bone is clearly visualized in both 2D and 3D images (arrows). (middle) Nasal bone defect in a case of trisomy 21. Nasal bone is completely missing in both 2D and 3D. (right) Nasal bone hypoplasia in a case of trisomy 21. Small nasal bone is visualized in both 2D and 3D.
A short maxillary length has been associated with trisomy 21 [Citation23]. Micrognathia can be detected as an isolated structural anomaly, as one of the features of a chromosomal abnormality, or a syndrome [Citation24]. Congenital micrognathia and lowest ears are frequently detected together as shown in , in cases with chromosomal aberrations and other syndromic diseases, because mandibula and ears arises from the first pharyngeal (branchial) arch. Assessment of the facial features, chin development and mandibular size by 3D ultrasound in the second and third trimesters has been reported [Citation25]. By using the surface mode and maximum mode (), the fetal profile and facial bone structure in normal and abnormal fetuses can be described in the first trimester.
Figure 34. Micrognathia and lowset ears at 13 weeks of gestation. Both images are from the same fetus. Micrognathia (arrowheads) and lowset ears (arrows) are clearly demonstrated by 3D reconstructed images.
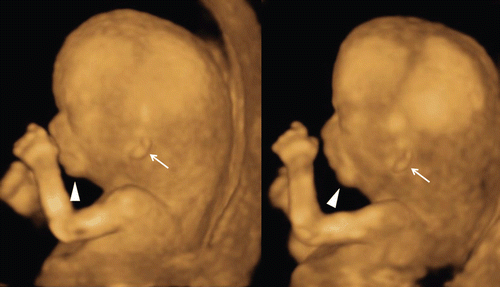
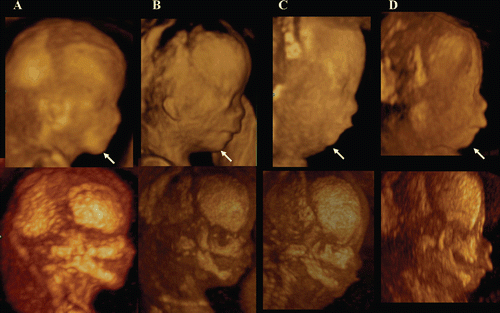
Figure 35. Fetal profile and facial bone development in normal fetus (A) and abnormal fetuses (B,C,D) at 12–13 weeks of gestation. A; Normal fetus, B; Trisomy 21 fetus, C; Trisomy 18 fetus, D; Mild micrognathia with normal chromosome. Lateral views of fetal profile (upper figures) show the difference of chin-angle. Maximum mode images (lower figures) indicate the hypoplastic maxilla and mandible in B,C,D.
Cleft lip and palate is usually demonstrated and diagnosed in the second and third trimesters. However, recent advances of the transvaginal 3D ultrasound have provided accurate and informative diagnostic images of cleft lip with orientation of left-sided, right-sided or bilateral cleft lip ( and ). Furthermore, the palate is still created during pregnancy but 3D reconstructed image can demonstrated cleft palate shown in and . Up-to-date tomographic ultrasound imaging can provide the precise demonstration of palate shown in and the possibility of early diagnosis of cleft palate.
Figure 36. Bilateral and unilateral cleft lip seen at 12 weeks of gestation. Left and middle; 3D reconstructed images of bilateral cleft lip seen in cases of trisomy 18 (left) and trisomy 21 (middle). Right; 3D reconstructed image of unilateral cleft lip seen as a single finding.
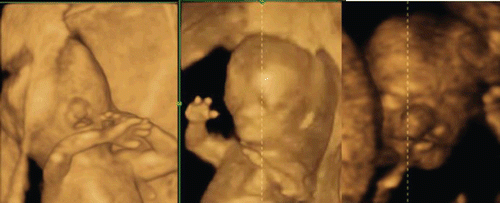
Figure 37. Left-sided cleft lip seen at 12 weeks of gestation. Left; 3D reconstructed images of bilateral cleft lip seen in cases of trisomy 21. Right; Macroscopic picture of aborted fetus.
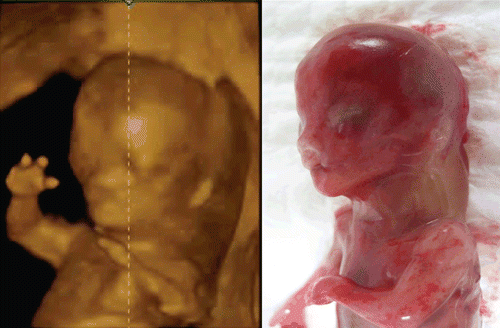
Figure 38. Bilateral cleft lip and palate at 13 weeks of gestation. Upper; Tomographic sagittal facial imaging of the normal-karyotype anomalous fetus. Lower; 3D reconstructed images. Oblique (left), frontal (middle) and maximum-mode frontal (right) views. Maximum image shows bilateral cleft of maxillary bone.
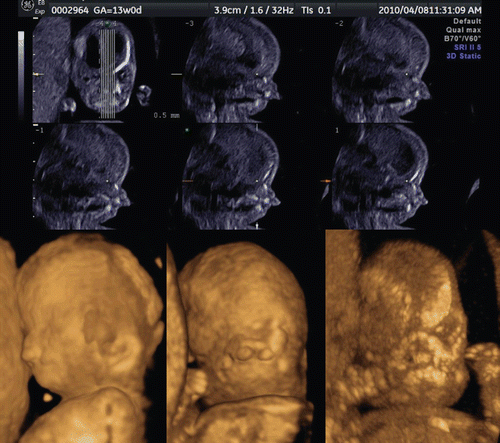
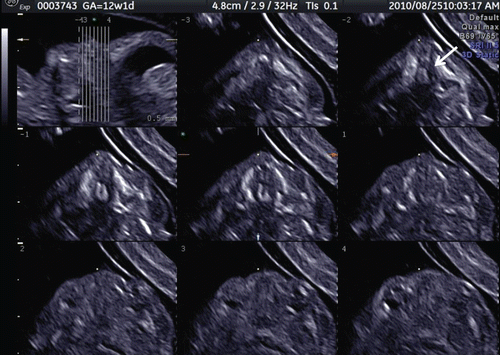
Figure 39. Tomographic ultrasound imaging of cleft plate at 12 weeks of gestation. Unilateral cleft palate is demonstrated (arrow).
Eyeballs and lenses are detectable by ultrasound from the late first trimester. Cataract is defined by the presence of any lens opacity. The incidence of congenital cataract ranges from 1 to 6 newborn infants out of 10,000 births [Citation26]. Cataract development is strongly linked to the embryonic ocular development. The lens differentiates from the surface ectoderm before the 6th week of gestation, explaining the absence of cataract in case of late first-trimester fetal infection [Citation27,Citation28]. A genetic cause is responsible for 30% of unilateral cataracts and 50% of bilateral cataracts. Prenatal diagnosis of fetal cataract was reported in the late second and third pregnancy [Citation29–33]. shows fetal bilateral cataract with microphthalmia as early as 14 weeks of gestation.
Limb abnormalities
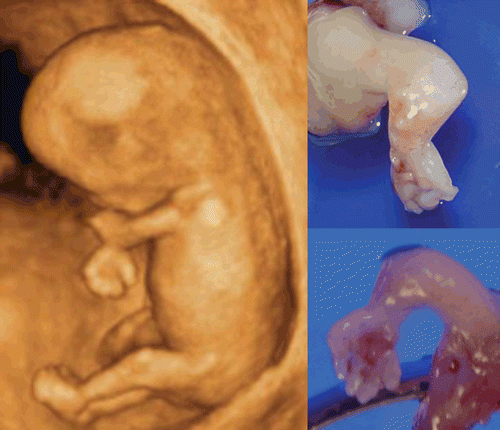
Limb abnormalities can occur as isolated findings or as one component of a syndrome or sequence. However, only 5% of congenital hand anomalies occur as part of a recognized syndrome [Citation34]. Overlapping fingers, wrist contracture () and forearm deformities are often associated with a chromosomal abnormality such as trisomy18. Most skeletal anomalies are recognizable in the second trimester. However, several reports on congenital skeletal abnormalities (such as sirenomelia and others) in the first trimester have been documented [Citation35–39]. Short limb abnormality of achondrogenesis in the first trimester was shown in . shows an 11-week fetus on 3D sonography with skeletal dysplasia of the bilateral upper extremities, and normal lower extremities. Clubfoot as shown in is not so common as clubhands in the first trimester. Finger abnormalities such as polydactyly, oligodactyly and syndactyly, are detectable from the late first trimester. In , polydactyly and syndactyly at 14 weeks in cases of holoprosencephaly are well demonstrated by 3D ultrasound.
Figure 41. Mild to moderate wrist contracture seen in cases of trisomy 18 at 12–13 weeks of gestation. Left; Mild wrist contracture is seen. Right; moderate wrist contracture is seen. Trisomy 18 was confirmed by chorionic villi sampling.
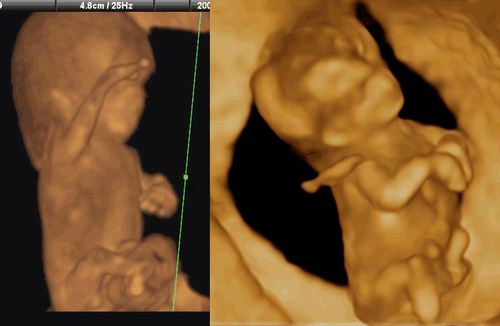
Figure 42. Short limb abnormality at 13 weeks of gestation. Short lower extremities with large abdomen is clearly demonstrated in the frontal (left) and lateral (right) views.
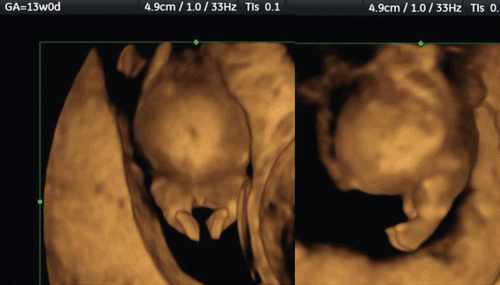
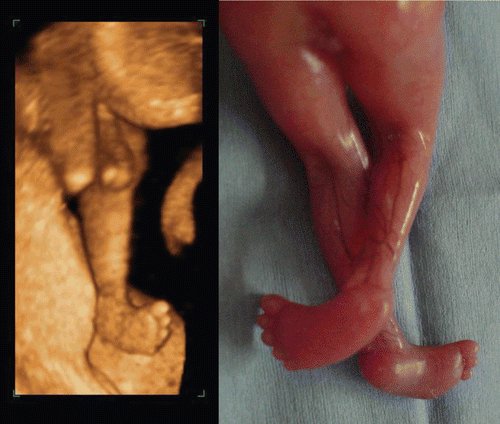
Figure 43. Upper limb abnormality at 11 weeks of gestation. 3D ultrasound revealed contracted elbow joint abnormality. Right figures show macroscopic appearance of upper limbs of aborted fetus.
Thoracoabdominal abnormalities
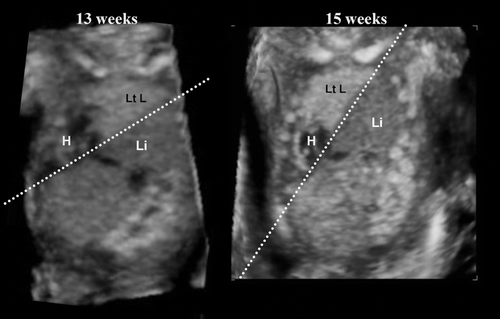
Congenital diaphragmatic hernia (CDH) occurs in 1 of every 2000–4000 live births and accounts for 8% of all major congenital anomalies [Citation40]. There are three types of CDH; posterolateral or Bochdalek hernia (occurring at approximately 6 weeks’ gestation), the anterior Morgagni hernia, and a hiatus hernia. The left-sided Bochdalek hernia occurs in approximately 90% of cases. Left-sided hernias allow herniation of both small and large bowel as well as intra-abdominal solid organs into the thoracic cavity. Early diagnosis of CDH in the first trimester has been reported [Citation41]. shows the thoraco-abdominal area of a fetus with a congenital diaphragmatic defect where the lung-liver border line acutely changes its angle from 13 to 15 weeks, due to progressive liver upward movement into the chest. Early diagnosis of this defect is important [Citation41].
Figure 46. Congenital diaphragmatic defect at 13 and 15 weeks of gestation. Referral case due to nuchal translucency of 3 mm at 11 weeks of gestation. (Left) frontal view at 13 weeks. Dextrocardia (H), liver-up (Li) and oppressed left lung (Lt L) are demonstrated. (Right) frontal view at 15 weeks. The line of Lung-liver border indicates acute angle changing in a short period due to progressive liver-up.
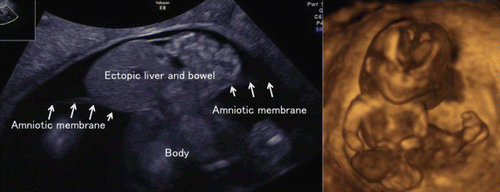
Omphalocele is often seen from the first trimester. Physiological umbilical hernia is usually observed around 8–10 weeks of gestation, however, umbilical hernia seen after the beginning of 12 weeks is definitely pathological (). Ectopic abdominal organs outside of amniotic cavity is often associated with short umbilical cord, abnormal limbs and this condition is called as short umbilical cord complex, limb body-wall complex or body-stalk anomaly as shown in and , different from amniotic band syndrome.
Figure 47. Omphalocele at 12 and 13 weeks of gestation 3D reconstructed ultrasound images clealy demonstrates omphalocele. Trisomy 18 was confirmed by chorionic villi sampling in both cases.
Urinary tract abnormality
Bladder extrophy () is a rare abnormality, which may be associated with cloaca malformation or the OEIS (omphalocele, bladder extrophy, imperforate anus, spine defect) complex. Prune-Belly syndrome () describes the triad of dilation of the urinary tract, a deficiency of the abdominal wall musculature, and failure of testicular descent. Hydronephrosis is the most common pathologic finding in the urinary tract on prenatal screening by ultrasonography. Fetal obstructive uropathy has become detectable earlier and fetal therapy of vesicoamniotic shunting (VAS) is the accepted procedure in well-defined cases. It has been reported that the long-term outcomes indicate that VAS at 18–30 weeks may not change the prognosis of renal function and that fetal surgery for obstructive uropathy should be performed only for the carefully selected patient who has severe oligohydramnios and “normal”-appearing kidneys [Citation42]. To preserve renal function, however, earlier VAS may be preferable ().
Figure 50. Bladder exstrophy in the first trimester. (upper) Cystic formation between bilateral umbilical arteries. No bladder is visible inside of the fetus. (lower) 3D US images at 10, 11 and 12 weeks from the left. Rapid increase in size of external bladder is clearly demonstrated.
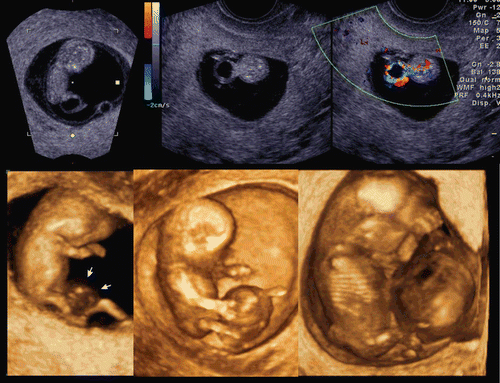
Figure 51. Prune-berry syndrome at 11 weeks of gestation (monochromatic figures) 3D orthogonal views of coronal (left upper), axial (middle upper) and sagittal (left lower) sections. Huge bladder of prune-berry shape and fragile abdominal wall are visible. (middle lower) 3D imaging of the fetus. (right) Macroscopic picture of aborted fetus.
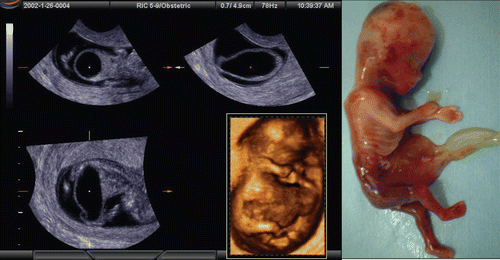
Figure 52. Huge bladder before and after vesicoamniotic shunt (VAS) operation. (left upper) 3D orthogonal view at 14 weeks of gestation. Huge bladder and bilateral hydronephrosis are demonstrated. Abdominal wall is not fragile like prune-berry syndrome. (right upper) sagittal image at 17 weeks before VAS. Bladder volume is 137.7 ml. (middle figures) Changing appearance of kidneys at 15,16 and 17 weeks from the left. Rapid thinning of renal parenchyma with progressive hydronephrosis is clearly demonstrated. (lower figures) Changing appearance of renal parenchyma (17, 18, and 20 weeks from the left) after VAS operation performed at 17 weeks. Renal parenchyma thickness was rapidly recovered after VAS procedure, with improvement of hydronephrosis [72].
![Figure 52. Huge bladder before and after vesicoamniotic shunt (VAS) operation. (left upper) 3D orthogonal view at 14 weeks of gestation. Huge bladder and bilateral hydronephrosis are demonstrated. Abdominal wall is not fragile like prune-berry syndrome. (right upper) sagittal image at 17 weeks before VAS. Bladder volume is 137.7 ml. (middle figures) Changing appearance of kidneys at 15,16 and 17 weeks from the left. Rapid thinning of renal parenchyma with progressive hydronephrosis is clearly demonstrated. (lower figures) Changing appearance of renal parenchyma (17, 18, and 20 weeks from the left) after VAS operation performed at 17 weeks. Renal parenchyma thickness was rapidly recovered after VAS procedure, with improvement of hydronephrosis [72].](/cms/asset/8a08d7cf-efaa-4cba-9984-a413431cad91/ijmf_a_636107_f0052_b.gif)
Conclusion
As illustrated by many papers published recently [Citation43–71], there has been an immense acceleration in the understanding of early human development. The anatomy and physiology of placental and embryonic development is a field where medicine exerts, its greatest impact on early pregnancy at present time, and it opens fascinating aspects of normal and abnormal embryonic differentiation. Clinical assessment of those stages of growth rely heavily on 3D/4D sonography, one of the most promising forms of noninvasive diagnostics and embryological phenomenon, once matters for textbooks, are now routinely recorded with outstanding clarity. Some advances deserve the adjective “breathtaking” including 4D parallel study of the structural and functional early human development.
During the first trimester of pregnancy, a unique and dramatic sequence of events occurs, defining the most critical and tenuous period of human development: the remarkable transformation of a single cell into a recognizable human being.
The possibility to describe the embryonic anatomy using the newer techniques has initiated a field called “SONOEMBRYOLOGY”.
3D and 4D ultrasounds play an important role in the early diagnosis and assessment of fetal abnormalities. Indeed, this diagnostic tool has moved embryology from postmortem studies to the in vivo environment even in the first semester of pregnancy.
With modern trend of shifting prenatal diagnosis at the earliest possible gestation, it is not far that 11–14 weeks scan becomes the first mini-anomaly scan to diagnose severe structural abnormalities giving the parents reassurance of the fetal well being.
Declaration of interest: The authors report no conflicts of interest.
References
- Timor-Tritsch IE, Peisner DB, Raju S. Sonoembryology: An organ-oriented approach using a high-frequency vaginal probe. J Clin Ultrasound 1990;18:286–298.
- Benoit B, Hafner T, Kurjak A, Kupesic S, Bekavac I, Bozek T. Three-dimensional sonoembryology. J Perinat Med 2002;30:63–73.
- O’Rahilly R, Müller F. Prenatal ages and stages-measures and errors. Teratology 2000;61:382–384.
- Pooh RK, Shiota K, Kurjak A. Imaging of the human embryo with magnetic resonance imaging microscopy and high-resolution transvaginal 3-dimensional sonography: Human embryology in the 21st century. Am J Obstet Gynecol 2011;204:77.e1–77.16.
- Kurjak A, Pooh RK, Merce LT, Carrera JM, Salihagic-Kadic A, Andonotopo W. Structural and functional early human development assessed by three-dimensional and four-dimensional sonography. Fertil Steril 2005;84:1285–1299.
- Kurjak A, Zudenigo D, Predanic M, Kupesic S. Recent advances in the Doppler study of early fetomaternal circulation. J Perinat Med 1993;21:419–439.
- Kurjak A, Schulman H, Predanic A, Predanic M, Kupesic S, Zalud I. Fetal choroid plexus vascularization assessed by color flow ultrasonography. J Ultrasound Med 1994;13:841–844.
- Pooh RK, Aono T. Transvaginal power Doppler angiography of the fetal brain. Ultrasound Obstet Gynecol 1996;8:417–421.
- Chaoui R, Levaillant JM, Benoit B, Faro C, Wegrzyn P, Nicolaides KH. Three-dimensional sonographic description of abnormal metopic suture in second- and third-trimester fetuses. Ultrasound Obstet Gynecol 2005;26:761–764.
- Kim MS, Jeanty P, Turner C, Benoit B. Three-dimensional sonographic evaluations of embryonic brain development. J Ultrasound Med 2008;27:119–124.
- Hata T, Dai SY, Kanenishi K, Tanaka H. Three-dimensional volume-rendered imaging of embryonic brain vesicles using inversion mode. J Obstet Gynaecol Res 2009;35:258–261.
- Jauniaux E, Gulbis B, Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus–a review. Placenta 2003;24 Suppl A:S86–S93.
- Küçük T, Duru NK, Yenen MC, Dede M, Ergün A, Baser I. Yolk sac size and shape as predictors of poor pregnancy outcome. J Perinat Med 1999;27:316–320.
- Lindsay DJ, Lovett IS, Lyons EA, Levi CS, Zheng XH, Holt SC, Dashefsky SM. Yolk sac diameter and shape at endovaginal US: Predictors of pregnancy outcome in the first trimester. Radiology 1992;183:115–118.
- Blaas HG, Eik-Nes SH, Isaksen CV. The detection of spina bifida before 10 gestational weeks using two- and three-dimensional ultrasound. Ultrasound Obstet Gynecol 2000;16:25–29.
- Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol 2004;191:45–67.
- Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K. Absence of nasal bone in fetuses with trisomy 21 at 11–14 weeks of gestation: An observational study. Lancet 2001;358:1665–1667.
- Zoppi MA, Ibba RM, Axiana C, Floris M, Manca F, Monni G. Absence of fetal nasal bone and aneuploidies at first-trimester nuchal translucency screening in unselected pregnancies. Prenat Diagn 2003;23:496–500.
- Cicero S, Rembouskos G, Vandecruys H, Hogg M, Nicolaides KH. Likelihood ratio for trisomy 21 in fetuses with absent nasal bone at the 11–14-week scan. Ultrasound Obstet Gynecol 2004;23:218–223.
- Cicero S, Avgidou K, Rembouskos G, Kagan KO, Nicolaides KH. Nasal bone in first-trimester screening for trisomy 21. Am J Obstet Gynecol 2006;195:109–114.
- Rembouskos G, Cicero S, Longo D, Vandecruys H, Nicolaides KH. Assessment of the fetal nasal bone at 11–14 weeks of gestation by three-dimensional ultrasound. Ultrasound Obstet Gynecol 2004;23:232–236.
- Benoit B, Chaoui R. Three-dimensional ultrasound with maximal mode rendering: A novel technique for the diagnosis of bilateral or unilateral absence or hypoplasia of nasal bones in second-trimester screening for Down syndrome. Ultrasound Obstet Gynecol 2005;25:19–24.
- Cicero S, Curcio P, Rembouskos G, Sonek J, Nicolaides KH. Maxillary length at 11–14 weeks of gestation in fetuses with trisomy 21. Ultrasound Obstet Gynecol 2004;24:19–22.
- Teoh M, Meagher S. First-trimester diagnosis of micrognathia as a presentation of Pierre Robin syndrome. Ultrasound Obstet Gynecol 2003;21:616–618.
- Tsai MY, Lan KC, Ou CY, Chen JH, Chang SY, Hsu TY. Assessment of the facial features and chin development of fetuses with use of serial three-dimensional sonography and the mandibular size monogram in a Chinese population. Am J Obstet Gynecol 2004;190:541–546.
- Francis PJ, Berry V, Bhattacharya SS, Moore AT. The genetics of childhood cataract. J Med Genet 2000;37:481–488.
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol 2001;231:63–76.
- Karkinen-Jääskeläinen M, Saxén L, Vaheri A, Leinikki P. Rubella cataract in vitro: Sensitive period of the developing human lens. J Exp Med 1975;141:1238–1248.
- Romain M, Awoust J, Dugauquier C, Van Maldergem L. Prenatal ultrasound detection of congenital cataract in trisomy 21. Prenat Diagn 1999;19:780–782.
- Pedreira DA, Diniz EM, Schultz R, Faro LB, Zugaib M. Fetal cataract in congenital toxoplasmosis. Ultrasound Obstet Gynecol 1999;13:266–267.
- Daskalakis G, Anastasakis E, Lyberopoulos E, Antsaklis A. Prenatal detection of congenital cataract in a fetus with Lowe syndrome. J Obstet Gynaecol 2010;30:409–410.
- Reches A, Yaron Y, Burdon K, Crystal-Shalit O, Kidron D, Malcov M, Tepper R. Prenatal detection of congenital bilateral cataract leading to the diagnosis of Nance-Horan syndrome in the extended family. Prenat Diagn 2007;27:662–664.
- Léonard A, Bernard P, Hiel AL, Hubinont C. Prenatal diagnosis of fetal cataract: Case report and review of the literature. Fetal Diagn Ther 2009;26:61–67.
- Bolitho DG. Hand, Congenital hand deformities. Emedicine 2006. http://www.emedicine.com/plastic/TOPIC298.HTM
- Carbillon L, Seince N, Largillière C, Bucourt M, Uzan M. First-trimester diagnosis of sirenomelia. A case report. Fetal Diagn Ther 2001;16:284–288.
- Monteagudo A, Mayberry P, Rebarber A, Paidas M, Timor-Tritsch IE. Sirenomelia sequence: First-trimester diagnosis with both two- and three-dimensional sonography. J Ultrasound Med 2002;21:915–920.
- Schiesser M, Holzgreve W, Lapaire O, Willi N, Lüthi H, Lopez R, Tercanli S. Sirenomelia, the mermaid syndrome–detection in the first trimester. Prenat Diagn 2003;23:493–495.
- Dugoff L, Thieme G, Hobbins JC. First trimester prenatal diagnosis of chondroectodermal dysplasia (Ellis-van Creveld syndrome) with ultrasound. Ultrasound Obstet Gynecol 2001;17:86–88.
- Percin EF, Guvenal T, Cetin A, Percin S, Goze F, Arici S. First-trimester diagnosis of Robinow syndrome. Fetal Diagn Ther 2001;16:308–311.
- Doyle NM, Lally KP. The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol 2004;28:174–184.
- Daskalakis G, Anastasakis E, Souka A, Manoli A, Koumpis C, Antsaklis A. First trimester ultrasound diagnosis of congenital diaphragmatic hernia. J Obstet Gynaecol Res 2007;33:870–872.
- Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: Long-term postnatal outcomes. Pediatrics 2001;108:E7.
- Pooh RK. Contribution of transvaginal high-resolution ultrasound in fetal neurology. Donald School Journal of Ultrasound in Obstetrics and Gynecology 2011;2:93–99.
- Comas C, Echevarria M, Rodriguez I, Sabria Serra, B. Analysis of quality of nuchal translucency measurements. Donald School Journal of Ultrasound in Obstetrics and Gynecology 2011;2:127–132.
- Pooh RK, Kurjak A. Fetal Neurology. Jaypee Brothers: New Delhi 2009.
- Kurjak A, Chervenak FA. Donald School Textbook of Ultrasound in Obstetrics and Gynecology, 3rd edition. Jaypee Brothers: New Delhi 2011.
- Kurjak A, Azumendi G, Andonotopo W, Salihagic-Kadic A. Three- and four-dimensional ultrasonography for the structural and functional evaluation of the fetal face. Am J Obstet Gynecol 2007;196:16–28.
- Pooh RK, Kurjak A. Recent advances in 3D assessment of various fetal anomalies. Donald School Journal of Ultrasound in Obstetrics and Gynecology 2009;3:1–25.
- Lausin I, Kurjak A. The early human development: Advances in its visualisation. Gynaecol Perinatol 2009;18:76–87.
- Pooh RK, Kurjak A. 3D and 4D sonography and magnetic resonance in the assessment of normal and abnormal CNS development: Alternative or complementary. J Perinat Med 2011;39:3–13.
- Kurjak A, Kupesic S, Di Renzo GC, Pooh RK, Kos M, Hafner T. Recent advances in perinatal sonography. Prenat Neonat Med 1998;3:194–207.
- Pooh RK. Early detection of fetal abnormality. Donald School Journal of Ultrasound in Obstetrics and Gynecology 2005;2:74–83.
- Pooh RK, Kurjak A. Normal and abnormal early human structural development. In: The Fetus in Three Dimensions: Imaging, Embryology and Fetoscopy. Kurjak A, Azumendi G, eds. Informa: London 2007, pp, 83–98.
- Pooh RK, Kurjak A, Tikvica A. Normal and abnormal brain vascularity. In: Fetal Neurology. Pooh RK, Kurjak A, (eds). Jaypee Brothers: New Delhi 2009, pp 39–58.
- Pooh RK, Kurjak A. Neuroscan of normal and abnormal vertebrae and spinal cord. In: Fetal Neurology. Pooh RK, Kurjak A, (eds). Jaypee Brothers: New Delhi 2009, pp. 141–159.
- Pooh RK, Shiota K, Kurjak A. Three-dimensional sonoembryology. In: Donald School Textbook of Ultrasound in Obstetrics and Gynecology, 3rd edition. Kurjak A, Chervenak FA, (eds). Jaypee Brothers: New Delhi 2011, pp. 540–558.
- Pooh RK, Kurjak A. 3D ultrasound in detection of fetal anomalies. In: Donald School Textbook of Ultrasound in Obstetrics and Gynecology, 3rd edition. Kurjak A, Chervenak FA (eds). Jaypee Brothers: New Delhi 2011, pp. 621–639.
- Ebrashy A, El Kateb A, Momtaz M, El Sheikhah A, Aboulghar MM, Ibrahim M, Saad M. 13–14-week fetal anatomy scan: A 5-year prospective study. Ultrasound Obstet Gynecol 2010;35:292–296.
- Tonni G, Ventura A, Bonasoni MP. Acrania/encephalocele sequence (exencephaly) associated with 92,XXXX karyotype: Early prenatal diagnosis at 9(+5) weeks by 3D transvaginal ultrasound and coelocentesis. Congenit Anom (Kyoto) 2009;49:113–115.
- Becker R, Wegner RD. Detailed screening for fetal anomalies and cardiac defects at the 11–13-week scan. Ultrasound Obstet Gynecol 2006;27:613–618.
- Carvalho MH, Brizot ML, Lopes LM, Chiba CH, Miyadahira S, Zugaib M. Detection of fetal structural abnormalities at the 11–14 week ultrasound scan. Prenat Diagn 2002;22:1–4.
- Saltvedt S, Almström H, Kublickas M, Valentin L, Grunewald C. Detection of malformations in chromosomally normal fetuses by routine ultrasound at 12 or 18 weeks of gestation—a randomised controlled trial in 39,572 pregnancies. BJOG 2006;113:664–674.
- Sepulveda W, Wong AE, Fauchon DE. Fetal spinal anomalies in a first-trimester sonographic screening program for aneuploidy. Prenat Diagn 2011;31:107–114.
- Antsaklis A, Anastasakis E, Komita O, Theodora M, Hiridis P, Daskalakis G. First trimester 3D volumetry. Association of the gestational volumes with the birth weight. J Matern Fetal Neonatal Med 2011;24:1055–1059.
- Tonni G, Ventura A, Centini G, De Felice C. First trimester three-dimensional transvaginal imaging of alobar holoprosencephaly associated with proboscis and hypotelorism (ethmocephaly) in a 46,XX fetus. Congenit Anom (Kyoto) 2008;48:51–55.
- Abu-Rustum RS, Daou L, Abu-Rustum SE. Role of first-trimester sonography in the diagnosis of aneuploidy and structural fetal anomalies. J Ultrasound Med 2010;29:1445–1452.
- Souka AP, Pilalis A, Kavalakis I, Antsaklis P, Papantoniou N, Mesogitis S, Antsaklis A. Screening for major structural abnormalities at the 11- to 14-week ultrasound scan. Am J Obstet Gynecol 2006;194:393–396.
- Ghi T, Pilu G, Savelli L, Segata M, Bovicelli L. Sonographic diagnosis of congenital anomalies during the first trimester. Placenta 2003;24 Suppl B:S84–S87.
- Bronshtein M, Zimmer EZ, Blazer S. The utility of detailed first trimester ultrasound examination in abnormal fetal nuchal translucency. Prenat Diagn 2008;28:1037–1041.
- Sciaky-Tamir Y, Cohen SM, Hochner-Celnikier D, Valsky DV, Messing B, Yagel S. Three-dimensional power Doppler (3DPD) ultrasound in the diagnosis and follow-up of fetal vascular anomalies. Am J Obstet Gynecol 2006;194:274–281.
- Dane B, Dane C, Sivri D, Kiray M, Cetin A, Yayla M. Ultrasound screening for fetal major abnormalities at 11–14 weeks. Acta Obstet Gynecol Scand 2007;86:666–670.