Abstract
The past decade has witnessed increasing global attention and political support for maternal, newborn and child health. Despite this increased attention, actual progress has been slow and sporadic: coverage of key maternal and newborn health interventions remains low and there are wide disparities in access to care, within and across countries. Strategies for improving maternal and newborn health are closely linked, and can be delivered most effectively through a continuum of care approach. While these interventions are largely known, there is little information on which interventions have a positive health impact for both women and newborns. This supplement identifies the interventions during the preconception, pregnancy, intrapartum and postnatal periods found to have a positive, synergistic effect on maternal and neonatal outcomes. These interventions are then grouped into packages of care for delivery at the community, health center or hospital levels.
Introduction
The past decade has ushered in unprecedented global commitment and attention to the health of mothers and their newborn children. The Millennium Development Goals (MDGs) set ambitious targets for reducing maternal and under-five child mortality, for achieving social and economic development and for ending poverty by the year 2015.
In spite of this increased attention, actual progress has been slow and sporadic: rates of decline in maternal, newborn and under-five mortality in several regions of the world remain insufficient to achieve these goals by 2015. Furthermore, progress is marked by inequities, not only across regions and countries, but also within countries where maternal and child health mortality rates and health indicators differ substantially by geographic location (higher in rural versus urban areas) as well as by socioeconomic status.
The burden of maternal and neonatal death and disability is heavily concentrated in developing countries, and persists despite the availability of simple, cost-effective and low-technology interventions [Citation1]. Coverage of key maternal and newborn health interventions in developing countries is low, and wide disparities exist within countries, across socioeconomic status, geographic location and educational status. In 2008, only 63% of deliveries in developing countries were attended by skilled health personnel, as opposed to 99% in developed regions [Citation2]. Furthermore, within developing regions, the proportion of women receiving the World Health Organization (WHO) recommended four or more antenatal visits is 67% in urban areas versus only 34% in rural areas. Training skilled birth attendants in neonatal resuscitation is also a missed opportunity: only 1 in 4 infants in six African countries is delivered by an attendant skilled in neonatal resuscitation and equipped with the appropriate supplies [Citation3].
While the interventions and strategies for ensuring maternal and newborn survival are largely known, few attempts have been made to identify which interventions affect both women and newborn children, analyze identified synergies between interventions, or assess the impact of efforts to integrate these interventions across the continuum of care. This special supplement identifies interventions that improve both maternal and neonatal health outcomes, focusing specifically on the interconnections between maternal and newborn health strategies and interventions. The paper provides an overview of the global burden of maternal and newborn death and disability, and highlights how an integrated, continuum of care approach can address this public health problem. It then highlights (see “Results” section) the evidence for interventions found to have a synergistic effect on maternal and neonatal outcomes during the preconception period, during pregnancy, during the intrapartum period and during the postnatal/postpartum period. The paper groups these interventions into proposed packages of care for delivery at the community, health center, or hospital levels, highlights gaps in knowledge and research, and provides recommendations for an integrated management of maternal and newborn health.
Burden of maternal and newborn death and disability
Worldwide, an estimated 250 000–280 000 women die every year [Citation4], while over 15 million suffer from long-term illnesses or disability due to complications of pregnancy and childbirth [Citation5]. Every year, an estimated 2.9 million babies die in the first 4 weeks of life [Citation6,Citation7]. Maternal health complications contribute to 1.5 million neonatal deaths during the first week of life and 1.4 million stillbirths [Citation5]. Data for neonatal morbidities is limited, and gaps in data from developing countries for both maternal and neonatal mortality/morbidity are a challenge, especially with such a large number of births taking place at home.
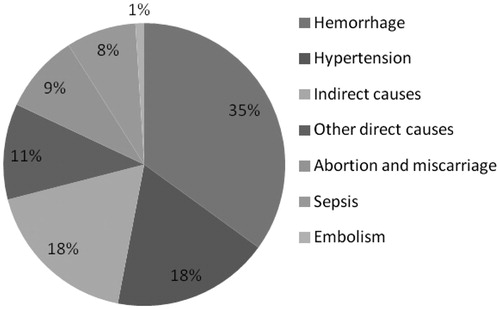
The majority of maternal deaths occur during labor, delivery, and the immediate postpartum period, with obstetric hemorrhage being the main medical cause of death (). Other direct causes of maternal mortality include hypertensive diseases, sepsis/infections and unsafe abortion. Maternal deaths due to indirect causes represent 18% of the global total; these include malaria, anemia, HIV and cardiovascular disease.
The main direct causes of neonatal death and morbidity are infections, complications arising from preterm birth, and intrapartum-related neonatal deaths; these account for nearly 80% of all neonatal deaths globally.
Approximately 99% of maternal and newborn deaths occur in low and middle income countries. The risk of adverse pregnancy outcomes is much higher in low and middle income countries (LMICs) as compared to developed countries. In 1990, maternal mortality was 115 times higher in developing countries compared to developed countries (n = 405 605 in developing; n = 3448 in developed countries); while the total number of maternal deaths decreased in 2011, the difference between developed and developing countries remained 100 times higher (n = 270 772 in developing; n = 2693). In select European countries, the risk of pregnancy-related maternal mortality is 1 in 20 000 as compared to 1 in 15 or 16 in high-risk countries, such as Somalia and Chad [Citation4].
In addition to the global burden of maternal death, maternal morbidities (including anemia, maternal depression, infertility, fistula, uterine rupture and scarring, and genital and uterine prolapse) can have a significant impact on health and well-being. These can result in women facing catastrophic health expenditures, and can also lead to significant losses of productivity. The global cost for maternal disability is estimated to be US $6.8 billion annually [Citation8].
The linkages between the health of women and newborns
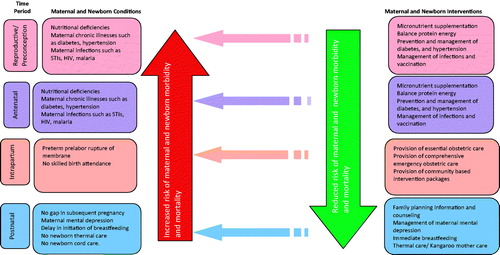
There is strong evidence on the close and inextricable relationship between maternal and newborn health [Citation9]. The major causes of maternal and newborn death and disability are linked, and pivotal time points for maternal and newborn health overlap, with childbirth being the most sensitive time period for both. describes these linkages. For example, the same set of interventions can prevent or treat the conditions resulting in maternal and newborn morbidity and mortality.
The interventions that women receive during pre-pregnancy, pregnancy, and childbirth have a beneficial health impact on their newborn children. For example, antenatal care and skilled birth attendance (SBA) not only address the three major causes of maternal mortality (bleeding, hypertensive diseases and infections), but also the three main causes of neonatal death (infections, complications arising from preterm birth and intrapartum-related neonatal deaths). As illustrated in , lower coverage of SBA correlates with higher neonatal mortality, with 77% of neonatal deaths occurring in countries where coverage of SBA is 50% or less. Simple treatments such as cleansing of the umbilical cord and promotion of immediate breastfeeding can prevent a significant portion of neonatal infections. Providing birth attendants with basic training and equipment (bag and mask) for neonatal resuscitation is a low-tech, low-cost opportunity for reducing intrapartum-related neonatal deaths. Complications from preterm birth and low birth weight (LBW) take the largest toll on newborn survival; while more advanced care may be required for those born before 33 weeks’ gestation, a meta-analysis suggested a 51% reduction in mortality for newborns weighing less than 2000 g through simple kangaroo mother care (KMC) or skin-to-skin care with the mother [Citation3,Citation6].
Table 1. Skilled attendance at birth correlated with neonatal mortality.
Even further along the continuum of care, a number of low-cost interventions including counseling and services for family planning, vaccines, antibiotics, insecticide-treated mosquito nets and micronutrient supplementation, along with the promotion of improved feeding and hygiene practices, can together make an effective impact on both mother and child [Citation10].
Improving a woman’s nutritional status has translational benefits for her child. As described in , good maternal health and nutrition are important contributors to neonatal and infant survival: when women are malnourished, ill or receive inadequate care, their newborns face a greater risk of disease and premature death [Citation11]. Maternal infections and other poor health conditions often contribute to neonatal morbidity and mortality (including stillbirths, neonatal deaths and other adverse clinical outcomes).
Box 1. Maternal and child undernutrition.
The linkages between maternal health and neonatal and child survival has also been shown statistically in several research studies. Newborns whose mothers have died during childbirth have a much greater chance of dying in their first year than those whose mothers remain alive. A study by Ronsmans et al. [Citation12], using data from population-based surveillance during 1982–2005 in Matlab, Bangladesh reported cumulative probabilities of survival and rates of age-specific death (according to the survival status of the mother during that period). The authors reported that the risk of death for a newborn whose mother has died increases up to 8-fold (risk ratio (RR) 8.35; 95% confidence interval (CI): 5.73 to 12.18); similarly, the risk of a death for infants age 1 to 5 months increases up to 27-fold if the mother had died (RR 27.61; 95% CI: 20.27 to 37.61) [Citation12].
The continuum of care for women and their children
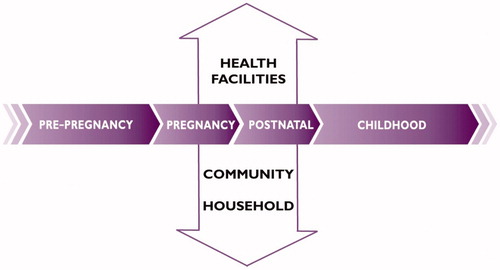
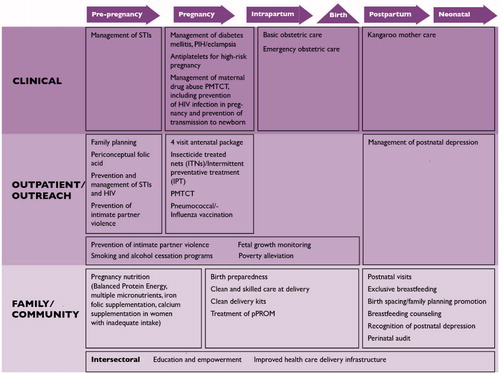
The continuum of care for reproductive, maternal, newborn and child health (RMNCH) is based on the concept that the health and well-being of women, newborns and children are closely linked and can be managed most effectively in an integrated way; interventions at one stage of the life cycle profoundly affect the rest (). During the last decade, the continuum of care approach has been identified as a core principle of RMNCH programs, as a way to reduce the burden of death and disability.
The continuum of care aims to bring together health care for women and their children, and avoid the separation or dichotomy in the care provided [Citation13]. It recognizes that the mother is not a lone entity; when she seeks medical attention at the primary care level, any opportunity to gain information regarding the health status of her child should not be missed, and vice versa. For example, when a woman seeks family planning or antenatal care (ANC), an unimmunized child can be screened and vaccinated appropriately, growth parameters can be assessed, and cases of malnutrition can be addressed. Similarly, when a woman seeks care for her infant or child, she can be asked about her interest in family planning, testing for HIV, or other health interventions. In this way, health intervention packages can be delivered to the whole family, across population groups.
The continuum of care occurs in two dimensions: time (throughout the lifecycle, i.e. adolescence, pregnancy, childbirth, the postnatal period, and childhood) and place (between places of care giving including households and communities, outpatient and outreach services, and clinical-care settings) [Citation13]. Linking interventions across the lifecycle allows the success of one stage to build on the previous one; for example, appropriate services to ensure proper nutrition during adolescence can prevent complications during pregnancy and reduce preterm birth. The second dimension of place entails linking essential, quality maternal and newborn health services across home, community and health facility settings. This dimension takes into consideration the gaps in care that occur at locations where care is most essential, in the home and community – since in many developing countries most women give birth at home [Citation10]. Within this continuum, the goal is to provide access to reproductive health care and safe pregnancy and childbirth for all women; and for all babies to grow into children who survive and thrive [Citation14].
Considering the interdependent relationship between maternal and neonatal health, an integrated approach appears to be the most pragmatic strategy for provision of maternal, newborn and child health care in low-resource settings. An integrated health system is potentially more cost effective and helps to maximize the use of limited health resources and provide a more seamless health care experience for clients – allowing health care providers at all levels to achieve a greater impact on the health of the people they serve. When linked together and delivered as integrated programs, high-impact maternal and newborn health interventions can lower costs, promote greater efficiencies and reduce duplication of efforts [Citation9]. Monitoring implementation of the continuum of care can also result in strengthening of health systems through public health planning, greater efficiency in training, supervision and tracking of performance. In addition, a continuum of interventions can bolster uptake and promote a continuation of healthy behaviors through increased opportunities for counseling [Citation15].
What has been done so far to improve maternal and newborn health?
Recent evidence, highlighted in the Lancet series (on neonatal survival, 2005; on maternal survival, 2006; on maternal and child under-nutrition, 2008; and on Alma Alta, 2008) as well as other sources, indicates that a package of interventions, if implemented at scale, could substantially reduce both newborn and maternal mortality. While the Lancet series have emphasized numerous interventions for maternal and newborn well being and survival, relatively few studies have examined how interventions affect both women and newborn. Almost no attempt has been made to identify synergies between interventions, or to assess the impact of integrating these interventions across the continuum of care. This supplement identifies interventions which have beneficial impacts on maternal and newborn health, and provides recommendations for their scale-up as packages of care.
Methods
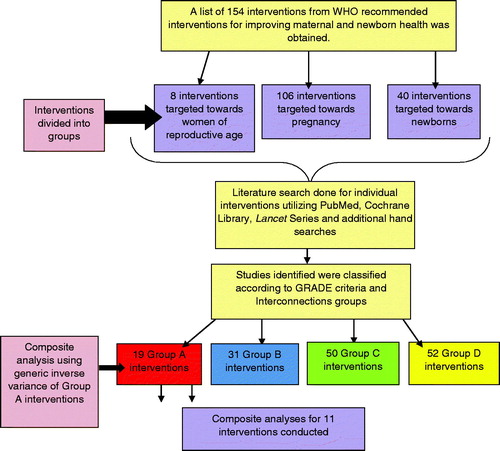
Based on the WHO recommended interventions for reducing maternal and newborn deaths and disability [Citation16], this review assessed a total of 154 maternal and newborn health interventions for their synergistic impact on maternal and newborn health (MNH) outcomes. Systematic reviews were conducted for these interventions; the interventions were then categorized according to their impact on maternal and neonatal mortality and morbidity. Biologically plausible maternal and neonatal mortalities and morbidities were pooled to assess additive or synergistic effects of interventions on composite maternal and neonatal/infant outcomes. Cases where interventions did not have significant impact on maternal or neonatal outcomes individually were analyzed for significant impacts in composite analyses. This review has updated and complemented existing literature, and identified key gaps in knowledge and priority areas for research for integrated management of maternal and newborn health.
Additional empirical work was undertaken to explore potential synergies with new and/or poorly researched areas such as opportunities for linking maternal health, nutrition and family planning interventions with perinatal and newborn outcomes. The review also grouped interventions into packages of care, and identified possible or actual strategies for delivering the interventions to populations of interest. lists the interventions included in the review.
Table 2. Landscape of interventions evaluated in our review (total number of interventions = 102).
Search strategy and selection criteria
An inventory of the interventions aimed to reduce maternal and neonatal morbidity and mortality was prepared using the WHO recommended interventions [Citation16]. Initially, reviews and studies for each of these interventions were identified from the Cochrane Library and the Lancet series (on neonatal survival, 2005; on maternal survival, 2006; on maternal and child under-nutrition, 2008; and on Alma Alta, 2008).
In addition, individual searches were conducted for any studies that may have been published after the last search dates of the systematic reviews. If no Cochrane reviews were identified for a given intervention, PubMed was searched for studies of that particular intervention. Exhaustive search strategies were employed using appropriate key words, accepted MeSH words, and combinations thereof. One search approach employed broad search terms (e.g. (“Pregnancy”[MeSH] OR mother* OR pregnancy OR maternal) AND (neonat* OR infan* OR child*), and was combined with search terms specific for interventions, (e.g. (“Iron”[MeSH] OR iron OR folate OR folic acid). To supplement the search, reports by agencies including WHO, UNFPA, UNICEF and the World Bank were also reviewed. A further snow-balling search was conducted through hand searching of references from identified studies. The principal focus was on collecting randomized controlled and quasi-randomized trials, where such studies were missing; other studies such as observational studies were also included.
Data abstraction, analysis of study quality and effect estimation
The quality of the systematic reviews were analyzed using the AMSTAR (Assessment of the Methodological Quality of Systematic Reviews) criteria [Citation17]. However, the quality of evidence was analyzed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation (GRADE) [Citation18,Citation19] criteria ()). Any discrepancies across the two methods were resolved through discussion within the review team.
Box 2. Criteria for determining quality of evidence for each intervention.
GRADE quality of evidence and GRADE recommendations were assessed for each intervention. The final recommendations were therefore made according to the GRADE criteria using a two-step process. First, the evidence was graded based on the quality of study design. Subsequently, the evidence was graded according to the following recommendations:
High: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: evidence will not be included in our analysis.
Following the grading, the interventions were further analyzed to assess their synergistic effect. Each intervention was studied for its impact on maternal, fetal, newborn or child outcomes. The synergies were classified as follows:
Mother and fetus
Mother and newborn
Mother and infants/children
The interventions were categorized into Interconnections Groups A, B, C, D or E, depending on whether statistically significant outcomes for mother and/or newborn were found. See for criteria used for classifications of the interventions.
Box 3. Interconnections groups.
Box 3 categorizes the level of evidence on the impact on maternal, fetal, neonatal, and infant mortality and stillbirths in an attempt to identify the most efficient and efficacious set of interventions. further illustrates the pathway for selection of interventions for meta-analyses. details the process for abstraction of studies.
Group A interventions were further analyzed to derive estimates for composite maternal and neonatal/infant mortality. This was performed via generic inverse variance to pool the risk ratios of individual outcomes into an effect for composite outcome using the software RevMan 5.1 [Citation20]. Heterogeneity of meta-analyses was assessed from the p-value (Chi square) and the I2 statistic and when found significant, random models were used to try to adjust for unexplained heterogeneity. details how Group A interventions span across the continuum of care.
The assessment of composite outcomes on maternal, fetal and neonatal mortality was followed by determining composite outcomes for corresponding morbidities. The combination of maternal and neonatal morbidity was based on a temporal and biological association between maternal morbidity and neonatal morbidity. For instance, in studies where the use of magnesium sulfate for preeclampsia was analyzed, the maternal morbidity was combined with neonatal outcome of Apgar scores. The aim of this exercise was to identify additive or synergistic impacts of an intervention on composite outcomes of maternal, neonatal and infant health parameters. An outcome was considered synergistic if it displayed statistically significant benefit in any of the composite outcomes of maternal and neonatal mortality/morbidity.
Results
The interventions found to have a positive synergistic impact on both maternal and neonatal mortality and morbidity are outlined below; they are grouped along the continuum of care (from reproductive/preconception, pregnancy, childbirth and postpartum).
Interventions during the reproductive/preconception period
Care during pregnancy has long established links with improved maternal and neonatal outcomes (see next section on the antenatal period). More recently, greater attention is also being paid to the period before pregnancy, specifically focusing on preconception care. The preconception period provides an opportunity to intervene earlier to optimize the health of potential mothers (and fathers) and to prevent harmful exposures from affecting the developing fetus. For example, early childbearing negatively affects educational and economic opportunities; women with lower education are less knowledgeable about health-prevention activities, and are more likely to live a life of poverty. Their children have fewer options and are also more likely to die. Young mothers are often not physically mature enough to deliver a baby, leaving them and their children at risk for death or disability from obstructed labor, fistula, premature birth or low birth weight.
For the purpose of this review, preconception care and its boundaries are defined as: “any preventive, promotive or curative health care intervention provided to women of childbearing age in the period before pregnancy (at least 2 years) or between consecutive pregnancies, to improve health-related outcomes for women (regardless of their pregnancy status), newborns or children up to 5 years of age” [Citation21].
This section identifies key interventions during the preconception period (such as birth spacing and preventing teenage pregnancy) which have synergistic effects on maternal and neonatal health outcomes. It further explores these interconnections by elucidating the effects of various interventions during the pre-pregnancy period on the woman and her newborn. These synergies can be used to guide implementation efforts and in scaling-up interventions in health programs.
Interventions evaluated and their assigned GRADE
enumerates the interventions evaluated in this analysis, reports impact on composite maternal and fetal/neonatal outcomes, and assigns each intervention to an Interconnection group (A, B, C or D).
Table 3. Individual interventions with composite maternal/fetal/neonatal impact estimates and their assigned GRADE.
Nutritional interventions
Peri-conceptual folic acid supplementation
The use of folic acid 3 months before conception is known to reduce the risk of first occurrence and a recurrence of neural tube defects (NTDs) [Citation22]. For this intervention, reviews were identified which looked at the impact of peri-conceptual folic acid supplementation on maternal, pregnancy and neonatal outcomes.
Impact estimates: Evidence from the Cochrane review by De-Regil et al. [Citation23] showed that peri-conceptual folic acid supplementation had a significant protective effect on NTDs (RR 0.28; 95% CI: 0.15 to 0.52). The review did not reveal any significant impact of folic acid supplementation on miscarriages (RR 1.10; 95% CI: 0.97 to 1.26) and stillbirths (RR 0.96; 95% CI: 0.51 to 1.83). Blencowe et al. [Citation24] also reported a significant reduction in occurrent neural tube defects with peri-conceptual folic acid supplementation (RR 0.38; 95% CI: 0.29 to 0.51). Bukowski et al. [Citation25] reported a significant decrease in the incidence of preterm births between 20 and 28 weeks (RR 0.22; 95% CI: 0.08 to 0.61). The same study also reported that the risk of spontaneous preterm birth decreased with the duration of peri-conceptual folate supplementation (p = 0.01) and was the lowest in women who used folate supplementation for 1 year or longer. Brouwer et al. [Citation26] also provides data on the potential long-term health benefits to mother in terms of significant reduction in the total plasma homocysteine levels (tHcy) with 400 mcg folic acid supplementation during 24 weeks of supplementation (p < 0.001). The lower plasma homocysteine reduces the risk of heart diseases and stroke.
Composite analysis: No composite outcomes were generated due to the lack of studies that looked into both maternal and neonatal outcomes simultaneously.
Key findings: Although the composite analysis did not produce results, this intervention did demonstrate benefits for the woman and the newborn individually. Thus, all women should be advised and given folate at least 3 months before conception until 3 months after conception to prevent the occurrence and/or recurrence of NTDs.
Maternal pre-pregnancy weight
Pre-pregnancy underweight poses major perinatal risks, e.g. stillbirths, preterm births, small for gestational age (SGA) and low birth weight babies [Citation27–34]. Pre-pregnancy overweight has been linked to two major causes of maternal mortality [Citation35,Citation36], i.e. hypertensive disorders of pregnancy [Citation35–39] and gestational diabetes mellitus [Citation36,Citation38], as well as an entire spectrum of adverse pregnancy outcomes [Citation27,Citation28,Citation39–44]. In order to define the categories of weight that are not normal, the WHO and the National Institutes of Health grouped weight into four categories according to individuals’ body mass index: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (30.0 kg/m2). This analysis examines the effect of pre-pregnancy underweight and overweight women on maternal, pregnancy and neonatal outcomes.
Under-weight
Impact estimates: In various studies, pre-pregnancy underweight was associated with an increased risk for preterm birth (OR 1.75; 95% CI: 1.13 to 2.71) [Citation45], (OR 1.41; 95% CI: 1.37 to 1.35) [Citation46], (OR 2.11; 95% CI: 1.03 to 4.32) [Citation47]. Pre-pregnancy underweight was also found to significantly increase the likelihood of SGA babies (OR 1.95; 95% CI: 1.52 to 2.50) [Citation48], (OR 1.90; 95% CI: 1.70 to 2.12) [Citation49], stillbirths (OR 1.39; 95% CI: 0.33 to 5.86) [Citation50] and LBW babies (OR 1.54; 95% CI: 1.04 to 2.28) [Citation51]. As expected, pre-pregnancy underweight reduced the risk of Cesarean section (OR 0.65; 95% CI: 0.44 to 0.95) [Citation52], hypertensive disorders of pregnancy (OR 0.71; 95% CI: 0.60 to 0.84) [Citation53] and gestational diabetes mellitus (GDM) (OR 0.22; 95% CI: 0.05 to 0.97) [Citation54].
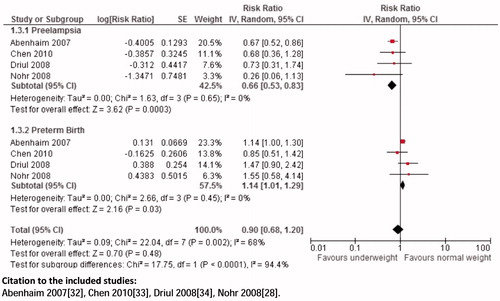
Composite analysis: The analysis showed a non-significant association of pre-pregnancy underweight and risk for preeclampsia and preterm birth (RR 0.90; 95% CI: 0.68 to 1.20) (), preeclampsia and LBW (RR 0.96; 95% CI: 0.09 to 10.35), preeclampsia and stillbirths (RR 0.52; 95% CI: 0.27 to 1.00), preeclampsia and SGA (RR 0.91; 95% CI: 0.20 to 4.17).
Key findings: The analysis did not reveal significant results of pre-pregnancy underweight on composite maternal and fetal/neonatal outcomes, although there were significant impacts on maternal and neonatal health outcomes individually. Since many studies have proven the morbid pregnancy outcomes of preconception underweight women, it is recommended that interventions for adequate nutrition be incorporated into health systems.
Overweight
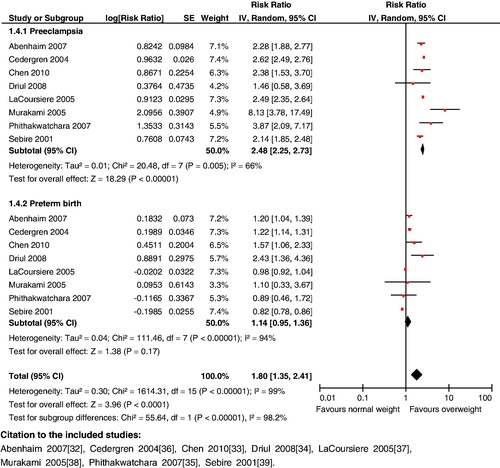
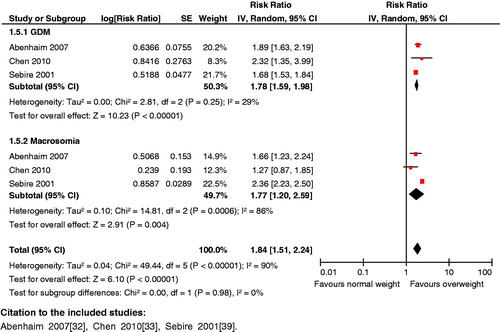
Impact analysis: Overweight women during pregnancy have been shown to have an increased risk of hypertension (OR 1.56; 95% CI: 1.35 to 1.80) [Citation53], preeclampsia (OR 2.38; 95% CI: 1.53 to 3.70) [Citation54] and gestational diabetes mellitus (OR 2.50; 95% CI: 2.10 to 2.98) [Citation49]. Overweight women are also at a higher risk for postpartum hemorrhage (OR 1.26; 95% CI: 1.03 to 1.54) [Citation53], another leading cause of maternal mortality. In terms of pregnancy outcomes, women with pre-pregnancy overweight have an increased likelihood of cesarian sections (OR 2.22; 95% CI: 1.45 to 3.40) [Citation55], preterm birth (OR 2.43; 95% CI: 1.36 to 4.36) [Citation56], stillbirths (OR 1.79; 95% CI: 1.59 to 2.02) [Citation57] and macrosomia (OR 1.66; 95% CI: 1.23 to 2.24) [Citation53].
Composite analysis: Pre-pregnancy overweight women are at a higher risk for the composite outcomes of hypertensive disorders and preterm birth (RR 1.46; 95% CI: 1.22 to 1.76), hypertensive disorders and stillbirths (RR 1.59; 95% CI: 1.15 to 2.21), preeclampsia and preterm birth (RR 1.80; 95% CI: 1.35 to 2.41) (), preeclampsia and stillbirths (RR 1.88; 95% CI: 1.44 to 2.46), gestational diabetes mellitus and macrosomia (RR 1.84; 95% CI: 1.51 to 2.24) ().
Key findings: The analysis revealed significant effects of pre-pregnancy overweight on maternal and neonatal outcomes. Maternal obesity has serious consequences for both mother and child; measures for controlling maternal weight therefore need to be prioritized.
Maternal age at conception: prevention of teenage pregnancy
Ten percent of women between the age of 14–49 years become mothers by the age of 16 in sub-Saharan Africa and south and south-east Asia. Adolescent pregnancy is dangerous for both the mother and the child, including obstructed and prolonged labor, high risk of development of vesico-vaginal fistulae, stillbirths and neonatal deaths, as well as preterm birth, low birth weight and asphyxia [Citation58].
Impact estimates: A 2012 Lancet review described various prevention policies that targeted adolescent health [Citation59]. It cited a study, which revealed that legal access to oral contraception without parental involvement was associated with an 8·5% decrease in birth rates [Citation60]. It also cited a randomized controlled trial by Campbell et al. [Citation61] which involved full-day, year-round child care given 5 d a week for 5 years (from age 0–5 years with a structured curriculum) and resulted in the intervention group mothers being less likely to become a repeat parent before age 20 years (26% versus 45%). This analysis reviewed a number of programs for preventing teenage pregnancy. One of these was implemented in multiple community centers and provided educational and vocational support, sex education, medical care, sports, arts, free STI testing and condoms [Citation62]. This program was successful in reducing the risk of teen pregnancy by 41%. Another highly successful program (risk reduction of 57%) focused on youth development through community service and personal development [Citation63].
Expanded sexual education programs delivered by adults did not show an effect in preventing adolescent pregnancy [Citation64–67], except in one study in Chile [Citation68]. Similarly, abstinence-focused education [Citation69,Citation70] and provision of free contraception alone [Citation71] did not show a significant effect in risk reduction for adolescent pregnancy. Of those studies evaluating contraceptive methods, hormonal implants were found to be extremely successful in preventing repeat teenage pregnancy, causing an 89% risk reduction [Citation72]. Contraceptive provision to adolescents might also be more successful if implemented in school-based health centers with case management provided by an onsite care provider [Citation73].
Composite analysis: No composite outcomes were generated due to the lack of studies which examined maternal and neonatal outcomes simultaneously.
Key findings: Various trials have shown that abstinence education, expanded sexual health education provided by adults and provision of contraception alone are not effective in reducing the incidence of teenage pregnancy. However, comprehensive interventions that involve program implementation in community centers, sexual and reproductive health services, contraceptive provision, and school-based education and youth development programs are highly effective in reducing the risk of teenage pregnancy.
Family planning
Both short and long inter-pregnancy intervals (IPIs) are known to be associated with adverse perinatal outcomes [Citation74,Citation75] such as preterm birth, low birth weight, SGA and perinatal death. For this review, the exposure IPI (from the end of one pregnancy to the beginning of another) was largely used, to accommodate those intervals where the preceding pregnancy may not have ended in a birth, and short (<6 months) and long (>60 months) intervals were compared to the “ideal” interval (which was usually 12–23 months).
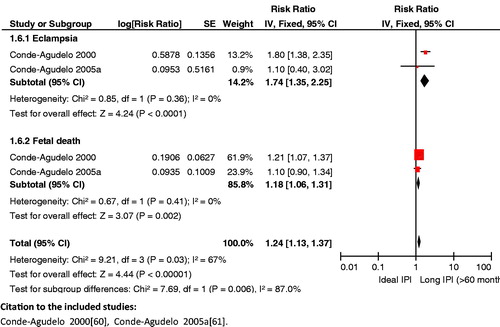
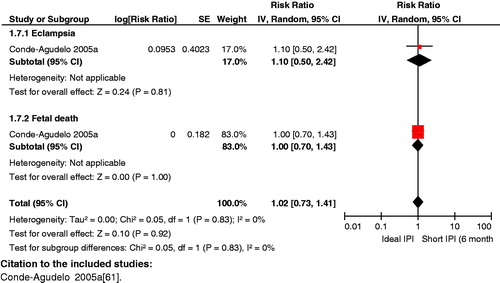
Impact analysis: Available data on birth spacing shows that short IPIs of <6 months as compared to IPIs of 18–23 months are associated with a statistically significant higher risk of low birth weight (OR 1.61; 95% CI: 1.39 to 1.86) [Citation76], preterm birth (OR 1.40; 95% CI: 1.24 to 1.58) [Citation76], and SGA infants (OR 1.26; 95% CI: 1.18 to 1.33) [Citation76]. As compared to IPI of <18 months, an IPI of 18–23 months was associated with a decreased risk of perinatal mortality (AOR 0.45; 95% CI: 0.20 to 0.98) [Citation77]. As far as maternal outcomes are concerned, it was observed that compared to an IPI of 18–23 months, an IPI of <6 months was also associated with significantly increased risk of maternal death (OR 2.54; 95% CI: 1.22 to 5.38) [Citation76], maternal anemia (OR 1.30; 95% CI: 1.18 to 1.43) [Citation76], and other obstetric morbidities including premature rupture of membranes (1.72; 95% CI: 1.53 to 1.93) [Citation76], puerperal endometritis (OR 1.33; 95% CI: 1.22 to 1.45) [Citation76] and third trimester bleeding (OR 1.73; 95% CI: 1.42 to 2.24) [Citation76].
Composite analysis: The analysis revealed some significant results for long IPIs, which included an increased risk for eclampsia and fetal death (RR 1.24; 95% CI: 1.13 to 1.37) () and for third trimester bleeding and fetal death (RR 1.13; 95% CI: 1.06 to 1.30) (). However, the impact of long IPIs on risk for maternal anemia and fetal death (RR 1.03; 95% CI: 0.99 to 1.06) failed to reach statistical significance for these composite outcomes. All the results regarding short IPIs failed to reach statistical significance.
Key findings: An IPI of less than 6 months is associated with adverse maternal outcomes (e.g. increased risk of anemia, obstetric morbidities and death) and poor fetal/neonatal outcomes (e.g. increased risk of preterm births, low birth weight and SGA infants). Ensuring adequate birth spacing (18–23 months) has also been seen to reduce the risk of perinatal mortality. An IPI of 18–23 months is recommended to decrease maternal and neonatal morbidity and mortality.
Pre-conception counseling
This analysis reviews counseling interventions aimed at women during the preconception and antenatal periods.
Impact estimates: Women exposed to preconception counseling were more likely to quit smoking before pregnancy (OR 2.94; 95% CI: 0.70 to 8.84) [Citation78], initiate breast feeding early (RR 1.49; 95% CI: 1.22 to 1.82) [Citation79] or use folic acid (RR 1.57; 95% CI: 1.10 to 2.24) [Citation80]. Women exposed to information on pre-conception health during routine family planning visits had a 51.8% (p = 0.06) greater likelihood of identifying their pregnancies as intended [Citation81]. This intervention was also associated with lower rates of ectopic pregnancy (0.2% compared to 0.8% in general population) [Citation82]. In a review by Bhutta et al. [Citation79], quarterly group education sessions facilitated by lady health workers and community health committees, including promotion of ANC and maternal health education, were associated with a reduction in stillbirth rate (RR 0.79; 95% CI: 0.68 to 0.92), neonatal mortality rate (RR 0.85; 95% CI: 0.76 to 0.96) and perinatal mortality rate (RR 0.83; 95% CI: 0.74 to 0.93).
Composite analysis: An analysis was not done as most studies targeted interventions to women, not only before conception but also before birth.
Key findings: Women who receive pre-conception counseling are more likely to change their behaviors and initiate breastfeeding early than women who did not receive counseling. Preconception counseling allows women to identify and reduce possible risk factors for poor maternal and newborn health outcomes before pregnancy.
Diabetes
Diabetes in pregnancy is associated with elevated rates of miscarriage [Citation83], pre-eclampsia [Citation84,Citation85], preterm labor, cesarean sections [Citation86,Citation87] and fetal malformation [Citation86,Citation88–90]. Optimal glycemic control during pregnancy may reduce these diabetes-related risks, but a more effective time to intervene could be before pregnancy. A number of systematic reviews on the effectiveness of preconception care in diabetic women show positive trends in maternal and newborn health outcomes [Citation86,Citation91,Citation92].
Impact analysis: Various studies report the advantages of preconception care for pre-gestational diabetes on maternal outcomes. A study by Tripathi et al. [Citation93] showed that diabetic women with preconception care were more likely to have better glycemic control 3 months preconception (OR 1.91, 95% CI 1.10–3.04) and in the first trimester (OR 2.05; 95% CI: 1.39 to 3.03). The study by Temple et al. [Citation94] showed that the risk of preterm births decreased in diabetic women receiving pre-pregnancy care (RR 0.64; 95% CI: 0.47 to 0.88). However, another study by the same author reported no difference in rates of pre-eclampsia (13.1 versus 12.6%) [Citation95] between the women who did and those who did not attend pre-pregnancy care. Another study reported a reduced risk of cesarean section in diabetic women receiving this intervention (RR 0.73; 95% CI: 0.56 to 0.95) [Citation96]. Preconception care for diabetic mothers was able to significantly reduce the occurrence of congenital malformations (RR 0.19 95% CI: 0.04 to 0.81) [Citation97] and perinatal mortality (RR 0.28; 95% CI: 0.08 to 0.94) [Citation97].
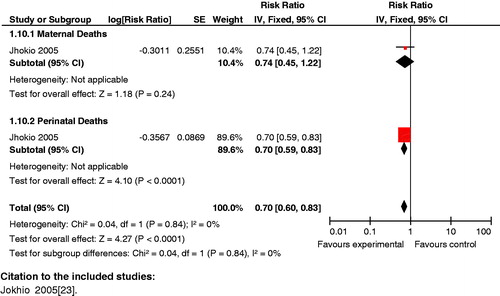
Composite analysis: The analysis showed that preconception care versus non-preconception care for diabetes significantly reduced the risk for HbA1C>7.8% for mothers in the first trimester and perinatal mortality (RR 0.14 95% CI: 0.05 to 0.41) (). However, the result for this intervention failed to reach significance for reduced risk for HbA1C>7.8% for mothers in the first trimester and congenital malformations in the neonate (RR 0.32 95% CI: 0.08 to 1.37).
Figure 12. Preconception care versus non-preconception care – HbA1C>7.8% in first trimester and perinatal mortality.
Key findings: Counseling of diabetic women should begin at the time of preconception rather than during pregnancy.
Infections: sexually transmitted infections
Sexually transmitted infections (STIs) during pregnancy are associated with adverse pregnancy outcomes – ranging from early abortion, stillbirth and premature births to congenital infections and infant death [Citation98,Citation99]. The opportune time to identify and address risk factors for poor reproductive health outcomes for women and babies is before conception [Citation100]. The literature was reviewed for the effects of gynecologic infections in women in the preconception period on maternal and newborn health outcomes, and to identify interventions which reduce these infections and any associated morbidity and mortality. Due to a shortage of preconception data being available, studies done among the general population were also included.
Impact estimates: In review of intervention treatment programs, Marion et al. [Citation101] stated that the estimated probability of a woman who was part of an STI management intervention program having an STI at month 15 after program initiation was 20% less than that for a woman who was not part of such a program. Mass treatment with antibiotics significantly dropped the rates of syphilis (OR 0.83; 95% CI: 0.73 to 0.96) [Citation102], gonorrhea (OR 0.43; 95% CI: 0.25 to 0.74) [Citation103], trichomoniasis (OR 0.64; 95% CI: 0.54 to 0.76) [Citation102] and bacterial vaginosis (OR 0.87; 95% CI: 0.83 to 0.91) [Citation102].
In reviewing behavioral interventions, Jemmott et al. [Citation104] reported significant 12-month STI reduction (12%) with intensive group skills counseling. Interventions concentrating on behavioral modifications for STIs promoted overall safer practices in the subjects, especially in terms of increase in condom use (OR 5.50; 95% CI: 2.80 to 10.80) [Citation105].
Composite analysis: No composite outcomes were generated due to the lack of studies which examined maternal and neonatal outcomes simultaneously.
Key findings: Mass treatment of STIs with antibiotics and behavioral/counseling interventions leads to a reduction in STI prevalence and incidence, respectively. Interventions targeting STIs also lead to a significant increase in safer sexual behaviors, especially condom use.
HIV/AIDS prevention strategies
Babies born with HIV are more likely to develop AIDS and have serious complications. Additionally, HIV-positive women are more likely to terminate their pregnancies, give birth to low birth weight babies, deliver preterm or experience stillbirths [Citation106–109]. For the purpose of this review, both therapeutic and preventive preconception measures to reduce HIV infection were examined.
Impact estimates: In reviewing preventive measures, Karim et al. [Citation110] showed that microbicides used by women significantly reduced the risk of HIV infection (RR 0.63; 95% CI: 0.42 to 0.92). However, various other studies reported no significant risk reduction for HIV with the use of microbicides [Citation111–112]. Condom use has been shown to be the most effective way to prevent HIV infection through sexual intercourse (RR 0.37; 95% CI: 0.15 to 0.91) [Citation113], (RR 0.13; 95% CI: 0.07 to 0.24) [Citation114]. Denison et al. [Citation115] stated that voluntary counseling and testing (VCT) recipients were significantly less likely to engage in unprotected sex when compared to behaviors before receiving VCT, or as compared to participants who had not received VCT (OR 1.69; 95% CI: 1.25 to 2.31). As far as therapeutic interventions were concerned, a study by Fang et al. [Citation116] where free Highly Active Anti-retroviral therapy (HAART) as provided to all HIV positive women showed that HIV transmission rate from mother to child decreased by 53% (95% CI: 31 to 65%).
Composite analysis: No composite outcomes were generated due to the lack of studies that looked into maternal and neonatal outcomes simultaneously.
Key findings: Therapeutic interventions such as antiretroviral therapy are successful in preventing transmission to partners and newborns. Ongoing trials may provide urgently needed evidence as to whether prophylactic or therapeutic use of antiretroviral drugs is safe and effective in women of reproductive age, and improves maternal and newborn health outcomes. Among preventive measures, condom and microbicide use reduced the risk of HIV transmission to the partner. Microbicides have been shown to prevent HIV infection in women; a number of clinical trials are underway to test the efficacy of a different ARV-based microbicides in preventing HIV. Voluntary counseling and testing has also been shown to lower the likelihood of having unprotected intercourse.
Maternal mental health
Evidence suggests that depression and anxiety during pregnancy and postpartum can severely impact family life, the mother–infant relationship and the future mental health of the child [Citation117–120]. Maternal antenatal depression generally has been highly correlated with preterm deliveries [Citation121].
Impact estimates: There is a serious lack of evidence of how pre-pregnancy mental ill-health and psychotropic drug use may affect pregnancy. Pre-pregnancy depression is significantly related to preterm births (OR 1.04; 95% CI: 1.02 to 1.07) [Citation122], and adolescent depression per se was significantly associated with an increased risk of miscarriages (AOR 2.25; 95% CI: 1.12 to 4.50) [Citation123]. In a study by Seth et al. [Citation124], adolescents with high levels of psychological distress were reported to have high-risk behavior, relative to those with low levels of psychological distress. They were more likely to have a biologically confirmed STI (adjusted odd ratio (AOR) 1.40, 95% CI: 1.01 to 1.94), to use condoms inconsistently in the past 2 months (AOR 1.50; 95% CI: 1.02 to 2.21), not use condoms during their last casual sexual encounter (AOR 1.89; 95% CI: 1.08 to 3.30) and have sex while high on alcohol or drugs in the past 2 months (AOR 1.47; 95% CI 1.07 to 2.02). When assessing for maternal morbidity, adolescent depression was positively associated with suffering from intimate partner violence (IPV) (AOR 3.47; 95% CI: 1.11 to 10.84) but not STIs (AOR 1.50; 95% CI: 0.83 to 2.72) [Citation123]. Silverman et al. [Citation125] concluded that a pre-existing psychiatric condition was one of the best predictors of the development of postpartum depression.
Key findings: Depression in adolescents significantly increases the risk of miscarriages, preterm births, IPV and high-risk behavior. The literature indicates that mental state at preconception is a good indicator of the development of postpartum depression.
Interventions during the antenatal period
ANC plays an important role in reducing maternal and newborn morbidity and mortality by promoting healthy behaviors and addressing a woman’s health care needs such as diet, exercise and rest. ANC also includes interventions for the prevention and treatment of human immunodeficiency virus (HIV) infection and other STIs, and chronic diseases during pregnancy. identifies the interventions evaluated in this paper, their reported impact on composite maternal and fetal/neonatal outcomes, and the Interconnection groups (A, B, C or D) to which they have been assigned.
Table 4. Antenatal Period: Individual interventions with Composite maternal/fetal/neonatal impact estimates and their assigned GRADE.
Nutritional interventions
Iron–folic acid supplementation
During pregnancy, women with iron-deficiency anemia have inadequate weight gain, a weakened immune system, heavy placenta (weighing more than 700 grams) and are more likely to have a preterm or low birth weight infant. [Citation126–129].
Impact estimates: A review by Yakoob et al. [Citation130] found that iron supplementation and iron/folate supplementation during pregnancy reduces the risk of iron deficiency anemia at term (RR 0.33; 95% CI: 0.16 to 0.69), and anemia at term (RR 0.27; 95% CI: 0.12 to 0.56), respectively. A review by Pena-Rosas et al. [Citation131] reported in cases where women were provided with iron supplementation during the antenatal period, there was no impact on the prevention of low birth weight (<2500 g) (RR 0.79; 95% CI: 0.61 to 1.03), very LBW (<1500 g) (RR 0.73; 95% CI: 0.31 to 1.74) or premature delivery (<37 weeks) (RR 0.85; 95% CI: 0.67 to 1.09).
Composite analysis: Results from composite analyses reveal that the use of iron–folic acid supplementation during pregnancy has a significant impact on anemia at term and low birth weight (RR 0.52; 95% CI: 0.31 to 0.88) (), and on anemia at term and preterm delivery (RR 0.55; 95% CI: 0.38 to 0.80). The intervention also displayed a significant impact on the composite outcome of maternal anemia at term and very premature delivery (RR 0.49; 95% CI: 0.32 to 0.75).
Figure 13. Iron supplementation versus none/placebo – anemia at term (Hb<110 g/l) and low birth weight.
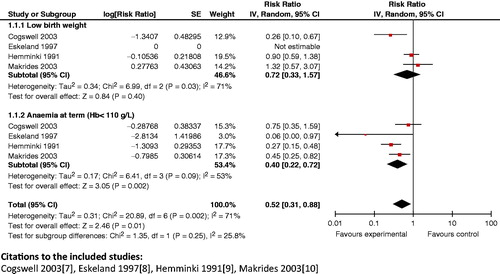
Key finding: Routine supplementation of iron–folic acid during pregnancy should be recommended.
Multiple micronutrient supplementation
Women of reproductive age, especially pregnant women, in developing countries are at risk of multiple micronutrient deficiencies, such as iron, folic acid, iodine, zinc, vitamins A and D, riboflavin, B6 and B12, with the likelihood of adverse effects on the mother and pregnancy outcomes [Citation132,Citation133].
Impact estimates: A review by Haider et al. [Citation134] showed that multiple micronutrient supplementation during pregnancy had no significant effect on maternal anemia in the third trimester compared to iron–folate (RR 1.03; 95% CI: 0.87 to 1.22). However, the same review showed a significant reduction in the risk of SGA infants with the use of multiple micronutrient supplements (RR 0.91; 95% CI: 0.86 to 0.96) compared to iron–folate supplementation [Citation134]. The impact of this intervention in another review by Imdad et al., showed no impact on stillbirths (RR 0.98; 95% CI: 0.88 to 1.10) [Citation135] or on perinatal mortality (RR 1.07; 95% CI: 0.92 to 1.25) [Citation135]. There was also no evident reduction in the risk of neonatal mortality (RR 1.05; 95% CI: 0.92 to 1.19) [Citation134].
Composite analysis: No composite outcomes were generated due to the lack of studies that looked into maternal and neonatal outcomes simultaneously.
Key findings: Studies show that multiple micronutrient supplementation in pregnancy is associated with significant reductions in SGA births. No effects on perinatal or neonatal mortality were found to be significant. Therefore, more research is required to study how maternal micronutrient supplementation can help improve both maternal and newborn health outcomes.
Balanced protein energy supplementation during pregnancy
Balanced protein/energy supplementation provides nutritional support for women whose protein intake is less than 25% of the total daily energy consumption. Women targeted for this intervention are those with a body mass index <18.5.
Impact estimates: A review by Imdad et al. [Citation136] showed a significant reduction in stillbirths (RR 0.55; 95% CI: 0.31 to 0.97) and SGA babies (RR 0.69; 95% CI: 0.56 to 0.85) with the use of balanced protein/energy supplementation during pregnancy. However, its impact on neonatal deaths failed to reach significance (RR 0.63; 95% CI: 0.37 to 1.06). A Cochrane review by Kramer et al. [Citation137] also had similar findings, highlighting that balanced energy/protein supplementation was associated with a reduction in the risk of SGA babies (RR 0.69; 95% CI: 0.56 to 0.85) and a lowered risk of stillbirths (RR 0.55; 95% CI: 0.31 to 0.97). The review also reported that the maternal group which received balanced protein supplements gave birth to babies with a higher mean birth weight as compared to the control group (Mean difference (MD) 37.62; 95% CI: −0.21 to 75.45) [Citation137]. This effect was more pronounced in malnourished women compared to adequately nourished women.
Composite analysis: No composite outcomes were generated due to the lack of studies that looked into maternal and neonatal outcomes simultaneously.
Key findings: More research is required to study how balanced protein/energy supplementation during pregnancy can help improve both maternal and newborn health outcomes.
ANC during pregnancy and four-visit focused ANC model
The aim of ANC is to detect or prevent and manage conditions that may adversely affect the health of the mother and/or fetus/newborn.
Impact analysis: A Cochrane review by Dowswell et al. [Citation138] showed a significant increase in the number of maternal deaths (RR 1.13; 95% CI: 0.5 to 2.57) with reduced number of antenatal visits as compared to standard ANC visits. However, availability of ANC services leads to an increase in the detection of anemia (from 26.3% pre-intervention to 41.3% post-intervention, p = 0.001) [Citation139] and pregnancy-induced hypertension (PIH) (11% in women attending one of the care programs versus 5.1% women not attending the program, P 0.03) [Citation139]. A study by Humphrey et al. [Citation140] also reported an increased risk of perinatal mortality (OR 6.30; 95% CI: 3.72 to 10.69) without ANC.
Composite analysis: No composite analysis was done for this intervention due to the lack of studies reporting impact estimates for both maternal and fetal/neonatal outcomes.
Key findings: ANC packages can help reduce maternal mortality and morbidities (e.g. those resulting from postpartum hemorrhage and anemia), and increase detection of pregnancy complications (e.g. pregnancy-induced hypertension (PIH)).
Newborn care preparedness: community-based intervention packages
To support the basic primary health care infrastructure, a range of community-based intervention packages have been introduced to train people in the community (e.g. community health workers (CHWs)) with background medical knowledge. These intervention packages deliver preconception, antenatal and postnatal care. Only studies which implemented packages of health interventions (packages included multiple interventions such as building community support groups and provision of home-based ANC, childbirth and postnatal services by CHWs) were considered eligible for inclusion. The interventions and packages of interventions reviewed included breastfeeding promotion, iron–folic acid distribution, among others, but in all cases the ultimate goal is to improve maternal, perinatal, and neonatal mortality and morbidity.
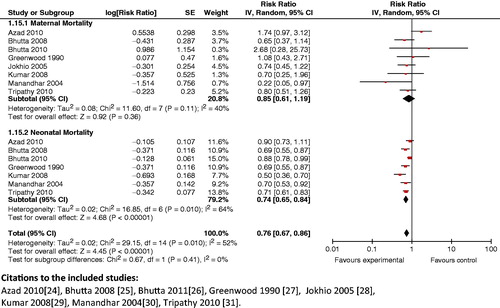
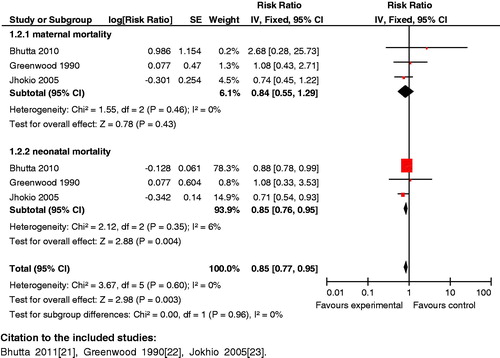
Impact analysis: The available data suggests that introduction of community-based intervention packages reduce maternal mortality (Kidney 2009: OR 0.62; 95% CI: 0.39 to 0.98) [Citation141,Citation142]. It was also seen that these packages improved breastfeeding practices (RR 0.81; 95% CI: 0.74 to 0.89) [Citation143] and other health care practices in pregnancy, such as increased rates of iron–folic acid supplementation in pregnant women from 32 to 76% (p < 0.001) [Citation144]. Use of community-based intervention packages also resulted in a significant decrease in the incidence of stillbirths (RR 0.84; 95% CI: 0.74 to 0.97) [Citation142], neonatal mortality (RR 0.76; 95% CI: 0.68 to 0.84) [Citation142] and perinatal mortality (RR 0.80; 95% CI: 0.71 to 0.91) [Citation142].
Composite analysis: The analysis showed a significant (RR 0.76; 95% CI: 0.67 to 0.86) () reduction in maternal and neonatal mortality with these packages. It also showed that providing access to community support and advocacy groups as part of packages markedly improved (RR 0.76; 95% CI: 0.66 to 0.88) maternal and neonatal mortality (). The composite impact of this intervention on health-seeking behavior for maternal and neonatal morbidity was also assessed; it revealed that health care seeking significantly improved (RR 1.99; 95% CI: 1.45 to 2.73).
Figure 15. Access to community support and advocacy groups in intervention package versus no package outcome: composite maternal and neonatal mortality.
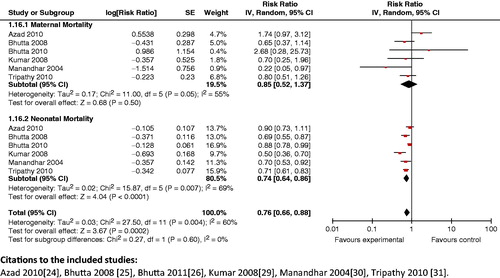
Key findings: The introduction of community-based intervention packages has been shown to reduce maternal and neonatal mortality, and improves breastfeeding and health-seeking practices. The results from this analysis support community-based intervention packages for a more holistic and self-sustaining approach to improving maternal and newborn health.
Management of STIs in pregnancy
STIs, other than HIV, are not adequately addressed by societies, government and health workers even though they have significant health impact on women and newborns [Citation145].
Syphilis
The WHO estimates that there are at least 12 million newly diagnosed cases of syphilis globally each year [Citation146]. Maternal syphilis causes adverse health outcomes in newborns.
Impact estimates: A recent review by Blencowe et al. [Citation147] reported that treatment with penicillin was associated with an 82% reduction in stillbirth (95% CI: 67 to 90%), a 64% reduction in preterm delivery (95% CI: 53 to 73%) and an 80% reduction in neonatal deaths (95% CI: 68 to 87%) There is also a strong trend towards reduced risk of pregnancy loss among women receiving multiple doses of penicillin for the treatment of syphilis (RR 0.63; 95% CI: 0.48 to 0.84) [Citation148]. Walker et al. [Citation149] reported that treating syphilis (complete or incomplete treatment with three penicillin doses) as a part of ANC reduced perinatal mortality by 63% (RR 0.37; 95% CI: 0.18 to 0.76).
Composite analysis: No composite analysis was done for this intervention due to the lack of studies providing impact estimates on both maternal and neonatal morbidity and mortality outcomes.
Key findings: The prevention and management of syphilis is a key intervention for preventing adverse outcomes in the mother, fetus and newborn. There is a dearth of studies that report both maternal and neonatal outcomes; additional research can help fill these gaps.
Gonorrhea
Impact analysis: A review by Brocklehurst et al. [Citation150] examined the use of penicillin for the treatment of gonorrhea versus any other antibiotic used; it was associated with a non-significant increased risk of failure to achieve microbiological cure (OR 2.49; 95% CI: 0.88 to 7.02). Other comparisons in the same review were amoxicillin and probenicid versus spectinomycin (OR 2.29; 95% CI: 0.74 to 7.08) and versus ceftriaxone (OR 2.29; 95% CI: 0.74 to 7.08), respectively, for the treatment of gonorrhea; these also did not reach significance when reporting the failure of microbiological cure in the mother.
Composite analysis: No composite analysis was done for this intervention due to the lack of studies providing impact estimates on both maternal and neonatal morbidity and mortality outcomes.
Chlamydia
Impact analysis: A review by Brocklehurst et al. [Citation151] showed that azithromycin is more effective than erythromycin for the treatment of chlamydial infection (Failure to achieve microbiological cure: RR 0.38; 95% CI: 0.19 to 0.74). No significant impact was found on neonatal mortality for any antibiotic therapy versus placebo for chlamydia infection (RR 7.43; 95% CI: 0.15 to 374.24).
Composite analysis: No composite analysis was done for this intervention due to the lack of studies providing impact estimates on both maternal and neonatal morbidity and mortality outcomes.
Trichomoniasis
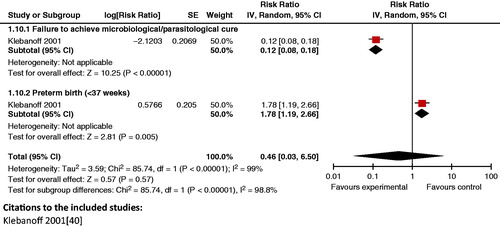
Impact analysis: For the treatment of trichomoniasis the review by Gülmezoglu et al. [Citation152] reported that metronidazole improved cure rates by 89% (RR 0.11; 95% CI: 0.08 to 0.17) during pregnancy. However, the treatment had no impact on neonatal outcomes such as preterm birth (<37 weeks) (RR 1.78; 95% CI: 1.19 to 2.66) and LBW (<2500g) (RR 1.38; 95% CI: 0.92 to 2.06).
Composite analysis: Treatment of trichomoniasis in pregnancy with metronidazole yielded non-significant impact on the composite outcome of failure to achieve parasitological/microbiological cure in the mother and neonatal morbidities, such as preterm birth (RR 0.46; 95% CI: 0.03 to 6.50) ().
Bacterial vaginosis
Impact analysis: A review by McDonald et al. [Citation153] suggests that antibiotic treatment given to women with bacterial vaginosis in pregnancy was associated with reduced failure of cure (OR 0.17; 95% CI: 0.15 to 0.20). There was no significant decrease in the risk of preterm birth (<37 weeks gestation) for any treatment versus no treatment or placebo (OR 0.91; 95% CI: 0.78 to 1.06).
Composite analysis: Treatment of bacterial vaginosis in pregnancy yielded a significant reduction (60%) on the composite outcome of failure of parasitological/microbiological cure in the mother and perinatal mortality (RR 0.40; 95% CI: 0.22 to 0.71) ().
Figure 17. Treatment of bacterial vaginosis in pregnancy – failure of microbiological cure and perinatal mortality.
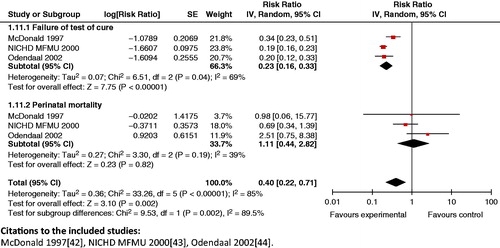
Key findings: Azithromycin was more effective than erythromycin for achieving microbiological cure. Treatment of bacterial vaginosis in pregnancy yielded an impact on the composite outcome of failure to achieve parasitological/microbiological cure in the mother and on perinatal mortality.
Prevention and management of HIV in pregnancy
The majority of HIV-infected children acquire the virus through mother-to-child transmission (MTCT), either during the intrapartum period or postnatally through breastfeeding. Antiretroviral therapy can reduce the risk of mother to child transmission of HIV. While studies show that nevirapine can decrease the risk of transmission of HIV-1 from mother to infant [Citation154,Citation155], the WHO Guidelines [Citation157] no longer recommend short course regimens of nevirapine due to evidence of increased resistance among pregnant women.
Antiretroviral therapy including HAART
In developed countries, HAART therapy has reduced vertical transmission from 1–2%; however, in developing countries HAART therapy is not widely available; instead, low-cost antiretroviral regimens are offered to pregnant women and their children to decrease transmission.
Impact analysis: A recent review by Siegfried et al. [Citation154] showed that the rates of stillbirths were reduced with the use of 3 Regimens of different antiretrovirals. The results from six studies were not pooled and RR ranged from 1.48 (95% CI: 0.25 to 8.58) to 2.99 (95% CI: 0.12 to 73.33). Volmink et al. [Citation155] stated that short courses of antiretroviral drugs are effective in reducing vertical transmission of HIV by 54% (RR 0.46; 95% CI: 0.35 to 0.60). In a randomized control trial (RCT), maternal HAART and daily infant Nevirapine for 28 weeks were effective in reducing HIV transmission during breastfeeding [Citation156].
According to the WHO 2010 guidelines [Citation157], lifelong ART for HIV-infected women in need of treatment is recommended for their own health, and is also safe and effective in reducing MTCT. For HIV-infected pregnant women who are not in need of ART for their own health, effective ARV prophylaxis is recommended to prevent HIV infection in their infants. In such women ARV prophylaxis should be started from as early as 14 weeks of gestation (second trimester) or as soon as feasible during pregnancy, labor, delivery, breastfeeding or thereafter. Two options are recommended for HIV-infected pregnant women who are not eligible for ART: option A is maternal AZT + infant ARV prophylaxis; option B is maternal triple ARV prophylaxis.
Composite analysis: The use of zidovudine for reducing MTCT in pregnancy did not yield significant results for the composite outcome for maternal and neonatal mortality (RR 1.44; 95% CI: 0.54 to 3.86).
Key findings: The evidence brought forth by this review revealed that courses of antiretroviral drugs reduced stillbirths as well as vertical transmission of HIV. To prevent MTCT in women who do not require ART for their own health, it is recommended that either maternal AZT + infant ARV prophylaxis or maternal triple ARV prophylaxis should be started by 14 weeks of gestation and continued during labor, delivery and breastfeeding.
Prevention of malaria in pregnancy
Malaria during pregnancy has adverse effects on the health of the mother and baby, including maternal anemia and low birth weight [Citation158]. Prevention of malaria during pregnancy, therefore, is an important strategy in endemic areas.
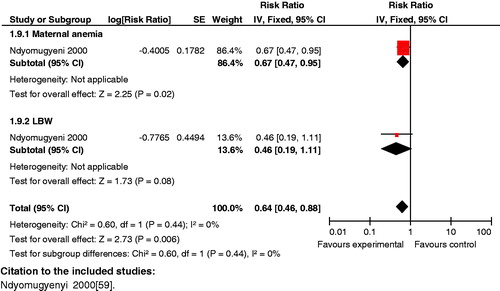
Impact analysis: Reviews show that administration of routine antimalarial drugs, such as prophylaxis, reduced placental parasitaemia [(RR 0.53; 95% CI: 0.33 to 0.86), (RR 0.48; 95% CI: 0.35 to 0.68) [Citation159,Citation160]] and maternal anemia (RR 0.90; 95% CI: 0.81 to 0.99) [Citation160] in women living in malarial endemic areas, especially when given to primigravidas and secondigravidas. When given during first and second pregnancy it also causes significant reduction in the risk of having low birth weight infants (RR 0.71; 95% CI: 0.55 to 0.92), (RR 0.11; 95% CI: 0.07 to 0.17) [Citation160,Citation161]. A similar decrease in the incidence of low birth weight was also noted by Eisele et al. (RR 0.65; 95% CI: 0.55 to 0.77) [Citation162]. The evidence regarding the effect of chemoprophylaxis on maternal mortality (RR 0.34; 95% CI: 0.04 to 3.27) [Citation159] and neonatal mortality (RR 1.02; 95% CI: 0.73 to 1.43) [Citation159] failed to reach significance.
Composite analysis: The results of antimalarial drug prevention for women in the first or second pregnancy failed to improve composite outcome of maternal and perinatal death (RR 0.70; 95% CI: 0.45 to 1.09). A significant composite outcome was generated for maternal anemia and low birth weight when the intervention was given to women in their first or second pregnancy (RR 0.64; 95% CI: 0.46 to 0.88), and for severe maternal anemia and low birth weight (RR 0.56; 95% CI 0.42 to 0.74) (), raising the possibility of its synergistic benefit on mother and neonate.
Figure 18. Antimalarial drug prevention versus none (women in 1st or 2nd pregnancy) outcome: severe maternal anemia and low birth weight.
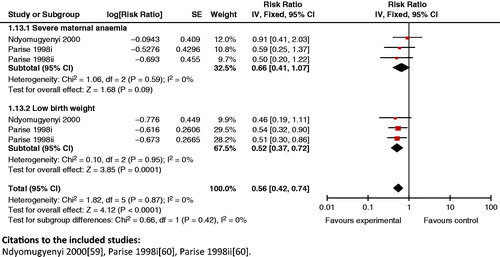
Key findings: These findings indicate that use of anti-malarial medications in women in the first or second pregnancy can have a composite impact on reducing maternal anemia and low birth weight, and severe anemia and low birth weight. However, the analysis showed no significant impact on combined maternal and neonatal/fetal mortality, highlighting the need for additional research to generate impact estimates.
Use of insecticide-treated nets in pregnancy
An insecticide-treated bed net (ITN) is a mosquito net that repels, disables, and/or kills mosquitoes coming into contact with insecticide on the netting material. Use of such nets is mostly applicable to areas where malaria is endemic.
Impact estimates: A study by Ter Kuile et al. [Citation163] showed that ITNs reduced the risk of maternal anemia (RR 0.80; 95% CI: 0.48 to1.33) [Citation163] and clinical illness (RR 0.72; 95% CI: 0.19 to 2.75) [Citation163]. It was seen in the review by Gamble et al. [Citation164] that the use of ITNs is very effective in reducing perinatal morbidity (reduction in LBW) (RR 0.77; 95% CI: 0.61 to 0.98) [Citation164] and mortality (RR 0.67; 95% CI: 0.47 to 0.97) [Citation164]. This intervention also improved the mean birth weight (increase by 55 g (95% CI: 21 to 88) [Citation164].
Composite analysis: The analyses showed a significant composite impact of ITNs on maternal anemia and low birth weight (RR 0.64; 95% CI: 0.46 to 0.88) (). However, the composite effect of ITNs on maternal malarial illness and fetal loss showed no impact (RR 0.84; 95% CI: 0.63 to 1.11).
Key findings: Use of ITNs results in a composite reduction of maternal anemia and LBW in the newborn. More targeted efforts are needed to increase coverage of ITN, and improve knowledge and practice regarding its use in pregnancy.
Management of complications during pregnancy
Management of diabetes
Pre-gestational diabetes mellitus is a risk factor for congenital malformations, hypertension, preeclampsia, macrosomia and intrauterine fetal death [Citation165]. Gestational diabetes and impaired glucose tolerance, like pre-gestational diabetes, are also commonly associated with adverse perinatal outcomes [Citation166].
Impact estimates:
Screening of diabetes in pregnancy: Recent data elucidates that risk factor screening versus routine screening for gestational diabetes mellitus (GDM) reduced the risk of an early delivery (MD −0.15; 95% CI: −0.27 to −0.53) [Citation167]. In a study by Hedderson et al. [Citation168], a rate of weight gain from 0.27 to 0.40 kg/week and 0.41 or more, was associated with an increased risk of developing GDM (OR 1.43; 95% CI: 0.96 to 2.14) and (OR 1.74; 95% CI: 1.16 to 2.60), respectively.
Treatment of diabetes in pregnancy: A review by Alwan et al. [Citation169] showed a reduction in maternal and perinatal morbidity with specific treatment, such as dietary advice and insulin for mild GDM compared to routine care. The risk for developing preeclampsia was also reduced (RR 0.65, 95% CI: 0.48 to 0.88) [Citation169] when any treatment is utilized versus none. Treatment with oral hypoglycaemic drugs versus insulin therapy reduces the risk of cesarean section (RR 0.46; 95% CI: 0.27 to 0.77) [Citation169]. Syed et al. [Citation170] showed that there was a non-significant reduction in the risk of stillbirths (RR 0.20; 95% CI: 0.03 to 1.10) when intensive management protocols were utilized as opposed to conventional management. Optimum control versus sub-optimum control of diabetes significantly reduced perinatal mortality (RR 0.4; 95% CI: 0.25 to 0.63) [Citation170].
Composite analysis: The analysis showed that a lower fasting glucose level had a synergistic positive impact on composite outcomes of GDM and large for gestational age/macrosomia ( and ).
Figure 20. Fasting glucose <95 mg/dl versus fasting plasma glucose ≥95 mg/dl – GDM and LGA/macrosomia.
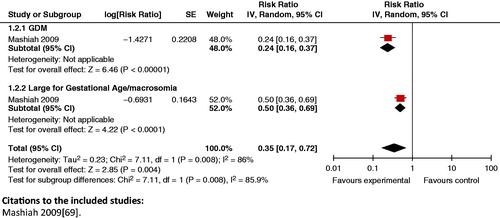
Figure 21. Fasting glucose <100 mg/dl versus fasting glucose ≥100 mg/dl in first trimester – GDM and LGA/macrosomia.
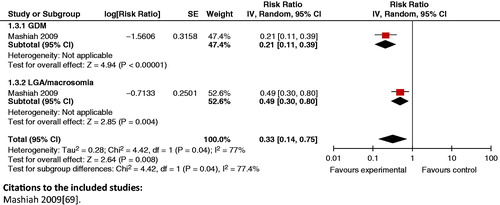
When comparing specific dietary advice versus routine ANC, no significant impact was seen on the risk of cesarian section and birth weight >90th percentile (RR 0.92, 95% CI: 0.55 to 1.53). A packaged treatment of GDM (dietary advice, glucose monitoring and insulin), described in the review by Crowther et al. [Citation171], had a significant positive impact on composite analyses of antenatal preeclampsia and neonatal convulsions (RR 0.70; 95% CI: 0.51 to 0.94) and cesarean section and birth weight >4000g (RR 0.64; 95% CI: 0.44 to 0.94).
Key finding: A packaged treatment consisting of dietary advice, glucose monitoring and insulin should be used in the management of gestational diabetes mellitus, especially during the first trimester. There is currently not enough evidence to assess the impact of different types/regimens of intensive treatment (such as oral hypoglycemic drugs and insulin) on individual and short- and long-term infant outcomes.
Recognition and prevention of PIH
PIH results in maternal morbidities (such as proteinuria, preeclampsia/eclampsia, severe hypertension, HELLP syndrome), neonatal morbidities (including preterm birth, low birth weight, and birth asphyxia) and stillbirth.
Calcium supplementation for hypertensive disorders in pregnant women with low/inadequate calcium intake
Studies have shown that women with a high calcium intake in their diet have a lower prevalence of preeclampsia and eclampsia [Citation172,Citation173].
Impact estimates: A review by Imdad et al. [Citation174] showed a significant reduction in preeclampsia (RR 0.41; 95% CI: 0.24 to 0.69) and gestational hypertension (RR 0.55; 95% CI: 0.36 to 0.85) in women of developing countries who were given calcium supplements during pregnancy. However, it failed to show any impact on the risk for low birth weight in developing countries (RR 0.81; 95% CI: 0.58 to 1.12). Hofmeyr et al. [Citation175], on the other hand, reported a reduction in rates of preeclampsia in women with low calcium intake (RR 0.36; 95% CI: 0.20 to 0.65). The same review also illustrated a decreased risk of having preterm births (RR 0.76; 95% CI: 0.60 to 0.97) for women who were given calcium supplementation. However, the effects on low birth weight births (RR 0.85; 95% CI: 0.72 to 1.01) and stillbirths (RR 0.90; 95% CI: 0.74 to 1.09) failed to reach significance.
Composite analysis: The use of calcium supplementation in women with low/inadequate calcium intake significantly lowers the composite outcome of the rates of preeclampsia and preterm birth (RR 0.85; 95% CI 0.77 to 0.93) (). It also reduces the risk of serious maternal and perinatal mortality (RR 0.83; 95% CI: 0.72 to 0.95).
Figure 22. Calcium supplementation in low calcium diet versus placebo – preeclampsia and preterm birth.
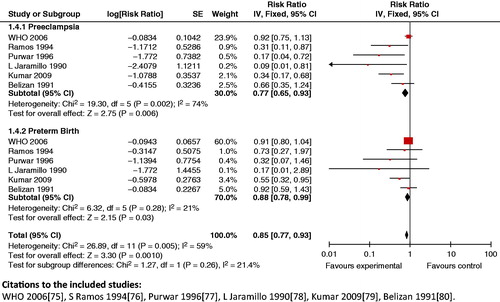
Key findings: Calcium supplementation in women with low calcium intake has been shown to reduce the composite risk of preeclampsia and preterm birth. It also significantly decreased composite maternal and perinatal mortality.
Antiplatelet agents in high-risk pregnancy
Preeclampsia leads to deficiency of prostacyclin, a vasodilator and produces excessive amounts of thromboxane, which acts by stimulating platelet aggregation and causing platelet-derived vasoconstriction [Citation176]. Antiplatelet agents, especially low-dose aspirin, can prevent or delay the development of preeclampsia.
Impact estimates: A review by Duley et al. [Citation177] identified that the use of antiplatelet agents had a significant impact on maternal outcomes of proteinuric preeclampsia (RR 0.83; 95% CI: 0.77 to 0.89) [Citation177]. It also reported reduction in the risk of fetal and neonatal deaths (RR 0.86; 95% CI: 0.76 to 0.98) when antiplatelet were used for primary prevention. A treatment with aspirin before 16 weeks was also linked with a significant reduction in the incidence of severe preeclampsia (RR 0.10; 95% CI: 0.01 to 0.74), gestational hypertension (RR 0.31; 95% CI: 0.13 to 0.78), and intrauterine growth retardation (IUGR) (RR 0.51; 95% CI: 0.28 to 0.92) [Citation178].
Composite analysis: The use of antiplatelet agents in high-risk pregnancies had a significant impact on maternal, neonatal and fetal mortality (RR 0.83; CI: 0.63 to 1.07) (). The use of antiplatelet agents did not have an impact on the composite risk of eclampsia and preterm birth (RR 0.94; 95% CI: 0.88 to 1.00). Similar effects were seen on the effect of eclampsia and neonates requiring admission to special care baby units (RR 0.95; with 95% CI: 0.89 to 1.01). In women with gestational hypertension, use of antiplatelet agents had significant impact on gestational hypertension and preterm birth (RR 0.50; 95% CI: 0.28 to 0.91) and gestational hypertension and SGA babies (RR 0.56; 95% CI: 0.38 to 0.82).
Figure 23. Antiplatelet agents versus none in high risk pregnancy – maternal, fetal and neonatal mortality.
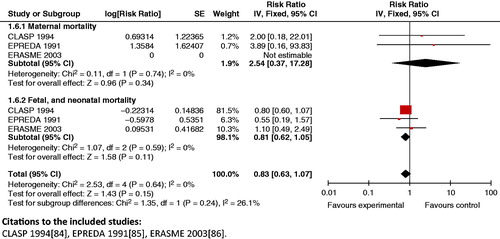
Key findings: Use of antiplatelet agents has many demonstrated benefits for maternal and newborn health, and is recommended for the prevention of preeclampsia, particularly in high-risk women.
The use of anti-hypertensive agents for mild to moderate hypertension during pregnancy
The use of anti-hypertensive agents to lower blood pressure in mild and moderate cases delays progression to severe disease; the occurrence of can lead to drastic complications for the pregnant woman and her baby.
Impact estimates: The review by Abalos et al. [Citation179] showed that the use of anti-hypertensives versus none for mild to moderate hypertension during pregnancy had significant impacts on severe hypertension (RR 0.50; 95% CI: 0.41 to 0.61) but no impact on perinatal mortality (RR 0.96; 95% CI: 0.60 to 1.54) and eclampsia (RR 0.34; 95% CI: 0.01 to 8.15).
Composite analysis: The use of anti-hypertensive agents in mild to moderate hypertension in pregnancy had a significant impact on the synergistic outcome for maternal severe hypertension and preterm birth (RR 0.84; 95% CI: 0.74 to 0.96) ().
Figure 24. Anti-hypertensive use in mild to moderate hypertension versus placebo – severe hypertension and preterm birth.
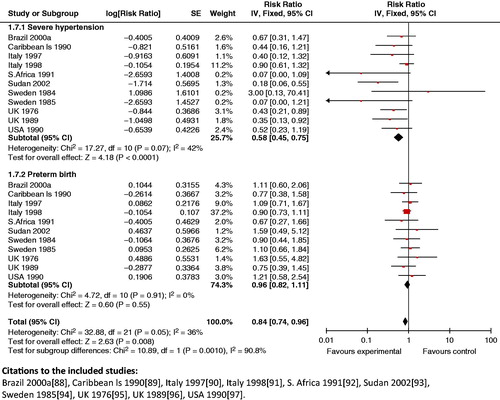
Treatment of mild to moderate hypertension in pregnancy with anti-hypertensive agents also showed a significant reduction in maternal severe hypertension and respiratory distress syndrome (RR 0.66; 95% CI: 0.48 to 0.90), and in the incidence of maternal severe hypertension and SGA babies (RR 0.81; 95% CI: 0.71 to 0.92). The analysis also showed that it had a significant impact on the synergistic outcome for maternal severe hypertension and perinatal mortality (RR 0.61; 95% CI: 0.48 to 0.76) ().
Figure 25. Anti-hypertensive use in mild to moderate hypertension versus placebo – severe hypertension and perinatal mortality.
Key findings: The use of antihypertensive drugs for mild to moderate hypertensive disorders in pregnancy is recommended for preventing the development of severe hypertension, which may result in hypertension-related disorders such as preeclampsia and eclampsia.
Magnesium sulfate for treatment of pregnancy-induced hypertension (PIH)/eclampsia
Anticonvulsants, such as magnesium sulfate, are used to treat eclamptic seizures, and have been administered to women with preeclampsia based on the premise that they would prevent the onset of seizures.
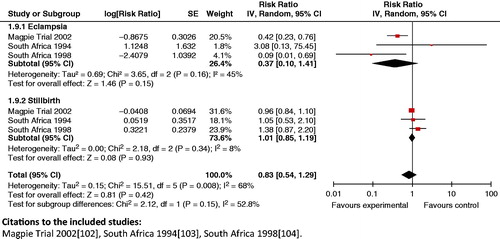
Impact analysis: A review by Duley et al. [Citation180] reported a reduction in maternal mortality by 50% (RR 0.50; 95% CI: 0.24 to 1.05) when magnesium sulfate versus phenytoin is used for eclampsia. The review also indicated that this drug is significantly better in preventing recurrence of seizures when compared to phenytoin (RR 0.34; 95% CI: 0.24 to 0.49) and diazepam (RR 0.44; 95% CI: 0.34 to 0.57). When comparing magnesium sulfate versus none/placebo for preeclampsia, Duley et al. [Citation180] reported a significant reduction in eclampsia (risk difference (RD) −0.01; 95% CI: −0.02 to −0.01).
Composite outcomes: While magnesium sulfate is clearly effective for treating eclampsia and pre-eclampsia in women, the analysis reveals that treatment of PIH/eclampsia with magnesium sulfate failed to show significant reduction on the composite outcome of eclampsia in the woman, on stillbirth (RR 0.83; 95% CI: 0.54 to 1.29) (), and neonatal morbidity: Apgar score <7 at 5 min (RR 0.69; 95% CI: 0.29 to 1.63).
Key findings: Magnesium sulfate has emerged as the drug of choice for eclampsia, as compared to other drugs, such as phenytoin and diazepam.
Lifestyle modification: smoking cessation during pregnancy
Smoking increases the risk of premature mortality among women (smoking is responsible for 1 in 20 deaths in women), and preterm birth and low birth weight among newborn babies. Counseling, cognitive behavioral therapy, social support and encouragement and the use of pharmacological agents (such as nicotine replacement therapy) have been shown to be effective in helping women stop smoking.
Impact analysis: The existing data shows that smoking cessation programs in pregnancy can reduce preterm birth (RR 0.86; 95% CI: 0.74 to 0.98) [Citation181] and low birth weight (RR 0.83; 95% CI: 0.73 to 0.95) [Citation181]. It is also shown to increase the mean birth weight (MD 39.26; 95% CI: 15.77 to 62.74) [Citation181] of newborns. A study by McCowan et al. [Citation182] showed higher rates of spontaneous preterm birth (AOR 3.21; 95% CI: 1.42 to 7.23) and SGA babies (AOR 1.76; 95% CI: 1.03 to 3.02) in smokers when compared to those women who had stopped smoking in pregnancy.
Composite analysis: No composite analysis was done for this intervention due to the lack of studies providing impact estimates on both maternal and neonatal morbidity and mortality outcomes.
Key findings: Smoking cessation programs have a significant positive impact on preterm birth, low birth weight and mean birth weight of newborn. These programs also significantly reduce the proportion of women who continue to smoke in late pregnancy.
Maternal drug use in pregnancy
Substance abuse during pregnancy has both immediate and long-term impact on a mother and her newborn. The most obvious effect of drug exposure to nicotine, opiates, alcohol or cocaine [Citation183] during pregnancy is generalized growth retardation and a small head circumference.
Impact analysis: A review by Doggett et al. [Citation184] studied the impact of home visits during pregnancy versus none for women with alcohol/substance abuse problems. Another study by Quinlivan et al. [Citation185], demonstrated a significant effect of home visits on failure to use postpartum contraceptives (RR 0.41; 95% CI: 0.20 to 0.82) and involvement with child protective services (RR 0.38; 95% CI: 0.20 to 0.74). According to a review by Sweeney et al. [Citation186], pregnant substance abusers who received prenatal care and intervention for drug treatment have significantly heavier infants and their gestational age was also 2 weeks longer (p-value for both <0.001). In a study by Armstrong et al. [Citation187], babies of mothers who had received treatment for substance abuse had lower rates of assisted ventilation after birth compared to women who had been screened but received no treatment (p = 0.01).
Composite analysis: No composite analysis was done for this intervention due to the lack of studies providing impact estimates on both maternal and neonatal morbidity and mortality outcomes.
Key findings: While current evidence does not support any one treatment modality, there are many potential advantages of treatment. Thus, it is imperative to treat pregnant women who abuse illicit drugs and alcohol. There is a dearth of studies focusing on interventions which reduce drug abuse during pregnancy; more research is needed to study the impact of interventions on maternal and newborn health outcomes.
Prevention of intimate partner violence (IPV)
IPV has been associated with poor health outcomes, e.g. increased risk of preterm labor, LBW infants and STIs.
Impact analysis: IPV in women is associated with increased risk of high blood pressure or edema (AOR 1.37 to 1.40), vaginal bleeding (AOR 1.54 to 1.66) and antenatal hospitalization not associated with delivery (AOR 2.39; 95% CI: 1.77 to 3.24) [Citation188]. Other studies have shown that IPV leads to increased risk of perinatal death (ARR 2.1; 95% CI: 1.3 to 3.4) [Citation189], preterm low birth weight births (ARR 2.4; 95% CI: 1.5 to 4.0) [Citation189], term low birth weight births (Coker 2004: ARR 1.9; 95% CI: 1.0 to 3; Lipsky 2004: OR 1.67; 95% CI: 1.12 to 2.49) [Citation189,Citation190] and preterm births (OR 1.37; 95% CI: 1.16 to 1.61) [Citation188].
Community education on topics related to maternal and neonatal health and the empowerment of women can help decrease IPV in women. A review by Ramsay et al. [Citation191] showed that intensive advocacy (12 h or more duration) results in a decrease in physical abuse in women leaving domestic violence shelters or refuges at 12 to 24 months follow-up (OR 0.43; 95% CI: 0.23 to 0.80) and improved quality of life at up to 12 months follow-up (weighted mean difference (WMD) 0.23; 95% CI: 0.00 to 0.46). A study by Kiely et al. [Citation192] reported a decrease in the number of recurrent episodes of IPV (OR 0.48; 95% CI: 0.29 to 0.80) [Citation192], fewer very preterm neonates (p 0.03) and an increase in mean gestational age (p 0.016) in women receiving intervention against IPV.
Composite analysis: A composite analysis could not be conducted because of lack of studies reporting maternal and neonatal outcomes simultaneously.
Key findings: Evidence has shown that IPV is associated with adverse pregnancy outcomes. Community education on maternal and neonatal health may decrease the incidence of IPV.
Vaccination in pregnancy
Influenza vaccination
Maternal influenza infection has been associated with an increased risk of maternal hospitalization, fetal malformation, and other illnesses. Immunization of pregnant women with inactivated trivalent influenza vaccine has been recommended in the United States for more than a decade and by the WHO since 2005.
Impact analysis: Zaman et al. [Citation193] showed that the rate of respiratory illness with fever was reduced by 36% (95% CI: 4 to 57%) in the group of pregnant women who received vaccination. Among infants of mothers who received influenza vaccine, there were fewer cases of laboratory-confirmed influenza than among infants in the control group with vaccine effectiveness 63% (95% CI: 5 to 85%) [Citation193].
Composite analysis: The analysis showed a significant (RR 0.69; 95% CI: 0.62 to 0.77) composite impact of vaccinating pregnant women on maternal and infant morbidity outcomes (i.e. maternal and infant febrile respiratory illness) (). The analysis showed that vaccination helped to reduce the risk of composite outcomes for maternal illness and infant clinic visits (RR 0.46; 95% CI: 0.47 to 0.76).
Figure 27. Influenza vaccination versus none – maternal febrile respiratory illness and infant febrile respiratory illness.
Key findings: Administering influenza vaccination to mothers during pregnancy has the potential to reduce febrile respiratory illness in both mother and infant. A strong case can be made for the use of H1N1 strain of influenza vaccine for pregnant women.
Pneumococcal vaccination
Pneumococcal vaccination during pregnancy is one means of preventing pneumococcal disease during the first months of life before the pneumococcal vaccine administered to the infant starts to produce protection [Citation194].
Impact analysis: Most studies on pneumococcal vaccination show significant positive effect on the immune response in mothers and neonates. This included a rise in the antibody levels of maternal blood (3.3-to-9.1-fold increase) [Citation195], breast milk (1.1 to 1.8 times higher) [Citation196] and cord blood (2 to 3 fold higher) [Citation197]. A review by Chaithongwongwatthana et al. [Citation194] also showed reduction in pneumococcal colonization in infants by 16 months of age and 22 months of age (RR 0.33; 95% CI: 0.11 to 0.98 and RR 0.28; 95% CI: 0.02 to 5.11, respectively).
Composite analysis: No composite analysis was done for this intervention because randomized controlled trials in this area are largely pilot studies and there is insufficient information regarding impact estimates.
Key findings: Pneumococcal vaccination during pregnancy has been shown to improve the immune response in mothers and neonates and can have potential health benefits for both. However, there is a paucity of trials focusing on both maternal and infant morbidity and mortality outcomes; further research is needed.
Interventions during the intrapartum period
The highest incidence of maternal and perinatal mortality occurs around the time of birth, with the majority of deaths occurring within the first 24 h after delivery. There exist effective interventions to avoid most of the deaths and disabilities attributable to childbirth. This section describes the interventions during the intrapartum period found to have synergistic effects on maternal and newborn health outcomes. The review further highlights the impact of these interventions on composite maternal and neonatal morbidity and mortality.
Interventions evaluated and their assigned GRADE
identifies the interventions evaluated in this paper, their reported impact on composite maternal and fetal/neonatal outcomes, and their assigned interconnection GRADES (A, B, C or D).
Table 5. Intrapartum Period: Individual interventions with Composite maternal/fetal/neonatal impact estimates and their assigned GRADE.
Treatment of preterm prelabor rupture of membranes (pPROM)
In 2010, more than 1 in 10 of the world’s births were premature, resulting in an estimated 15 million preterm births globally [Citation198,Citation199]. Most of these preterm births are linked to pPROM [Citation200]; infection plays an important role in pPROM, either as a cause or a consequence [Citation201]. Antibiotics can prevent in-utero infections from occurring and thus prevent preterm births [Citation202].
Impact analysis: The Cochrane review by Kenyon et al. [Citation203] reported reduced risk of chorioamnionitis and neonatal infection including pneumonia when any antibiotic was compared with a placebo to treat pPROM (RR 0.66; 95% CI: 0.46 to 0.6 and RR 0.67; 95% CI: 0.52 to 0.85). Antibiotics for pPROM also resulted in significant decrease in neonatal mortality (RR 0.90; 95% CI: 0.72 to 1.12) [Citation204] and neonatal morbidities like respiratory disease (RR 0.88; 95% CI: 0.80 to 0.97) [Citation204] and postnatal infections (RR 0.61; 95% CI: 0.48 to 0.77) [Citation204]. Long-term follow-up at seven years of age showed little effect on the functional impairment (functional impairment derived from the mark III Multi-Attribute Health Status classification system) in children [Citation205].
Composite analysis: Treatment of pPROM with any antibiotic yielded a significant decrease in the composite outcome of maternal chorioamnionitis and neonatal infection (RR 0.71; 95% CI: 0.60 to 0.83) ().
Figure 28. Any antibiotic versus none – chorioamnionitis and neonatal infection including pneumonia.
Key findings: While antibiotics can be recommended for treating pPROM, additional research is needed to establish which antibiotic has the best impact on maternal and neonatal health outcomes.
Role of trained traditional birth attendants
Every year, nearly 53 million women give birth without the help of a skilled birth attendant. Many of these women deliver at home with a traditional birth attendant (TBA). Training these birth attendants in clean delivery techniques and referral can reduce maternal and neonatal morbidity and mortality.
Impact analysis: A review by Sibley et al. demonstrated that provision of trained TBAs had a significant impact on stillbirths (OR 0.69; 95% CI: 0.57 to 0.83), neonatal deaths (OR 0.71; 95% CI: 0.61 to 0.82) and perinatal deaths (OR 0.70; 95% CI: 0.59 to 0.83) [Citation206]. Similar results for neonatal mortality were also reported by other studies and reviews (RR 1.08; 95% CI: 0.33 to 3.53 [Citation207] and RR 0.88; 95% CI: 0.78 to 0.99 [Citation79,Citation208]. The impact of this intervention on maternal mortality failed to reach statistical significance (OR 0.74; 95% CI: 0.45 to 1.22) [Citation206]. However, assistance by trained TBAs at births significantly reduced the odds of postpartum hemorrhage (OR 0.61; 95% CI: 0.47 to 0.79) [Citation206].
Composite analysis: Training TBAs had a significant impact on composite maternal and neonatal mortality (RR 0.85; 95% CI: 0.77 to 0.95) (). Similar analysis also showed significant impact on combined maternal and perinatal deaths (RR 0.70; 95% CI: 0.60 to 0.83) ().
Key findings: These results indicate that TBA training programs can help to reduce maternal and neonatal mortality, and highlight the critical role of trained TBAs within the health system.
Skilled birth attendance
Maternal deaths due to obstetric complications (e.g. sepsis, postpartum hemorrhage, obstructed labor) can be prevented or managed if women have access to skilled birth attendance.
Impact analysis: McClure et al. [Citation209] undertook a sensitivity analysis and showed that when <42% of the population had access to SBA, there was no significant decrease in maternal deaths, but when access exceeded 42% there was a decrease of 0.15 maternal deaths per 1000 births (p < 0.0001) for each 1% increase in the presence of SBA. Provision of SBA also showed a significant impact on the incidence of stillbirths (OR 0.77; 95% CI: 0.69 to 0.85) [Citation210] and perinatal deaths (OR 0.88; 95% CI: 0.82 to 0.95) [Citation210]. A review by Darmstadt et al. [Citation211] indicates a significant impact in providing community-based skilled care attendance on perinatal (RR 0.88; 95% CI: 0.83 to 0.95) and neonatal mortality (RR 0.87; 95% CI: 0.7 to 0.97).
Composite analysis: A composite analysis could not be conducted as there was lack of data reporting impact estimates for maternal and neonatal outcomes in studies focusing on SBA.
Key findings: Reviews show that access to SBA not only decreases maternal mortality but also decreases stillbirths and reduces perinatal mortality. Efforts need to be made to develop studies that measure the composite impact of this intervention on maternal and neonatal outcomes.
Essential obstetric care
For the purposes of this review, the components included in essential obstetric care are: provision of clean delivery kits (CDKs), use of partogram in labor, and active management of the third stage of labor. Each of these interventions is described in detail below.
Provision of clean delivery kits
Each year about 60 million women in developing countries give birth with the assistance of an untrained attendant or family member, or with no help at all [Citation212]. Most of these deliveries take place at home, where the risk of infection is high. Many of these deaths can potentially be averted by providing clean delivery kits (CDKs) at the community level.
Impact estimates: The review by Hundley et al. [Citation213] identified intervention packages which include CDKs to reduce risk for maternal puerperal sepsis (OR 0.17; 95% CI: 0.13 to 0.23) [Citation214], neonatal infectious morbidity such as sepsis (RR 0.12; 95% CI: 0.02 to 0.93) [Citation215], and omphalitis (OR 0.42; 95% CI: 0.18 to 0.97 [Citation215]; (OR 0.08; 95% CI: 0.03 to 0.19) [Citation216,Citation217]. Available studies show that the provision of CDKs has a statistically significant impact on perinatal/neonatal mortality ((OR 0.7; 95% CI: 0.59 to 0.82) [Citation79]; (RR 0.17; 95% CI: 0.13 to 0.23) [Citation214,Citation218,Citation219]; (OR 0.78; 95% CI: 0.50 to 1.21) [Citation29]). Another review by Hundley et al. [Citation220] discussed that the levels of birth kit use vary considerably (8–99%) in more than 50 low resource countries; with higher levels being reported where birth kits are distributed free as part of a research program. A recent review of three community-based RCTs carried out between 2000 and 2008 in India, Bangladesh, and Nepal found that each additional clean delivery practice (using a plastic sheet during delivery, a boiled blade to cut the cord, a boiled thread to tie the cord, and antiseptic to clean the umbilicus) was associated with a 16% relative reduction in neonatal mortality (OR 0.84, 95% CI: 0.77 to 0.92) [Citation221].
Composite analysis: The analysis shows a statistically significant positive effect (RR 0.21; 95% CI: 0.09 to 0.47) of this intervention on combined maternal and neonatal morbidity ().
Key findings: Use of CDKs leads to significant reduction in combined maternal and neonatal morbidity. A pooled analysis from three countries indicates a significant impact of clean delivery practices on neonatal mortality.
Partograph use
A partograph provides a continuous pictorial overview of labor, including maternal condition, fetal condition, and labor progress. Although a useful tool, its role in improving maternal health outcomes has been debated.
Impact analysis: Lavender et al. [Citation222] showed no impact of partograph use on maternal outcomes (cesarean section: RR 0.64; 95% CI: 0.24 to 1.70) or on neonatal health benefits (Apgar score less than seven at 5 min: RR 0.77; 95% CI: 0.29 to 2.06; admission to special care nursery: RR 0.94; 95% CI: 0.51 to 1.75). The only possible advantage of use of a partograph was reduction in the length of labor (p < 0.001 in primigravida and p < 0.01 in multigravida) [Citation223].
Composite analysis: An analysis of the composite impact of partograph use on cesarean section rates and low Apgar scores demonstrated an insignificant impact (RR 0.67; 95% CI: 0.33 to 1.35).
Key findings: Based on the studies reviewed, the use of the partograph during labor does not have positive health benefits for mother or newborn; future trials are needed to support its continued use.
Active management of third stage of labor
More than half of maternal deaths during childbirth occur within 24 h of childbirth; the most common cause of death is excessive blood loss, or postpartum hemorrhage (PPH) [Citation224]. Active management of the third stage of labor can prevent PPH due to uterine atony; the components of active management typically includes administration of a uterotonic agent (oxytocin is the drug of choice) within 1 min after birth of the baby and after ruling out the presence of another baby.
Impact analysis: Active management is superior to expectant management; active management significantly reduced the risk for PPH (RR 0.38; 95% CI: 0.32 to 0.46) [Citation225], severe PPH (RR 0.38; 95% CI: 0.1 to 0.97) [Citation225], need for blood transfusion (RR 0.34; 95% CI: 0.22 to 0.53) [Citation225] and postpartum anemia (RR 0.40; 95% CI: 0.29 to 0.55) [Citation225]. A multicenter, non-inferiority, randomized controlled trial [Citation226] on women expecting to vaginally deliver singleton babies found no impact of placental delivery with gravity and maternal effort (simplified package) or controlled cord traction applied immediately after uterine contraction and cord clamping (full package) on blood loss (RR 1·09; 95% CI: 0·91 to 1·31).
Composite analysis: A composite analysis could not be conducted because active management of the third stage of labor largely impacts the mother. There is no evidence regarding its impact on neonatal outcomes.
Key findings: Because of the potential maternal health benefits, active management of the third stage of labor is highly recommended.
Comprehensive emergency obstetric care
Emergency obstetric care (EmOC) is a package of medical interventions required to treat the major obstetric complications. In this analysis, comprehensive EmOC was identified as: lower-segment cesarean section (LSCS), post-term induction of labor, instrumental delivery and emergency transport systems.
Lower-segment cesarean section (LSCS)
Lack of access to, and availability of, high-quality EmOC, especially cesarean section, has been considered as a risk factor for intrapartum stillbirths, particularly those associated with prolonged/obstructed labor [Citation227].
Impact analysis: The recent review from the Lancet Stillbirth series [Citation228] reported that with each 1% increase in cesarean section births, intrapartum stillbirths decreased by 1·61 per 1000 births. However, in a study by Lumbiganon et al. [Citation229], the risk of maternal mortality and morbidity index was increased for operative vaginal delivery (i.e. use of forceps or vacuum) (adjOR 2.1; 95% CI: 1.7 to 2.6), and all types of cesarean section (antepartum without indication: OR 2.7; 95% CI: 1.4 to 5.5; antepartum with indication: OR 10.6; 95% CI: 9.3 to 12.0; intrapartum without indication: OR 14.2; 95% CI: 9.8 to 20.7; intrapartum with indication: OR 4.5; 95% CI: 13.2 to 16.0). In the same study, for breech presentation, cesarean section, either antepartum (OR 0.2; 95% CI: 0.1 to 0.3) or intrapartum (OR 0.3; 95% CI: 0.2 to 0.4), was associated with improved perinatal outcomes, but also with an increased risk of stay at the neonatal intensive care unit (NICU) (OR 2.0; 95% CI: 1.1 to 3.6 and OR 2.1; 95% CI: 1.2 to 3.7, respectively).
Composite analysis: No composite analysis was done for this intervention.
Key findings: Cesarean sections reduce the risk of intrapartum stillbirths and improve perinatal mortality. Given that randomized controlled trials of LSCS are unlikely, further research may be needed to identify the composite impact of this intervention on maternal and neonatal outcomes from time-series analysis. In some parts of the developing world (in Latin America and in Asia), there are very high rates of cesarean section which represent an unnecessary increased risk for women and their babies.
Post-term induction of Labor
Evidence from the literature indicates that mother and infant are at increased risk of adverse events when the pregnancy continues beyond term (defined as 41 completed weeks for the purpose of this analysis). A policy of labor induction at 41 weeks is compared with expectant management (until an indication for birth arises).
Impact analysis: In one review, women at 37 to 40 completed weeks were more likely to have a cesarean section with expectant management than those in the labor induction group (RR 0.58; 95% CI: 0.34 to 0.99) [Citation230]. Another review by Hussain et al. [Citation231] showed that post-term induction of labor was associated with fewer perinatal deaths for the post-term group (RR 0.31; 95% CI: 0.11 to 0.88).
Composite analysis: A meta-analysis was done for the comparison of a policy of labor induction at 41 completed weeks versus expectant management. The combination of maternal morbidity of cesarean section with neonatal morbidity of Apgar score <7 at 5 min revealed significant reduction with labor induction compared with expectant management at 41 completed weeks of gestation (RR 0.87; 95% CI: 0.79 to 0.97). The combination of maternal morbidity of cesarean section with neonatal morbidity of NICU admission revealed significant beneficial effects for induction of labor at 41 weeks of gestation (RR 0.9; 95% CI: 0.83 to 0.96). Significant results were seen for the composite outcome of cesarean section and perinatal mortality for induction of labor at 41 completed weeks of gestation (RR 0.83; 95% CI: 0.74 to 0.92) ().
Figure 32. Induction of labor at or beyond term versus expectant management at 41 completed weeks and more – c-section and perinatal mortality.
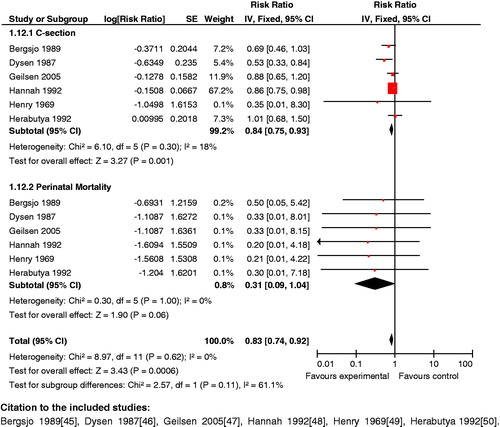
Key findings: Based on the research reviewed, women at 37 to 40 completed weeks were more likely to have a cesarean section with expectant management than those in the labor induction group. Cesarean section and perinatal mortality is reduced with labor induction at 41 weeks compared to expectant management of labor. A policy of labor induction at 41 completed weeks is therefore recommended for preventing maternal and neonatal morbidity.
Instrumental/assisted delivery
The main instruments used for assisted vaginal deliveries are obstetric forceps or vacuum. There is limited data on the incidence of instrumental vaginal births in low-income countries, but in the industrialized world, the rates range from 5 to 20% of all births [Citation232,Citation233].
Impact analysis: Johanson et al. [Citation234] showed that vacuum extraction was associated with significantly less maternal injuries compared to forceps (OR 0.41; 95% CI: 0.33 to 0.50). However, it had a higher incidence of fetal retinal hemorrhages and cephalhaematoma. In another review, delivery with the use of forceps versus any type of delivery was associated with a reduced risk of failed delivery (RR 0.65; 95% CI: 0.45 to 0.94) [Citation235].
Composite analysis: For the comparison of vacuum extraction versus forceps delivery, composite outcomes were assessed for severe maternal injury and perinatal mortality; these were statistically significantly reduced (RR 0.49; 95% CI: 0.35 to 0.70) (). Further analyses of the impact of vacuum extraction versus forceps delivery on the composite outcomes of cesarean section and perinatal mortality and cesarean section and cephalhaematoma did not reveal significant results (RR 0.57; 95% CI: 0.31 to 1.05 and RR 1.20; 95% CI: 0.65 to 2.61, respectively).
Figure 33. Vacuum extraction versus forceps delivery – severe maternal injury and perinatal mortality.
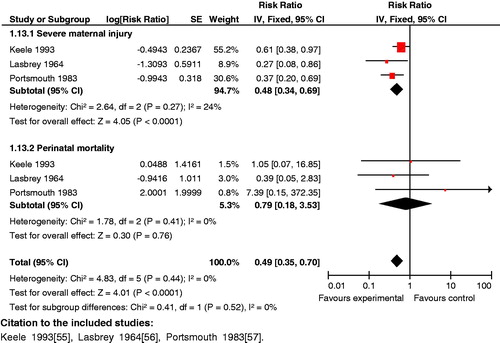
Key findings: Analysis revealed that the use of vacuum extraction displayed a reduction in the composite risk for severe maternal injury and perinatal mortality compared to the use of forceps.
Emergency transport systems
In developing countries, one of the major barriers to access maternal and newborn health care services is the lack of available transportation. This leads to unfavorable outcomes, including maternal and newborn-related mortality and morbidity, especially during emergency situations. Developing emergency transport/transfer systems tailored to the geographic area and needs of the community is therefore one strategy to increase access to maternal and newborn health services.
Impact analysis: A number of studies have examined the role of ambulance care in national programs aimed at attempting to addressing maternal mortality [Citation236]. In the Sierra Leone and Ugandan RESCUER programs, enhanced communication systems and emergency transport via 4-wheel-drive ambulances significantly increased obstetric referrals (from 0.9 to 2.6% per month), and reduced maternal case fatality (from 20 to 10%) [Citation237]. In the developed world, there is a well-developed system of emergency transport services supported by both hospitals and government; the same cannot be said for developing countries, where health budgets are insufficient to support this intervention [Citation238]. There is a dearth of studies providing data for this key intervention. Data from observational studies suggest that effective transport systems help to reduce the delays faced by patients experiencing obstetric emergencies. Use of simpler means of transport such as motorcycle ambulances in resource-poor regions may be more efficient than state-of-the-art ambulances. No composite analysis was done for this intervention.
Key findings: Literature suggests that ambulance care can reduce maternal mortality; this intervention can also address intrapartum-associated neonatal complications such as intrapartum stillbirths and birth asphyxia. There are limited studies on this subject and further research may be needed to determine combined benefits on maternal and newborn health outcomes.
Perinatal audit
Perinatal audit is the careful review and evaluation of care given to the pregnant woman, the fetus, and the neonate. Audits of maternal and perinatal mortality can be performed at the facility or at the community. Perinatal audit at the community level aims to evaluate national guidelines for maternal and newborn care; it is conducted at three levels, with each level adding depth to the audit. The first level involves recording the number of maternal/perinatal deaths in an area. The second level categorizes the causes of death, and the final level identifies potentially avoidable factors or suboptimal care. At the community level, an audit is done to evaluate national guidelines for maternal and newborn care, whereas, at the facility level it is performed to review the deficiencies/success in providing care.
Impact analysis: A study from Gaudeloupe [Citation239] showed a 25% reduction in perinatal mortality rate with the application of perinatal audit. Studies by Hawthorne et al. [Citation240] and Krue et al. 241 also showed significant reduction in the perinatal mortality rate with population based perinatal audit. A meta-analysis of seven studies by Pattinson et al. [Citation242] indicated a reduction in perinatal mortality of 30% (95% CI: 21 to 38%) after introduction of facility based perinatal audit. A Cochrane review did not identify any trial on the impact of critical clinical based audit on maternal and perinatal mortality [Citation61]. No composite analysis could be done for this intervention.
Key finding: Perinatal audit has been shown to reduce perinatal mortality rate in a number of studies. It is an essential tool for analyzing the causes of maternal and perinatal death. As with any audit system, feedback is essential to gauge its effectiveness. Efforts should identify which tools can gauge its effectiveness, and can provide an accurate estimate of its coverage in the population.
Interventions during the postpartum/postnatal period
The first 6 weeks following childbirth – defined as the postnatal or postpartum period – is dangerous for both mother and baby. More than 60% of maternal deaths occur during this time; half of all postpartum maternal deaths occur during the first week after childbirth, with the majority of these occurring during the first 24 h [Citation243]. An infant’s chance of dying is also highest during the postnatal period, with the most vulnerable time being the first 24 h following birth. In fact, 75% of all newborn deaths occur by the end of the first week after delivery [Citation7]. While the postpartum period represents a critical opportunity to safeguard the health and survival of a mother and her newborn, many women and newborns receive little or no postpartum care, and some do not receive any postpartum care at all. Given this high level of vulnerability, the postnatal period represents an ideal time within the continuum of care for delivering interventions to improve the health of women and their newborns. This section defines the interconnections between maternal and neonatal interventions delivered during the postnatal period, some of which are directed to the mother (e.g. recognition and treatment of postpartum depression) and others to the newborn (e.g. thermal care for newborn, early initiation of breastfeeding).
Interventions evaluated and their assigned GRADE
summarizes the relevant postpartum and newborn care interventions reviewed in this paper, reports their impact on composite maternal and fetal/neonatal outcomes, and identifies their assigned Interconnection groups (A, B, or C). Grade A interventions and their effect on maternal and neonatal death and disability are described below.
Table 6. Postnatal/Postpartum Period: Individual interventions with Composite maternal/fetal/neonatal impact estimates and their assigned GRADE.
Recognition and treatment of mild to moderate postnatal depression
Maternal mental health problems pose a huge human, social, and economic burden to women, their infants, their families, and society, and constitute a major public health challenge [Citation244]. The most common mental disorders associated with pregnancy and childbirth include postpartum depression, anxiety disorders and postnatal psychosis. The onset of depression in women increases during pregnancy and in the postpartum period. This review looks at the impacts of pharmacological and non-pharmacological treatment of maternal depression on maternal and neonatal health.
Impact analysis: Two reviews identified on this topic [Citation245,Citation246] indicated that use of pharmacological methods to treat postnatal depression displayed a significant decrease in evidence of depression post-treatment (clinical global impression scale: RR 0.46; 95% CI: 0.26 to 0.83). With regards to non-pharmacological treatment, Rahman et al. [Citation247] stated that cognitive behavior therapy, administered by primary health workers who were trained to deliver the psychological intervention, for the mothers led to a reduction in infant outcome of diarrhea (diarrhea episodes at 12 months: OR 0.6; 95% CI: 0.39 to 0.98), an improvement in complete immunization at 12 months (OR 2.5; 95% CI: 0.79 to 3.18), and increased contraceptive use in mothers at 12 months (OR 1.6; 95% CI: 1.20 to 2.27). A review by Sukran et al. [Citation248] found a significant association of maternal depression with underweight (OR: 1.5; 95% CI: 1.2 to 1.8) and stunting (OR: 1.4; 95% CI: 1.2 to 1.7) in children. A composite analysis could not be generated due to the lack of studies reporting simultaneous outcomes for the mother and the newborn child.
Key findings: Studies have shown that pharmacological treatment for depression has significantly reduced depression post-treatment. Literature has also shown that non-pharmacological treatment of maternal postnatal depression has a positive impact on subsequent child outcomes such as diarrhea and completion of routine immunizations. This treatment is also associated with increased contraceptive use in women. Although there is a dearth of systematic reviews on interventions related to maternal mental health, available evidence suggests that recognition and treatment of postnatal depression benefits both mother and child in the long term.
Support and promotion of early initiation and continued breastfeeding
The safest and most successful way of assuring proper growth and development is providing the infant with exclusive breast milk from the first hour of birth through 6 months of age [Citation249–251]. In developing countries alone, if mothers start practicing early initiation of breastfeeding, an estimated 1.45 million lives can be saved annually by reducing deaths due to lower respiratory tract infections and diarrheal disorders [Citation252]. Women also benefit from early breastfeeding, both in the short and long term. In this review the early initiation of breastfeeding is defined as breastfeeding that takes place within the first 24 h after birth; late or delayed initiation is breastfeeding that takes place after 24 h of birth.
Impact analysis: Two recent reviews assessed the impact of breastfeeding on neonatal outcomes: a review by Imdad et al. [Citation253] found that if promotion of initiation of breastfeeding was given in prenatal and postnatal periods there was a 12% statistically significant increase in any breastfeeding rates at 6 months (RR 1.12; 95% CI: 1.01 to 1.24). A Cochrane review by Lewin et al. [Citation254] also showed that counseling of pregnant women led to an increase in the incidence of early initiation of breastfeeding (RR 1.45; 95% CI: 1.14 to 1.84). A study by Edmond et al. [Citation255] reported a marked dose response relationship of increased risk of neonatal mortality with increasing delay in initiation of breastfeeding from 1 h to day 7. They reported that overall late initiation (after day 1) was associated with a 2.4 fold increase in risk. Similar findings were also reported in a study from Southern Nepal [Citation256] and they found higher mortality among late (>24 h) compared with early (<24 h) initiators (RR: 1.41; 95% CI: 1.08 to 1.86).
In the short-term, with breastfeeding begun shortly after delivery, the mother is likely to recover more rapidly from the stress of parturition; the uterus contracts, stimulated by the oxytocin released during lactation, thereby reducing blood flow, preventing anemia as iron stores are less depleted. As far as long-term outcomes are concerned, literature has shown that breastfeeding reduces fertility in the mother, perhaps for several months, affording a natural form of birth spacing. A Cochrane review by Wijden et al. [Citation257] concluded that fully breastfeeding women who remain amenorrhic have a very small risk of becoming pregnant in the first 6 months after delivery when relying on lactational subfertility [Citation258–265]. Pooled estimates from the Lancet Maternal and Child Undernutrition Series [Citation266] show that individual counseling increased the odds of exclusive breast feeding by a factor of 3.45 in the neonatal period (OR 3.45; 95% CI: 2.20 to 5.42) and by a factor of 1.93 at 6 months of age (OR 1.93; 95% CI: 1.18 to 3.15). This series also showed similar results in the analyses for group counseling, which improved the odds of exclusive breast feeding in the neonatal period (OR 3.88; 95% CI: 2.09 to 7.22) and at 6 months of age (OR 5.19; 95% CI: 1.90 to 14.15) [Citation266]. Breastfeeding has shown improvement in maternal weight loss at 4 to 6 months after delivery (MD −0.42; 95% CI: 0.02 to 0.82) [Citation267]. It has also been seen to have a significant impact on reducing breast cancer risk for the mother (OR 0.6; 95% CI: 0.5 to 0.9) [Citation268]. In a study published by the Collaborative Group on Hormonal Factors in Breast Cancer, it was observed that the relative risk of breast cancer decreased by 4.3% (95% CI: 2.9 to 5.8; p < 0.0001) for every 12 months of breastfeeding in addition to a decrease of 7.0% (5.0 to 9.0; p < 0.0001) for each birth [Citation269]. However, a Cochrane review by Crepinsek et al. [Citation270] found a non-significant impact of breastfeeding education on the risk of mastitis at day 7 (RR: 3.75; 95% CI: 0.35 to 40.70) and day 30 (RR: 0.93; 95% CI: 0.17 to 4.95) after delivery. A composite analysis was not possible due to the lack of trials focusing on maternal and neonatal health outcomes simultaneously.
Key findings: Early initiation and sustained exclusive breastfeeding for 6 months has wide-ranging beneficial effects for the mother, including an increased rate of postpartum weight loss, reduced blood loss after parturition, reduced fertility and decreased risk of breast cancer. A decrease in neonatal mortality has also been reported. Counseling of pregnant women regarding early initiation of breastfeeding has been recognized as an important tool for promoting exclusive breastfeeding practices, both in the neonatal period and at 6 months of age.
Thermal care or kangaroo mother care (KMC) for preterm and/or low birth weight babies
More than 1 in 10 of the world’s babies born in 2010 were born prematurely, resulting in estimated 15 million preterm births (defined as before 37 weeks of gestation) [Citation271]. Prematurity is the second-leading cause of death in children under-5 and the single most important cause of death in the critical first month of life [Citation272]. It is also the most important risk factor for neonatal deaths from infection [Citation6,Citation273], and for developing hypothermia. A number of measures have been proposed to maintain infants’ body temperature outside of incubators. These include: skin-to-skin care, extra clothing/bedding, warming up the nursery, heating the bed mattress and KMC. KMC is a technologically simple method developed by Colombian pediatrician Edgar Ray in 1970 to prevent the incidence of hypothermia in preterm babies as a result of incubator shortage.
Impact estimates: A Cochrane review by McCall et al. [Citation2] stated that the effect of barriers to heat loss such as plastic wrap applied in the delivery suite versus routine care significantly decreased the risk of hypothermia on admission to the neonatal intensive care unit (NICU) [core body temperature <36.5 °C or skin temperature <36 °C (RR 0.66; 95% CI: 0.51 to 0.84]. The same review reported a significant reduction in hypothermia [skin temperature <35.5 °C for two consecutive recordings (RR 0.09; 95% CI: 0.01 to 0.64 and RR 0.09; 95% CI: 0.01 to 0.64) with the use of skin-to-skin care versus routine care. Other modalities of external heat sources such as transwarmer (sodium acetate gel) mattresses also had a reduction on the risk of hypothermia on admission to NICU [core body temperature <36.5 °C (RR 0.3; 95% CI: 0.11 to 0.83) or skin temperature <36 °C (RR 0.30; 95% CI: 0.11 to 0.83)] when compared with routine care. A recent Cochrane review found a significant positive effect of early skin-to-skin contact on breastfeeding rates at one to four months post birth (RR 1.27; 95% CI: 1.06 to 1.53) [Citation274].
The review by Lawn et al. [Citation275] reported that it can lead to significant reduction in the risk of neonatal mortality <2000 g (RR 0.49; 95% CI: 0.29 to 0.82) and severe neonatal morbidity (RR 0.34; 95% CI: 0.18 to 0.65). Moreover, KMC was also found to increase rates of breastfeeding (RR 1.20; 95% CI: 1.01 to 1.43), and mother–infant attachment (RR 6.24; 95% CI: 5.57 to 6.91) [Citation276]. No studies were identified which explored the effects of KMC on women. Hence, composite analyses were not done for this intervention due to the lack of studies looking at the impact of KMC on women and infants simultaneously.
Key findings: A range of interventions, other than primary care designed for prevention of hypothermia, applied after birth in the delivery suite, may be beneficial in practice. These include: plastic wraps and bags, skin-to-skin contact, transwarmer mattresses, etc. These interventions keep infants warmer and lead to higher temperatures on admission to the NICU and to decreased incidence of hypothermia. KMC has been shown to significantly reduce the risk of neonatal mortality and severe neonatal morbidity. In light of the clear benefits for neonatal health, KMC should be routinely provided to babies <2000 g at birth in facilities in low- income countries, where other options for care of preterm babies are limited. No study reported the impact of neonatal thermal care on mothers.
Cross cutting interventions: training CHWs to provide interventions during the antenatal, childbirth and postnatal periods
Interventions which train and deploy human resources, specifically CHWs, were also included in this review. These health workers provide interventions at community level during the antenatal, childbirth, and postnatal periods.
Community based intervention packages (community mobilization and home visitation via trained CHWs)
To support the basic primary health care infrastructure, a range of different approaches have been developed to train CHWs to provide specific tasks related to antenatal and postnatal care [Citation216,Citation277]. These trained CHWs deliver these interventions to the community via different strategies, such as community mobilization and home visitation. Only studies which implemented packages of health interventions were considered eligible for inclusion. Such packages have been known to improve maternal and neonatal health.
Impact estimates: A systematic review by Kidney et al. [Citation278] stated that improving community based perinatal care practices (educating lay birth attendants on basic concepts of maternal and neonatal care, delivery practices and providing referral to complicated cases) had a significant impact on reducing maternal mortality (OR 0.62; 95% CI: 0.39 to 0.98). They also found that minimal goal-oriented versus usual ANC showed no difference in maternal mortality (RR 1.09; 95% CI: 0.53 to 2.25). The Cochrane review by Lassi et al. [Citation279] reported a non-significant reduction in maternal mortality (RR 0.77; 95% CI: 0.59 to 1.02), but significant reductions in perinatal mortality (RR 0.80; 95% CI: 0.71 to 0.91), stillbirths (RR 0.84; 95% CI: 0.74 to 0.97) and neonatal mortality (RR 0.76; 95% CI: 0.68 to 0.84) [Citation279] with the use of community based intervention packages.
Key findings: The available data suggests that introduction of community-based intervention packages via training CHWs can improve maternal, perinatal and neonate health. The results from recent reviews are very promising; these reviews recommend community-based packages be an integral part of primary health care in developing countries.
Training health personnel in basic neonatal resuscitation
Birth asphyxia is a major cause of neonatal mortality in developing countries and can result in permanent brain damage if the baby survives. Training health personnel in basic resuscitation techniques is a key strategy for improving birth asphyxia-related outcomes, and for maternal resuscitation-related emergencies. If health personnel are trained in neonatal resuscitation, they can be equipped to deal with maternal emergencies during childbirth or in the postpartum period.
Impact analysis: A review by Lee et al. [Citation280] found a significant effect of training community midwives on reducing intrapartum-related neonatal mortality (RR 0.78; 95% CI: 0.64 to 0.95) and all-cause early neonatal mortality (RR 0.82; 95% CI: 0.75 to 0.90). Another study found a reduced perinatal mortality by 19% among newborns delivered by TBAs trained in resuscitation compared to TBAs trained for mouth-to-mouth breathing [Citation281]. Other studies have shown that implementation of neonatal resuscitation guidelines resulted in a 66% reduction in early neonatal mortality rate [Citation282] and significant reduction in asphyxia-related deaths [Citation283].
Key finding: Training health care personnel in basic neonatal resuscitation has shown beneficial impact on neonatal health. Therefore, training health care providers is one of the many other interventions that can positively impact neonatal and perinatal health.
Postnatal visits
Home visits by trained CHWs to promote preventive care and to provide curative newborn care has been shown to be efficacious at reducing perinatal and neonatal mortality.
Impact analysis: A key benefit of postnatal visits is an improvement in exclusive breastfeeding for the first 6 months. In a study by Bashour et al. [Citation284], rates of exclusive breast feeding amongst women who received four, one or no postnatal visits were 28.5, 30, and 20%, respectively. The study reported higher rates of detection of mothers being pallor during the postnatal visits (p = 0.004); however, it had no impact on identifying and improving other maternal morbidities or improving contraceptive rates. In another study, postnatal visits on days 1 and 2 of life had significant effects on neonatal mortality (infants receiving a visit on day 1 versus none: HR 0.33; 95% CI: 0.23 to 0.46) [Citation227].
Key finding: Postnatal visits have shown to improve exclusive breastfeeding, which should be initiated as soon as possible after birth or after returning home from the facility. With regards to the timing of postnatal visits, a visit within the first 48 h after birth is most effective in reducing newborn mortality. Postnatal visits by CHWs also provide potential opportunities to detect maternal and neonatal morbidities.
Management of neonatal pneumonia and sepsis
Neonatal sepsis is defined as a clinical syndrome of bacteremia with systemic signs and symptoms of infection in the first 4 weeks of life. Infection remains a major cause of illness and death in the neonatal period [Citation285,Citation286]. Both pneumonia and neonatal sepsis can be treated in a hospital or community setting. The intervention has no benefit to mothers; however, effective management of neonatal sepsis by CHWs may indicate its potential beneficial impact in management of maternal peripartum infections, such as vaginal tract infections discussed earlier.
Impact estimates: When considering hospital management, a Cochrane review on monotherapy [Timentin] versus combination therapy [Piperacillin and Gentamicin] for early onset neonatal sepsis (<48 h) showed no significant effect on mortality in the first 28 d of life (RR 0.75; 95% CI: 0.19 to 2.90) [Citation287] or on rate of treatment failure (RR 1.25; 95% CI: 0.19 to 8.39) [Citation287]. Similarly, another Cochrane review on beta-lactam antibiotics versus combination therapy with a beta-lactam plus an aminoglycoside for late onset neonatal sepsis found no significant effect on mortality prior to discharge (RR 0.17; 95% CI: 0.01 to 3.23) [Citation288] or treatment failure (RR 0.17; 95% CI: 0.01 to 3.23) [Citation288]. With regards to community management of neonatal sepsis and pneumonia with antibiotics (cotrimoxazole, ampicillin or penicillin) versus no antibiotics, treatment with antibiotics resulted in a significant reduction in all-cause neonatal mortality (RR 0.73; 95% CI: 0.65 to 0.82) [Citation289] and pneumonia-specific mortality (RR 0.58; 95% CI: 0.43 to 0.78) [Citation289]. A study by Zaidi et al. [Citation290] stated that among 434 (61.6%) infants recruited at clinics randomized to 7 d of antibiotic therapy, there were 9% failures with penicillin-gentamicin, 15% with ceftriaxone and 18% with Trimethoprim-sulfamethaxole-gentamicin (TMP-SMX-gentamicin). Treatment failure was significantly higher with TMP-SMX-gentamicin compared with penicillin-gentamicin (RR 2.03; 95% CI: 1.09 to 3.79). By 14 d, mortality was 5.58 times in the ceftriaxone group compared to TMP-SMX-gentamicin group (95% CI: 1.26 to 24.72). This study also stated that when hospitalization of sick infants is unfeasible, outpatient therapy with injectable antibiotics is an effective option.
Key findings: Antibiotics have a clear role in the treatment of pneumonia and sepsis in low-income areas and can be effectively administered in homes via trained health workers. Management of neonatal and maternal infections can be packaged together and delivered through health workers considering its individual effectiveness. In terms of recommending a particular regimen of antibiotics for out-patient-based management of neonatal sepsis or pneumonia, Procaine penicillin-gentamicin is superior to TMP-SMX-gentamicin while Ceftriaxone is a more expensive option, and may be less effective, although this requires further research.
Discussion
There is strong evidence demonstrating the interdependent relationship between maternal and neonatal health. Strategies for improving maternal and newborn health are therefore closely related, and integrating care for women and children along the continuum of care has the potential for accelerating progress towards MDGs 4 and 5. The continuum of care not only spans time (preconception, pregnancy, childbirth, and the postnatal period), as well as the levels of care (household, community and health facilities).
The effective delivery of interconnected interventions can have a cross-generational impact on the health and wellbeing of women and newborns. They can promote greater efficiency by maximizing synergies and combining or linking services that are more efficient if delivered together. For example, by ensuring that postnatal visits address the health needs of both women and newborns, health workers can provide information about breastfeeding, immunization and raise awareness of danger signs in newborns, and also check for any signs of infection in both mother and newborn. Community-based intervention packages have been shown to reduce maternal mortality, perinatal mortality, stillbirths and neonatal mortality [Citation279]. Similarly, nutritional interventions for women prior to conception, such as folic acid supplementation, have long-term health benefits for women and also promote healthier development of the fetus [Citation291].
This paper has highlighted those interventions which have been shown to have an impact on improving both maternal and newborn health outcomes (19 Group A interventions and 10 Group B-I interventions), and could be potentially recommended for deployment and scaling-up in developing country health services. These interventions address different aspects of the continuum of care, in terms of level of care (community, health center, and hospital) and time (from pre-pregnancy to the postnatal period). Some interventions require advanced technical expertise and skills (e.g. management of diabetes), whereas many others can be provided at the community level (e.g. counseling for immediate and exclusive breastfeeding). This paper identifies these strategies and interventions, and groups them into packages of care for delivery at the community, health center or hospital level.
The review found that while the effects of maternal health interventions on neonatal outcomes have been relatively well studied, the converse is not the case; there is a dearth of studies examining the benefits of neonatal interventions for both maternal and neonatal health. Building on the evidence reviewed in this paper, the following recommendations suggest a way forward in integrating and packaging maternal and newborn health across the continuum of care. What follows below is a summary of the “Group A” interventions found to have a positive, synergistic effect on the health and survival of both women and newborns during the pre-pregnancy, antenatal, intrapartum and postpartum periods:
Pre-pregnancy and reproductive health interventions
Peri-conceptual folic acid has a significant protective effect on neural tube defects (NTDs). The use of folic acid 3 months before conception is known to reduce the risk of first occurrence and a recurrence of NTDs. The benefit of folic acid supplementation is evident in both women who had a previous pregnancy and were affected by it (recurrent NTDs), as well as those without prior history of NTDs. All women should be advised and provided with folic acid least 3 months before conception until 3 months after conception to prevent the occurrence and/or recurrence of NTDs. No recommendation can be made regarding the impact of this intervention on stillbirths because evidence is insufficient.
Appropriate birth spacing (each pregnancy to be 18–24 months apart) is strongly recommended across the reproductive age groups for its impact on both maternal and neonatal/child health.
Prevention and management of pre-gestational diabetes has a positive impact on maternal and newborn health outcomes. Therefore, it is strongly advisable to screen, detect and manage diabetes in women before pregnancy.
ANC interventions
Well-designed, good-quality ANC during pregnancy can reduce maternal mortality and morbidities and increase detection of pregnancy complications. ANC can also reduce the risk of preterm birth, perinatal mortality, and low birth weight infants.
Smoking cessation programs in pregnancy have been shown to reduce preterm birth and reduce the incidence of low birth weight. Smoking cessation and relapse prevention strategies should be routine parts of ANC.
Daily routine supplementation of iron and folic acid is a standard practice during pregnancy. Because of the combined benefit that iron supplementation has for both the woman and child, it is recommended that this practice should be continued. However, further trials are needed to explore the appropriate dosing of iron, especially in resource-poor developing countries. There is no evidence regarding benefit of giving folic acid in the antenatal period if it has not been given prior to conception.
Given the significant positive impact of the management of gestational diabetes mellitus (GDM) on maternal and neonatal outcomes, we suggest that a packaged treatment of GDM (dietary advice, glucose monitoring, and insulin) be delivered to pregnant women especially during the first trimester.
Prevention and management of STIs (syphilis, gonorrhea, bacterial vaginosis, and chlamydia) during pregnancy reduces poor health outcomes in women, the fetus, and the newborn. Treating women early in the pregnancy effectively prevents infection in the fetus, and helps reduce the occurrence of disease in the women.
Improving the uptake of and adherence to antiretroviral therapy (ART) to prevent mother-to-child transmission (PMTCT) of HIV is strongly recommended. Maternal ART during pregnancy, and through breastfeeding, is most effective for maternal health, and for reducing HIV transmission and infant death.
Recognition and management of hypertensive disorders in pregnancy can lead to reductions in maternal mortality and neonatal morbidities. These interventions are both preventive (calcium supplementation and anti-platelet agents in high-risk pregnancies) and therapeutic (anti-hypertensive drugs to mild to moderate hypertensive disorders in pregnancy). The use of magnesium sulfate is recommended for treatment of severe pregnancy-induced hypertension (PIH) and eclampsia. This intervention not only serves as prophylaxis in high-risk patients, but also has benefits that spill over to the fetus, lowering the incidence rate of NICU admission.
Use of ITNs can reduce maternal anemia and low birth weight infants. Targeted efforts are required to increase ITN coverage, and to promote knowledge and practice regarding ITN use in pregnancy.
Community-based packages (including establishing women’s support groups and empowering women on childbirth and newborn care preparedness) which provide health education and outreach provides a holistic and self-sustaining approach for increasing appropriate healthy behaviors, including utilization of appropriate health services, that benefit both the mother and fetus/newborn.
Intrapartum interventions
Many interventions during the intrapartum period can be packaged under the category of essential obstetric care. These can be further classified into basic and emergency obstetric care as outlined below:
Basic obstetric care
The use of clean delivery kits is recommended for home births, as it has potential positive health benefits to the mother and neonate in terms of reduced risk of infection.
Maternal deaths due to obstetric complications such as sepsis and postpartum hemorrhage can be prevented or managed if women have access to SBA during childbirth, including hygienic delivery practices and active management of third stage of labor, which help prevent complications. Although the evidence found was of low quality, additional research is needed to generate estimates that would make composite analyses feasible. Skilled attendance denotes the presence of a health professional with midwifery skills, as well as the enabling environment they need in order able to perform capably.
Emergency obstetric care
A policy of labor induction at 41 completed weeks is recommended to prevent maternal and neonatal morbidity.
Provision of cesarean section can reduce maternal mortality and the risk of intrapartum stillbirths. Instrumental vaginal delivery is an important option for the management of obstructed labor. The use of vacuum extraction over forceps is recommended for averting perinatal injury. Further studies are needed to examine these outcomes in a variety of settings and to assess the external validity of findings.
Postnatal/postpartum interventions
Although there is a dearth of systematic reviews on maternal mental health interventions, recognition and treatment of postnatal depression is essential for the benefit of both the woman and child.
Breastfeeding has well-established health benefits for both the woman and her newborn, including long-term benefits to the woman. Efforts to promote breastfeeding such as mass media awareness programs, individual/group counseling, and baby-friendly hospital initiatives should be continued and, as appropriate, expanded.
Home visits should be initiated as soon as possible after birth or after returning home from the health facility. A visit within the first 24 h after birth is likely to be most effective in improving maternal and newborn health. Visits on day 3 and day 7 are also recommended, as deemed appropriate.
Research limitations and evidence gaps
This review included systematic analyses for each intervention which have plausible benefits for both mothers and newborns; however, a number of limitations were identified in the review; including:
Lack of information. For many of the interventions, studies examined only outcomes related to either the mother or the newborn, and in many cases there were limitations in interpretation of effects related to absence of evidence, rather than there being evidence of the absence of an effect.
Ethical concerns do not permit RCTs for certain interventions, e.g. screening and treatment of syphilis in pregnancy, emergency obstetric care, and family planning. This limited the data available for composite analyses for these interventions. For interventions where conducting RCTs was ethically feasible, there was a lack of research reporting the evidence of impact on both maternal and neonatal outcomes simultaneously, such as breastfeeding promotion, smoking cessation in pregnancy, and peri-conceptual folic acid. This restricted the pool of studies for the composite analysis.
Proposed packages of care
The interventions identified in this series can be bundled together and delivered in various packages to increase efficiency and generate a synergistic effect. illustrates a framework for how the different packages can be delivered and the level of care at which each can be provided. In these seven packages of care, several means of delivery exist, and should be adopted in a context-specific manner. Critical to the success of delivering these packages, however, will be the support of key players, including CHWs, trained TBAs, and medical attendants in first-level facilities as providers of care. Furthermore, these key players must be coordinated and trained through institutional structures in the form of non-government organizations (NGOs), local government health policies, as well as national ministries of health and training institutes. Funding must also be carefully detailed to ensure that each of the packages can be sustained as a whole; in the instance that funding is insufficient for any single component of the packages, the entire continuum can be severely undermined. Furthermore, in order for coverage of these interventions to be high, it is important to ensure affordability of these services, and this may require establishment of strategies such as vouchers, conditional cash transfers, or health equity funds to ensure that women have financial access to services.
Undoubtedly, implementation of multi-level integration required for the continuum of care is challenging. The feasibility of providing packaged interventions is influenced by the strength of the existing health system and available resources in a given community, district, or country. However, the interventions within the packages identified are, to a large extent, low-tech, low-cost and have been implemented in an effective manner with strong impact. As with other integrative health strategies, these packages should be implemented incrementally, with close coordination and strengthening of the existing health system beginning at the community and district level. Creation of these packages can also aid in the reallocation of limited resources in a more effective and evidence-based manner. Below are the seven proposed packages of care, which include all Group A interventions, as well as those categorized as Group BI.
General supportive care
This package supports the health of the woman and child during pregnancy and during the postpartum period through prevention of unhealthy behaviors and conditions, including depression, which can be provided through community-based support groups. Interventions include smoking cessation in pregnancy, prevention of IPV, prevention of maternal drug abuse during pregnancy, recognition and treatment of postpartum depression, screening and detection of pre-gestational diabetes, and recognition of general mental health problems.
Advice and counseling for smoking cessation can be administered at the community level. Detection and treatment of pre-gestational diabetes and mental health issues and postnatal depression should be performed at the level of outpatient clinics or, preferably, within a tertiary setting. Prevention and treatment of drug abuse involves the health sector but also requires support from the community.
Maternal nutrition support package
This package consists of key nutrition interventions, including peri-conceptual folic acid supplementation, multiple micronutrient supplementation during pregnancy, balanced protein energy supplementation during pregnancy, iron–folic acid supplementation during pregnancy, calcium supplementation in pregnant women with low/inadequate calcium intake. These interventions can be both promoted and delivered at the community level. Supplementation can also be administered via outpatient programs.
Improving quality of basic ANC
ANC can be delivered at the outpatient level. An effective ANC package consists of birth preparedness, disease detection, complication readiness, and counseling and health promotion. An antenatal package includes interventions which detect and treat pregnancy-related infections, such as malaria, STIs and HIV. Case management of illness, such as malaria, pneumonia, and diarrhea, can be performed in the outpatient setting, whereas management of HIV and STIs should preferably be done at a tertiary care center. Other conditions affecting pregnancy, such as anemia, pre-eclampsia and malnutrition, can be screened at outpatient visits. At the community level, women can also be counseled on the benefits of breastfeeding by both CHWs and personnel in outreach/outpatient programs.
Expanded ANC package
Routine pregnancy care can be further improved by expanding the components of the ANC package; these include prevention and management of diabetes during pregnancy, treatment of pregnancy-induced hypertension (PIH) with magnesium sulfate and anti-hypertensive drugs, and use of antiplatelet agents for prevention of pre-eclampsia in high-risk pregnancies. These interventions are more appropriate at the tertiary care level after thorough clinical and laboratory assessment, but could be adapted for implementation at lower levels of the health care system. Another emerging intervention that has displayed improved maternal outcomes are influenza vaccination during pregnancy, which can be administered at the level of the community or via outpatient/outreach programs.
Community-based intervention packages and support groups
Community-based intervention strategies provide support to the basic primary health care infrastructure. These community based interventions include developing support groups for women for education and empowerment, and training CHWs to deliver antenatal, intrapartum, and postnatal care at the household level, and to identify, manage, and refer complicated cases to the health facility. Available data suggest that the introduction of community-based intervention packages have the potential to decrease maternal and neonatal mortality. It can also improve breastfeeding practices and increase referrals to health facilities for pregnancy-related complications and other health care services recommended during pregnancy, such as iron–folic acid supplementation.
Childbirth care package
A range of interventions for each stage of labor can improve the health outcomes for woman and child. However, for the purpose of this review, we grouped interventions under basic and comprehensive emergency obstetric care, which can be provided by skilled birth attendants. Some interventions such as cesarean section and instrumental delivery generally require tertiary level care. Others such as emergency transport systems can be established at the grassroots level within the community. These interventions should be considered essential components of any childbirth care package. These include basic obstetric care, emergency obstetric care (lower segment cesarean section, active induction of post term pregnancy), treatment of preterm prelabor rupture of membranes (pPROM), SBA (doctors, nurses, midwives), and training TBAs in clean delivery and referral.
Postnatal care
In the postnatal period, interventions such as breastfeeding, thermal care, KMC and family planning can be provided by CHWs. These interventions can be integrated into another important postpartum intervention, namely, postnatal visits. At the level of the tertiary care center, postnatal care, particularly for low birth weight infants and newborns at-risk for HIV and malaria, can be provided.
This review has identified a number of interventions which have beneficial health impacts on both mother and newborn. These interventions should be integrated in health policies and programs as part of a continuum of care approach. The integration of maternal and newborn health strategies has the potential to improve efficiency, save scarce resources, and ultimately improve maternal and newborn outcomes. This approach is critical to achieving MDGs 4 and 5 – ensuring that we not only save lives but also address overall health and well-being.
A call for scaling up these interventions
This series has highlighted the interventions which have been shown to have an impact on improving both maternal and newborn health outcomes, and could be potentially recommended for deployment and scaling-up in developing country health services. Policy and regulations are crucial to the implementation of any intervention, therefore, these interventions should be reviewed in light of existing standards and guidelines and be deployed and updated to ensure that priority lifesaving interventions reach women and their newborns.
Declaration of interest
The authors declare that there are no conflicts of interest. We express our gratitude to the Bill & Melinda Gates Foundation for their financial support of this work.
Acknowledgements
The authors wish to thank the Editors of the Supplement (Gary Darmstadt, France Donnay, and Ann Starrs) for their careful review and valuable comments. Special thanks also goes to Family Care International staff, in particular Hilary Lawton, who provided editorial support and assistance.
References
- USAID. Two Decades of Progress: USAID's Child Survival and Maternal Health Program. 2009. Available from: http://pdf.usaid.gov/pdf_docs/PDACN044.pdf [last accessed 21 Mar 2013]
- McCall EM, Alderdice F, Halliday HL, et al. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010;3:Art. No. CD004210
- Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3.6 Million neonatal deaths – what is progressing and what is not? Semin Perinatol 2010;34:371–86
- WHO. Trends in maternal mortality: 1990 to 2010. WHO, UNICEF, UNFPA and The World Bank estimates. Geneva, Switzerland; 2012
- Boama V, Arulkumaran S. Safer childbirth: a rights-based approach. Int J Gynaecol Obstet 2009;106:125–7
- Lawn JE, Cousens S, Zupan J. 4 Million neonatal deaths: When? Where? Why? Lancet 2005;365:891–900
- Lozano R, Wang H, Foreman KJ, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 2011;378:1139–65
- Stanton ME. A case for investment in maternal survival and health. USAID; 2010. Available from: http://www.wilsoncenter.org/sites/default/files/Mary%20Ellen%20Stanton%20Presentation.pdf [last accessed 12 Jun 2012]
- Bhutta ZA, Lassi ZS, Blanc A, Donnay F. Linkages among reproductive health, maternal health, and perinatal outcomes. Semin Perinatol 2010;34:434–45
- Weeks JR, Hill AG, Getis A, Stow D. Ethnic residential patterns as predictors of intra-urban child mortality inequality in Accra, Ghana. Urban Geogr 2006;27:526–48
- Tinker A, Ransom E. Healthy mothers and healthy newborns: the vital Link, in Population Reference Bureau and Saving Newborn Lives Initiative. April 2002. Available from: http://www.prb.org/pdf/HealthyMothers_Eng.pdf [last accessed 11 Sep 2011]
- Ronsmans C, Chowdhury ME, Dasgupta SK, et al. Effect of parent's death on child survival in rural Bangladesh: a cohort study. Lancet 2010;375:2024–31
- Kerber KJ, de Graft-Johnson JE, Bhutta ZA, et al. Continuum of care for maternal, newborn, and child health: from slogan to service delivery. Lancet 2007;370:1358–69
- PMNCH. Opportunities for Africa's Newborns: practical data, policy and programmatic support for newborn care in Africa. Cape Town, South Africa: PMNCH, Save the Children, UNFPA, UNICEF, USAID, WHO; 2006
- PMNCH. Conceptual and Institutional Framework. Geneva, Switzerland: Partnership for Maternal, Newborn and Child Health; 2006
- World Health Organization. Department of Making Pregnancy Safer. Integrated Management of Pregnancy and Childbirth. WHO Recommended Interventions for Improving Maternal and Newborn Health. 2007. Available from: http://whqlibdoc.who.int/hq/2007/WHO_MPS_07.05_eng.pdf [last accessed 23 May 2012]
- Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004;4:38
- Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011
- Bhutta Z, Dean S, Imam A, Lassi Z. A systematic review of preconception risks and interventions. Karachi: The Aga Khan University; 2011
- Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ 1993;306:1645–8
- De-Regil LM, Fernandez-Gaxiola AC, Dowswell T, Pena-Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2010;10:Issue 10. Art. No. CD007950
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 2010;39:i110–21
- Bukowski R, Malone FD, Porter FT, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med 2009;6:e1000061
- Brouwer IA, van Dusseldorp M, Thomas CM, et al. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J Clin Nutr 1999;69:99–104
- Filler G, Rayar MS, da Silva O, et al. Should prevention of chronic kidney disease start before pregnancy? Int Urol Nephrol 2008;40:483–8
- Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity 2006;14:165–71
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164–70
- Hoff GL, Cai J, Okah FA, Dew PC. Pre-pregnancy overweight status between successive pregnancies and pregnancy outcomes. J Womens Health 2009;18:1413–17
- Joseph NP, Hunkali KB, Wilson B, et al. Pre-pregnancy body mass index among pregnant adolescents: gestational weight gain and long-term post partum weight retention. J Pediatr Adolesc Gynecol 2008;21:195–200
- Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;9:140–50
- Han Z, Mulla S, Beyene J, et al. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011;40:65–101
- Siega-Riz AM, Corrine Giannini RD. Promoting healthy weight in women. NC Med J 2009;70:449--53
- Doherty DA, Magann EF, Francis J, et al. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynecol Obstet 2006;95:242–7
- Lu GC, Rouse DJ, DuBard M, et al. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol 2001;185:845–9
- Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001;91:436–40
- Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev 2007;8:385–94
- Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am J Clin Nutr 2003;77:931–6
- Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lact 2004;20:18–29
- Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia 2006;61:36–48
- Chan B, Zacharin M. Maternal and infant outcome after pamidronate treatment of polyostotic fibrous dysplasia and osteogenesis imperfecta before conception: a report of four cases. J Clin Endocrinol Metab 2006;91:2017–20
- Rayco-Solon P, Fulford AJ, Prentice AM. Maternal preconceptional weight and gestational length. Am J Obstet Gynecol 2005;192:1133–6
- Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol 2002;100:959–64
- Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology 2010;21:243–52
- Salihu HM, Lynch ON, Alio AP, et al. Extreme maternal underweight and feto-infant morbidity outcomes: a population-based study. J Matern Fetal Neonatal Med 2009;22:428–34
- Johnson TS, Rottier KJ, Luellwitz A, Kirby RS. Maternal prepregnancy body mass index and delivery of a preterm infant in Missouri 1998–2000. Public Health Nurs 2009;26:3–13
- Ota E, Haruna M, Suzuki M, et al. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bull World Health Organ 2011;89:127–36
- Nohr EA, Vaeth M, Baker JL, et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750
- Chu SY, Bachman DJ, Callaghan WM, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med 2008;358:1444–53
- Frederick IO, Williams MA, Sales AE, et al. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J 2008;12:557–67
- Dietz PM, Callaghan WM, Morrow B, Cogswell ME. Population-based assessment of the risk of primary cesarean delivery due to excess prepregnancy weight among nulliparous women delivering term infants. Matern Child Health J 2005;9:237–44
- Abenhaim HA, Kinch RA, Morin L, et al. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch Gynecol Obstet 2007;275:39–43
- Chen CW, Tsai CY, Sung FC, et al. Adverse birth outcomes among pregnancies of teen mothers: age specific analysis of national data in Taiwan. Child Care Health Dev 2010;36:232–40
- Phithakwatchara N, Titapant V. The effect of pre-pregnancy weight on delivery outcome and birth weight in potential diabetic patients with normal screening for gestational diabetes mellitus in Siriraj Hospital. J Med Assoc Thai 2007;90:229–36
- Driul L, Cacciaguerra G, Citossi A, et al. Prepregnancy body mass index and adverse pregnancy outcomes. Arch Gynecol Obstet 2008;278:23–6
- Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 2004;103:219–24
- Haldre K, Rahu K, Karro H, Rahu M. Is a poor pregnancy outcome related to young maternal age? A study of teenagers in Estonia during the period of major socio-economic changes (from 1992 to 2002). Eur J Obstet Gynecol Reprod Biol 2007;131:45–51
- Catalano RF, Fagan AA, Gavin LE, et al. Worldwide application of prevention science in adolescent health. Lancet 2012;379:1653–64
- Guldi M. Fertility effects of abortion and birth control pill access for minors. Demography 2008;45:817–27
- Campbell FA, Ramey CT, Pungello E, et al. Early childhood education: young adult outcomes from the Abecedarian Project. Appl Dev Sci 2002;6:42–57
- Philliber S, Kaye J, Herrling S. The national evaluation of the Children’s Aid Society Carrera-Model Program to prevent teen pregnancy. Accord (NY): Philliber Research Associates; 2001
- Allen JP, Philliber S, Herrling S, Kuperminc GP. Preventing teen pregnancy and academic failure: experimental evaluation of a developmentally based approach. Child Dev 1997;68:729–42
- Eisen M, Zellman GL, McAlister AL. Evaluating the impact of a theory-based sexuality and contraceptive education program. Fam Plann Perspect 1990;22:261–71
- Howard M, McCabe JB. Helping teenagers postpone sexual involvement. Fam Plann Perspect 1990;22:21–6
- Mitchell-DiCenso A, Thomas BH, Devlin MC, et al. Evaluation of an educational program to prevent adolescent pregnancy. Health Educ Behav 1997;24:300–12
- Ross DA. Approaches to sex education: peer-led or teacher-led? PLoS Med 2008;5:e229
- Cabezon C, Vigil P, Rojas I, et al. Adolescent pregnancy prevention: an abstinence-centered randomized controlled intervention in a Chilean public high school. J Adolesc Health 2005;36:64–9
- Anderson R, Lois N, Koniak-Griffin D, et al. Evaluating the outcomes of parent–child family life education. Res Theory Nurs Pract 1999;13:211–34
- Kirby D, Korpi M, Barth RP, Cagampang HH. The impact of the postponing sexual involvement curriculum among youths in California. Fam Plann Perspect 1997;29:100–8
- Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol 2007;109:181–8
- Stevens-Simon C, Kelly L, Singer D. Preventing repeat adolescent pregnancies with early adoption of the contraceptive implant. Fam Plann Perspect 1999;31:88–93
- Blank L, Baxter SK, Payne N, et al. Systematic review and narrative synthesis of the effectiveness of contraceptive service interventions for young people, delivered in educational settings. J Pediatr Adolesc Gynecol 2010;23:341–51
- Winikoff B. The effects of birth spacing on child and maternal health. Stud Fam Plann 1983;14:231–45
- Rousso D, Panidis D, Gkoutzioulis F, et al. Effect of the interval between pregnancies on the health of mother and child. Eur J Obstet Gynecol Reprod Biol 2002;105:4–6
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809–23
- Huttly SR, Victora CG, Barros FC, Vaughan JP. Birth spacing and child health in urban Brazilian children. Pediatrics 1992;89:1049–54
- De Jong-Potjer LC, Elsinga J, Le Cessie S, et al. GP-initiated preconception counselling in a randomised controlled trial does not induce anxiety. BMC Fam Pract 2006;7:66
- Bhutta ZA, Soofi S, Cousens S, et al. Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial. Lancet 2011;377:403–12
- Manandhar DS, Osrin D, Shrestha BP, et al. Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet 2004;364:970–9
- Moos MK, Bangdiwala SI, Meibohm AR, Cefalo RC. The impact of a preconceptional health promotion program on intendedness of pregnancy. Am J Perinatol 1996;13:103–8
- Czeizel AE. Ten years of experience in periconceptional care. Eur J Obstet Gynecol Reprod Biol 1999;84:43–9
- Mathiesen ER, Ringholm L, Damm P. Stillbirth in diabetic pregnancies. Best Pract Res Clin Obstet Gynaecol 2011;25:105–11
- Ganesh KS, Unnikrishnan B, Nagaraj K, Jayaram S. Determinants of Pre-eclampsia: a case-control study in a district hospital in South India. Indian J Community Med 2010;35:502–5
- Shamsi U, Hatcher J, Shamsi A, et al. A multicentre matched case control study of risk factors for preeclampsia in healthy women in Pakistan. BMC Womens Health 2010;10:14
- Ray JG, O'Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM 2001;94:435–44
- Walkinshaw SA. Pregnancy in women with pre-existing diabetes: management issues. Semin Fetal Neonatal Med 2005;10:307–15
- Kitzmiller JL, Buchanan TA, Siri K, et al. Pre-conception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care 1996;19:514–41
- Ray JG, Vermeulen MJ, Meier C, Wyatt PR. Risk of congenital anomalies detected during antenatal serum screening in women with pregestational diabetes. QJM 2004;97:651–3
- Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies. Diabetes Care 2004;27:2819–23
- Wahabi HA, Alzeidan RA, Bawazeer GA, et al. Preconception care for diabetic women for improving maternal and fetal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2010;10:63
- Leguizamon G, Igarzabal ML, Reece EA. Periconceptional care of women with diabetes mellitus. Obstet Gynecol Clin North Am 2007;34:225–39
- Tripathi A, Rankin J, Aarvold J, et al. Preconception counseling in women with diabetes. Diabetes Care 2010;33:586–8
- Temple RC, Aldridge VJ, Murphy HR. Prepregnancy care and pregnancy outcomes in women with type 1 diabetes. Diabetes Care 2006;29:1744–9
- Temple RC, Aldridge V, Stanley K, Murphy HR. Glycaemic control throughout pregnancy and risk of pre-eclampsia in women with type I diabetes. BJOG 2006;113:1329–32
- Garcia-Patterson A, Corcoy R, Rigla M, et al. Does preconceptional counselling in diabetic women influence perinatal outcome? Ann Ist Super Sanita 1997;33:333–6
- Boulot P, Chabbert-Buffet N, d'Ercole C, et al. French multicentric survey of outcome of pregnancy in women with pregestational diabetes. Diabetes Care 2003;26:2990–3
- Eng TR, Butler WT, eds. The hidden epidemic: confronting sexually transmitted diseases. Institute of Medicine, Division of Health Promotion and Disease Prevention. Washington, DC: National Academy Press; 1997
- Gounden YP, Moodley J. Exposure to human immunodeficiency virus among healthcare workers in South Africa. Int J Gynecol Obstet 2000;69:265–70
- Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: Fall 2006. Top HIV Med 2006;14:125–30
- Marion LN, Finnegan L, Campbell RT, Szalacha LA. The Well Woman Program: a community-based randomized trial to prevent sexually transmitted infections in low-income African American women. Res Nurs Health 2009;32:274–85
- Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet 1999;353:525–35
- Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet 2003;361:645–52
- Jemmott Iii JB, Jemmott LS, Braverman PK, Fong GT. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic: a randomized controlled trial. Arch Pediatr Adolesc Med 2005;159:440--9
- van Deventer HW, Hall MD, Orlowski RZ, et al. Clinical course of thrombocytopenia in patients treated with imatinib mesylate for accelerated phase chronic myelogenous leukemia. Am J Hematol 2002;71:184–90
- Hankins C, Tran T, Lapointe N. Sexual behavior and pregnancy outcome in HIV-infected women. JAIDS 1998;18:479
- Markson LE, Turner BJ, Houchens R, et al. Association of maternal HIV infection with low birth weight. JAIDS 1996;13:227--34
- Temmerman M, Plummer FA, Mirza NB, et al. Infection with HIV as a risk factor for adverse obstetrical outcome. AIDS 1990;4:1087–93
- Martin R, Boyer P, Hammill H, et al. Incidence of premature birth and neonatal respiratory disease in infants of HIV-positive mothers. J Pediatr 1997;131:851–6
- Karim AQ, Abdool KSS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168--74
- McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 2010;376:1329–37
- Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials 2007;2:e27
- Ahmed S, Lutalo T, Wawer M, et al. HIV incidence and sexually transmitted disease prevalence associated with condom use: a population study in Rakai, Uganda. AIDS 2001;15:2171–9
- Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002;1:Issue 1. Art. No. CD003255
- Denison JA, O’Reilly KR, Schmid GP, et al. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS Behav 2008;12:363–73
- Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis 2004;190:879–85
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent–child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Dev Psychopathol 2005;17:145–65
- Halbreich U. The association between pregnancy processes, preterm delivery, low birth weight, and postpartum depressions: the need for interdisciplinary integration. Am J Obstet Gynecol 2005;193:1312–22
- Cohen JA. Treating traumatized children: current status and future directions. J Trauma Dissociation 2005;6:109–21
- Ross DS, Jones JL, Lynch MF. Toxoplasmosis, cytomegalovirus, listeriosis, and preconception care. Matern Child Health J 2006;10:189–93
- Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med 2006;68:938–46
- Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health 2009;18:803–11
- Jonsson U, Bohman H, Hjern A, et al. Intimate relationships and childbearing after adolescent depression: a population-based 15 year follow-up study. Soc Psychiatry Psychiatr Epidemiol 2010;46:711–21
- Seth P, Raiji PT, DiClemente RJ, et al. Psychological distress as a correlate of a biologically confirmed STI, risky sexual practices, self-efficacy and communication with male sex partners in African-American female adolescents. Psychol Health Med 2009;14:291–300
- Silverman ME, Loudon H. Antenatal reports of pre-pregnancy abuse is associated with symptoms of depression in the postpartum period. Arch Womens Ment Health 2010;13:411–15
- Garn SM, Ridella SA, Petzold AS, Falkner F. Maternal hematologic levels and pregnancy outcomes. Semin Perinatol 1981;5:155–62
- Kandoi A, Bhatia BD, Pandey LK, et al. Cellular immunity status in anaemia in pregnancy. Indian J Med Res 1991;94:11–15
- Prema K, Ramalakshmi BA, Madhavapeddi R, Babu S. Immune status of anaemic pregnant women. Br J Obstet Gynaecol 1982;89:222–5
- Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr 1992;55:985–8
- Yakoob MY, Bhutta ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011;11:S21
- Pena-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev 2009;4:Issue 4. Art. No. CD004736
- Black RE. Micronutrients in pregnancy. Br J Nutr 2001;85:193--7
- Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60
- Haider B, Yakoob M, Bhutta Z. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health 2011;11:S19
- Imdad A, Yakoob MY, Bhutta Z. The effect of folic acid, protein energy and multiple micronutrient supplements in pregnancy on stillbirths. BMC Public Health 2011;11:S4
- Imdad A, Bhutta Z. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011;11:S17
- Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev 2003;4:Issue 4. Art. No. CD000032
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev 2010;10:Issue 10. Art. No. CD000934
- Rumbold AR, Cunningham J. A review of the impact of antenatal care for Australian Indigenous women and attempts to strengthen these services. Matern Child Health J 2008;12:83–100
- Humphrey MD, Keating SM. Lack of antenatal care in far north Queensland. Aust N Z J Obstet Gynaecol 2004;44:10–13
- Kidney E, Winter HR, Khan KS, et al. Systematic review of effect of community-level interventions to reduce maternal mortality. BMC Pregnancy Childbirth 2009;9:2
- Lassi ZS, Haider BA, Bhutta ZA. Evaluation of community-based intervention package for preventing maternal morbidity and mortality and improving neonatal outcomes. International Initiative for Impact Evaluation (unpublished); 2010
- Britton C, McCormick FM, Renfrew MJ, et al. Support for breastfeeding mothers. Cochrane Database Syst Rev 2007;1:Issue 1. Art. No. CD001141
- Guyon AB, Quinn VJ, Hainsworth M, et al. Implementing an integrated nutrition package at large scale in Madagascar: the Essential Nutrition Actions framework. Food Nutr Bull 2009;30:233–44
- Brandt A, Jones D, Holmes KK, et al. Historical perspectives on sexually transmitted diseases: challenges for prevention and control. In: Holmes KK, Sparling PM, Mardh PA, Lemon S,eds. Sexually transmitted diseases, 3rd ed. New York: McGraw-Hill; 1999:15–21
- Hill-Jones B, Noble R. Global prevalence and incidence of selected curable sexually transmitted infections overview and estimates. Geneva: World Health Organization; 2001
- Blencowe H, Cousens S, Kamb M, et al. Lives saved tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related stillbirths and neonatal mortality. BMC Public Health 2011;11:S9
- Myer L, Abdool Karim SS, Lombard C, Wilkinson D. Treatment of maternal syphilis in rural South Africa: effect of multiple doses of benzathine penicillin on pregnancy loss. Trop Med Int Health 2004;9:1216–21
- Walker GJ, Walker DG. Congenital syphilis: a continuing but neglected problem. Semin Fetal Neonatal Med 2007;12:198–206
- Brocklehurst P. Antibiotics for gonorrhoea in pregnancy. Cochrane Database Syst Rev 2002;2:Issue 2. Art. No. CD000098
- Brocklehurst P, Rooney G. Interventions for treating genital chlamydia trachomatis infection in pregnancy. Cochrane Database Syst Rev 2000;2:Issue 2. Art. No. CD000054
- Gulmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011;5:Issue 5. Art. No. CD000220
- McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2007;1:Issue 1. Art. No. CD000262
- Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev 2011;7:Issue 7. Art. No. CD003510
- Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane database of systematic reviews 2007;1:Issue 1. Art. No. CD003510
- Chasela C, Hudgens M, Jamieson D, et al. Both maternal HAART and daily infant nevirapine (NVP) are effective in reducing HIV-1 transmission during breastfeeding in a randomized trial in Malawi: 28 week results of the Breastfeeding, Antiretroviral and Nutrition (BAN) Study. 2009
- WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. Geneva: WHO; 2010
- WHO. Malaria in pregnancy. Available from: http://www.who.int/malaria/high_risk_groups/pregnancy/en/ [last accessed 2 Oct 2012]
- Garner P, Gulmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;4:Issue 4. Art. No. CD000169
- ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 2007;297:2603–16
- Gies S, Coulibaly SO, Ouattara FT, D'Alessandro U. Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weight. Trop Med Int Health 2009;14:174–82
- Eisele TP, Larsen D, Steketee RW. Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas. Int J Epidemiol 2010;39:i88
- ter Kuile FO, Terlouw DJ, Phillips-Howard PA, et al. Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg 2003;68:50–60
- Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med 2007;4:e107
- Casson IF, Clarke CA, Howard CV, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 1997;315:275–8
- Russell MA, Carpenter MW, Coustan DR. Screening and diagnosis of gestational diabetes mellitus. Clin Obstet Gynecol 2007;50:949–58
- Tieu J, Middleton P, McPhee AJ, Crowther CA. Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database Syst Rev 2010;2:Issue 2. Art. No. CD007222
- Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010;115:597–604
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev 2009;3:Issue 3. Art. No. CD003395
- Syed M, Javed H, Yakoob MY, Bhutta Z. Effect of screening and management of diabetes during pregnancy on stillbirths. BMC Public Health 2011;11:S2
- Crowther CA, Hiller JE, Moss JR, et al. Australian Carbohydrate Intolerance Study in Pregnant Women Trial G. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86
- Hamlin RHJ. Prevention of pre-eclampsia. Lancet 1962;1:864–5
- Belizan JM, Villar J. The relationship between calcium intake and edema-, proteinuria-, and hypertension-getosis: an hypothesis. Am J Clin Nutr 1980;33:2202–10
- Imdad A, Jabeen A, Bhutta Z. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: a meta-analysis of studies from developing countries. BMC Public Health 2011;11:S18
- Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hyper-tensive disorders and related problems. Cochrane Database Syst Rev 2010;8:Issue 8. Art. No. CD001059
- Bussolino F, Benedetto C, Massobrio M, Camussi G. Maternal vascular prostacyclin activity in pre-eclampsia. Lancet 1980;2:702
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2007;2:Issue 2. Art. No. CD004659
- Bujold E, Morency AM, Roberge S, et al. Acetylsalicylic acid for the prevention of preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery Doppler: a systematic review and meta-analysis. J Obstet Gynaecol Can 2009;31:818–26
- Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2007;1:Issue 1. Art. No. CD002252
- Duley L, Henderson-Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev 2010;10:Issue 10. Art. No. CD000128
- Lumley J, Chamberlain C, Dowswell T, et al. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2009;3:Issue 3. Art. No. CD001055
- McCowan LM, Dekker GA, Chan E, et al. Spontaneous preterm birth and SGA infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ 2009;338:b1081
- Hoegerman G, Wilson CA, Thurmond E, Schnoll SH. Drug-exposed neonates. West J Med 1990;152:559–64
- Doggett C, Burrett S, Osborn DA. Home visits during pregnancy and after birth for women with an alcohol or drug problem. Cochrane Database Syst Rev 2005;4:Issue 4. Art. No. CD004456
- Quinlivan J, Box H, Cooke S, Evans S. What happens to adolescent mothers (WHAM) – a randomised controlled trial of a home visiting intervention. 2000 March 12–15; Brisbane, Australia
- Sweeney PJ, Schwartz RM, Mattis NG, Vohr B. The effect of integrating substance abuse treatment with prenatal care on birth outcome. J Perinatol 2000;20:219–24
- Armstrong MA, Gonzales Osejo V, Lieberman L, et al. Perinatal substance abuse intervention in obstetric clinics decreases adverse neonatal outcomes. J Perinatol 2003;23:3–9
- Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S. states: associations with maternal and neonatal health. Am J Obstet Gynecol 2006;195:140–8
- Coker AL, Sanderson M, Dong B. Partner violence during pregnancy and risk of adverse pregnancy outcomes. Paediatr Perinat Epidemiol 2004;18:260–9
- Lipsky S, Holt VL, Easterling TR, Critchlow CW. Police-reported intimate partner violence during pregnancy and the risk of antenatal hospitalization. Matern Child Health J 2004;8:55–63
- Ramsay J, Carter Y, Davidson L, et al. Advocacy interventions to reduce or eliminate violence and promote the physical and psychosocial well-being of women who experience intimate partner abuse. Cochrane Database Syst Rev 2009;3:Issue 3. Art. No. CD005043
- Kiely M, El-Mohandes AA, El-Khorazaty MN, Gantz MG. An integrated intervention to reduce intimate partner violence in pregnancy: a randomized controlled trial. Obstet Gynecol 2010;115:273–83
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555–64
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev 2006;1:Issue 1. Art. No. CD004903
- Quiambao BP, Nohynek H, Kayhty H, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 2003;21:3451–4
- Lehmann D, Pomat WS, Riley ID, Alpers MP. Studies of maternal immunisation with pneumococcal polysaccharide vaccine in Papua New Guinea. Vaccine 2003;21:3446–50
- Shahid NS, Hoque SS, Begum T, et al. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 1995;346:1252–7
- Howson CP, Kinney MV, Lawn JE, eds. PMNCH, Save the Children, WHO. Born too soon: the global action report on preterm birth. Geneva: World Health Organization; 2012
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72
- Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet 1995;346:1271–9
- Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2003;2:Issue 2. Art. No. CD001058
- Howson C, Kinney M, Lawn JE. March of Dimes, PMNCH, Save the Children, WHO. Born too soon: the global action report on preterm birth. Geneva: World Health Organization; 2012
- Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2010;2:Issue 2. Art. No. CD001058
- Cousens S, Blencowe H, Gravett M, Lawn JE. Antibiotics for pre-term pre-labor rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. Int J Epidemiol 2010;39:i134–43
- Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labor: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319–27
- Sibley LM, Sipe TA, Brown CM, et al. Traditional birth attendant training for improving health behaviours and pregnancy outcomes. Cochrane Database Syst Rev 2007;3:Issue 3. Art. No. CD005460
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis* 1. Am J Obstet Gynecol 2000;183:1124–9
- Greenwood AM, Bradley AK, Byass P, et al. Evaluation of a primary health care programme in The Gambia. I. The impact of trained traditional birth attendants on the outcome of pregnancy. J Trop Med Hyg 1990;93:58–66
- McClure EM, Goldenberg RL, Bann CM. Maternal mortality, stillbirth and measures of obstetric care in developing and developed countries. Int J Gynaecol Obstet 2007;96:139–46
- Yakoob MY, Ali MA, Ali MU, et al. The effect of providing skilled birth attendance and emergency obstetric care in preventing stillbirths. BMC Public Health 2011;11:1–8
- Darmstadt GL, Lee AC, Cousens S, et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynaecol Obstet 2009;107:S89–112
- World Health Organization. WHO annual report 2007. Making pregnancy safer. 2007. Available from: http://www.who.int/making_pregnancy_safer/documents/report_2007/en/index.html [last accessed 2 Jan 2013]
- Hundley VA, Avan BI, Braunholtz D, Graham WJ. Are birth kits a good idea? A systematic review of the evidence. Midwifery 2011;28:204–15
- Jokhio AH, Winter HR, Cheng KK. An intervention involving traditional birth attendants and perinatal and maternal mortality in Pakistan. N Engl J Med 2005;352:2091–9
- Garner P, Lai D, Baea M, et al. Avoiding neonatal death: an intervention study of umbilical cord care. J Trop Pediatr 1994;40:24–8
- Bhutta Z, Darmstadt G, Haws R, et al. Delivering interventions to reduce the global burden of stillbirths: improving service supply and community demand. BMC Pregnancy Childbirth 2009;9:S7
- Winani S, Wood S, Coffey P, et al. Use of a clean delivery kit and factors associated with cord infection and puerperal sepsis in Mwanza, Tanzania. J Midwifery Womens Health 2007;52:37–43
- Meegan ME, Conroy RM, Lengeny SO, et al. Effect on neonatal tetanus mortality after a culturally-based health promotion programme. Lancet 2001;358:640–1
- Kapoor SK, Reddaiah VP, Lobo J. Control of tetanus neonatorum in a rural area. Indian J Pediatr 1991;58:341–4
- Hundley VA, Avan BI, Braunholtz D, et al. Lessons regarding the use of birth kits in low resource countries. Midwifery 2011;27:e222–30
- Seward N, Osrin D, Li L, et al. Association between clean delivery kit use, clean delivery practices, and neonatal survival: pooled analysis of data from three sites in South Asia. PLoS Med 2012;9:e1001180
- Lavender T, Hart A, Smyth RM. Effect of partogram use on outcomes for women in spontaneous labor at term. Cochrane Database Syst Rev 2008;4:Issue 4. Art. No. CD005461
- Javed I, Bhutta S, Shoaib T. Role of partogram in preventing prolonged labor. J Pak Med Assoc 2007;57:408–11
- AbouZahr C. Global burden of maternal death and disability. Br Med Bull 2003;67:1
- Abalos E. Active versus expectant management of the third stage of labor: RHL commentary (last revised: 2 March 2009). The WHO Reproductive Health Library; Geneva: World Health Organization; 2009
- Gulmezoglu AM, Lumbiganon P, Landoulsi S, et al. Active management of the third stage of labor with and without controlled cord traction: a randomised, controlled, non-inferiority trial. Lancet 2012;379:1721–7
- Baqui AH, Ahmed S, El Arifeen S, et al. Effect of timing of first postnatal care home visit on neonatal mortality in Bangladesh: a observational cohort study. BMJ 2009;339:b2826
- Bhutta ZA, Yakoob MY, Lawn JE, et al. Stillbirths: what difference can we make and at what cost? Lancet 2011;377:1523–38
- Lumbiganon P, Laopaiboon M, Gulmezoglu AM, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007–08. Lancet 2010;375:490–9
- Gülmezoglu AM, Crowther CA, Middleton P. Induction of labor for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2006;4:Issue 4. Art. No. CD004945
- Hussain AA, Yakoob MY, Imdad A, Bhutta Z. Elective induction for pregnancies at or beyond 41 weeks of gestation and its impact on stillbirths: a systematic review with meta-analysis. BMC Public Health 2011;11:S5
- Janni W, Schiessl B, Peschers U, et al. The prognostic impact of a prolonged second stage of labor on maternal and fetal outcome. Acta Obstet Gynecol Scand 2002;81:214–21
- Roberts CL, Algert CS, Carnegie M, Peat B. Operative delivery during labor: trends and predictive factors. Paediatr Perinat Epidemiol 2002;16:115–23
- Johanson RB, Menon V. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev 1999;2:Issue 2. Art. No. CD000224
- O'Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev 2010;11:Issue 11. Art. No. CD005455
- Koblinsky MA, Campbell O. Factors affecting the reduction of maternal mortality. In: Koblinsky MA, ed. Reducing maternal mortality, learning from Bolivia, China, Egypt, Honduras, Indonesia, Jamaica, and Zimbabwe. Washington, DC: World Bank; 2003
- Samai O, Sengeh P. Facilitating emergency obstetric care through transportation and communication, Bo, Sierra Leone. The Bo PMM Team. Int J Gynaecol Obstet 1997;59:S157–64
- Babinard J, Roberts P. Maternal and child mortality development goals: what can the transport sector do? The World Bank Group (Transport papers). 2006. Available from: http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTTRANSPORT/EXTTSR/0, contentMDK:20835940∼menuPK:2274762∼pagePK:210058∼piPK:210062∼theSitePK:463716,00.html [last accessed 17 Nov 2012]
- De Caunes F, Alexander GR, Berchel C, et al. The Guadeloupean perinatal mortality audit: process, results, and implications. Am J Prev Med 1990;6:339–45
- Hawthorne G, Robson S, Ryall EA, et al. Prospective population based survey of outcome of pregnancy in diabetic women: results of the Northern Diabetic Pregnancy Audit, 1994. BMJ 1997;315:279–81
- Krue S, Linnet KM, Holmskov A, Nielsen JP. Perinatal audit i Viborg Amt 1994–1996. Ugeskr Laeger 1999;161:31–3
- Pattinson R, Kerber K, Waiswa P, et al. Perinatal mortality audit: counting, accountability, and overcoming challenges in scaling up in low-and middle-income countries. Int J Gynaecol Obstet 2009;107:S113–22
- Jamison DT, Richard GF, Malegapuru WM, et al. Disease and mortality in sub-Saharan Africa. Geneva: World Bank Publications; 2006
- Alder J, Fink N, Bitzer J, et al. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Maternal-Fetal Neonatal Med 2007;20:189–209
- Hoffbrand S, Howard L, Crawley H. Antidepressant treatment for post-natal depression. Cochrane Database Syst Rev 2001;2:Issue 1. Art. No. CD002018
- Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev 2007;3:Issue 3. Art. No. CD006309
- Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008;372:902–9
- Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ 2011;89:607–15
- Gartner LM, Morton J, Lawrence RA, et al. American Academy of Pediatrics Section on B. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506
- Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the US Preventive Services Task Force. Ann Intern Med 2008;149:565–82
- Morrow AL, Guerrero ML, Shults J, et al. Efficacy of home-based peer counselling to promote exclusive breastfeeding: a randomised controlled trial. Lancet 1999;353:1226–31
- Lauer JA, Betran AP, Barros AJD, De Onis M. Deaths and years of life lost due to suboptimal breast-feeding among children in the developing world: a global ecological risk assessment. Public Health Nutr 2006;9:673–85
- Imdad A, Yakoob MY, Bhutta Z. Effect of breastfeeding promotion interventions on breastfeeding rates, with special focus on developing countries. BMC Public Health 2011;11:S24
- Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev 2010;3:Issue 3. Art. No. CD004015
- Edmond KM, Zandoh C, Quigley MA, et al. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics 2006;117:e380–6
- Mullany LC, Katz J, Li YM, et al. Breast-feeding patterns, time to initiation, and mortality risk among newborns in southern Nepal. J Nutr 2008;138:599–603
- Van der Wijden C, Kleijnen J, Van den Berk T. Lactational amenorrhea for family planning. Cochrane Database Syst Rev 2003;4:Issue 4. Art. No. CD001329
- Bracher M. Breastfeeding, lactational infecundity, contraception and the spacing of births: implications of the Bellagio Consensus Statement. Health Transition Rev 1992;2:19–47
- Hardy E, Santos LC, Osis MJ, et al. Contraceptive use and pregnancy before and after introducing lactational amenorrhea (LAM) in a postpartum program. Adv Contraception 1998;14:59–68
- Laukaran VH, Labbok MH. The lactational amenorrhoea method re-examined: a response to Bracher's simulation models. Health Transition Rev 1993;3:97–100
- Kennedy KI, Rivera R, McNeilly AS, et al. Rejoinder to Trussell and Santow. Health Transition Rev 1991;1:107–10
- Labbok M. Rejoinder to Trussell and Santow. Health Transition Rev 1991;1:111–14
- Kennedy KI, Kotelchuck M, Visness CM, et al. Users' understanding of the lactational amenorrhea method and the occurrence of pregnancy. J Hum Lactation 1998;14:209–18
- Potter RG, Masnick GS, Gendell M. Postamenorrheic versus postpartum strategies of contraception. Demography 1973;10:99–112
- Trussell J. Methodological pitfalls in the analysis of contraceptive failure. Stat Med 1991;10:201–20
- Bhutta ZA, Ahmed T, Black RE, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40
- Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2004:Issue 1. Art. No. CD003517
- Gao YT, Shu XO, Dai Q, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer 2000;87:295–300
- Moller T, Olsson H, Ranstam J. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187–95
- Crepinsek MA, Crowe L, Michener K, Smart NA. Interventions for preventing mastitis after childbirth. Cochrane Database Syst Rev 2010;8:Issue 8. Art. No. CD007239
- Blencowe H, Howson M, Kinney JL. March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61
- Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol 2006;35:706–18
- Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev 2010;5:Issue 5. Art. No. CD003519
- Blencowe H, Lawn J, Vandelaer J, et al. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol 2010;39:i102–9
- Conde-Agudelo A, Belizãn JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011;3:Issue 3. Art. No. CD002771
- Bhutta ZA, Ali S, Cousens S, et al. Alma-Ata: rebirth and revision 6 interventions to address maternal, newborn, and child survival: what difference can integrated primary health care strategies make? Lancet 2008;372:972–89
- Kidney E, Winter H, Khan K, et al. Systematic review of effect of community-level interventions to reduce maternal mortality. BMC Pregnancy Childbirth 2009;9:2
- Lassi ZS, Haider BA, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database Syst Rev 2010;11:Issue 11. Art. No. CD007754
- Lee ACC, Cousens S, Darmstadt GL, et al. Care during labor and birth for the prevention of intrapartum-related neonatal deaths: a systematic review and Delphi estimation of mortality effect. BMC Public Health 2011;11:S10
- Kumar R. Effectiveness of training traditional birth attendants for management of asphyxia neonatorum using resuscitation equipment. Prenat Neonatal Med 1998;3:255–60
- Zhu XY, Fang HQ, Zeng SP, et al. The impact of the neonatal resuscitation program guidelines (NRPG) on the neonatal mortality in a hospital in Zhuhai, China. Singapore Med J 1997;38:485–7
- Deorari AK, Paul VK, Singh M, Vidyasagar D. News from the regions-newsletter from India. The national movement of neonatal resuscitation in India. J Trop Pediatr. 2000;46:315–17
- Bashour HN, Kharouf MH, Abdulsalam AA, et al. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nurs 2008;25:115–25
- Freedman RM, Ingram DL, Gross I, et al. A half century of neonatal sepsis at Yale: 1928 to 1978. Arch Pediatr Adolesc Med 1981;135:140
- Gladstone IM, Ehrenkranz RA, Edberg SC, Baltimore RS. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr Infect Dis J 1990;9:819–25
- Mtitimila EI, Cooke RW. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database Syst Rev 2004;4:Issue 4. Art. No. CD004495
- Gordon A, Jeffery HE. Antibiotic regimens for suspected late onset sepsis in newborn infants. Cochrane Database Syst Rev 2005;3:Issue 3. Art. No. CD004501
- Zaidi AKM, Saeed MA, Bhutta ZA, Thaver D. Community based management of neonatal sepsis in developing countries. Cochrane Database Syst Rev 2009:Issue 1. Art. No. CD007646
- Zaidi AKM, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J 2012;31:667–72
- Horton R. Maternal and child undernutrition: an urgent opportunity. Lancet 2008;371:179