Abstract
Objective: To prospectively determine the prognostic value of maternal plasma concentrations of placental growth factor (PlGF), soluble endoglin (sEng) and soluble vascular endothelial growth factor receptors-1 and -2 (sVEGFR-1 and -2) in identifying patients with suspected preeclampsia (PE), who require preterm delivery (PTD) or develop adverse outcomes.
Study design: This prospective cohort study included 85 consecutive patients who presented to the obstetrical triage area at 20–36 weeks with a diagnosis of “rule out PE.” Patients were classified as: 1) those who remained stable until term (n = 37); and 2) those who developed severe PE and required PTD (n = 48). Plasma concentrations of PlGF, sEng and sVEGFR-1 and -2 were determined by ELISA.
Results: Patients with PlGF/sVEGFR-1 ≤0.05 multiples of the median (MoM) or PlGF/sEng ≤0.07 MoM were more likely to deliver preterm due to PE [adjusted odd ratio (aOR) 7.4 and 8.8], and to develop maternal (aOR 3.7 and 2.4) or neonatal complications (aOR 10.0 and 10.1). Among patients who presented <34 weeks of gestation, PlGF/sVEGFR-1 ≤ 0.035 MoM or PlGF/sEng ≤0.05 MoM had a sensitivity of 89% (16/18), specificity of 96% (24/25) and likelihood ratio for a positive test of 22 to identify patients who delivered within 2 weeks. The addition of the PlGF/sVEGFR-1 ratio to standard clinical tests improved the sensitivity at a fixed false-positive rate of 3% (p = 0.004) for the identification of patients who were delivered due to PE within 2 weeks. Among patients who had a plasma concentration of PlGF/sVEGFR-1 ratio ≤0.035 MoM, 0.036-0.34 MoM and ≥0.35 MoM, the rates of PTD <34 weeks were 94%, 27% and 7%, respectively.
Conclusions: The determination of angiogenic/anti-angiogenic factors has prognostic value in patients presenting to the obstetrical triage area with suspected PE for the identification of those requiring preterm delivery and at risk for adverse maternal/neonatal outcomes.
Introduction
Preeclampsia (PE), one of the “great obstetrical syndromes” [Citation1–4], is characterized by systemic intravascular inflammation [Citation5–7], endothelial cell dysfunction [Citation8–11], excessive thrombin generation [Citation12–18], an anti-angiogenic state [Citation19–40] and is often associated with multiple organ involvement [Citation41–60]. However, PE is fundamentally a placental disease [Citation37,Citation61–63] which manifests itself, in most cases, by involvement of the vascular (i.e. hypertension) and renal systems (i.e. proteinuria). The diagnosis of PE typically requires the presence of hypertension and proteinuria [Citation64]. Sometimes, patients who are subsequently diagnosed with PE present initially with either hypertension [Citation65–68] or proteinuria [Citation69–73], and may have some signs or symptoms indicating yet, they do not meet the full diagnostic criteria for PE [Citation74]. Indeed, even in some cases of HELLP syndrome, patients present without hypertension and proteinuria, and only develop these two abnormalities if pregnancy is allowed to continue [Citation43,Citation75]. In addition, when PE involves organs other than the vascular system and the kidney, it becomes the “great imitator” [Citation76]. Such cases are frequently misdiagnosed and initially thought to have disorders unrelated to pregnancy. Hence, variability in clinical presentation presents a management challenge.
While approximately 1 in 10 pregnant women exhibit some signs and/or symptoms associated with PE, only 20–25% of these patients will eventually be diagnosed as having the disease [Citation77–79]. Women with signs and/or symptoms associated with PE (i.e. elevated blood pressure, headache, abdominal pain, edema, etc.) are frequently referred to an obstetrical triage area for assessment of multiple maternal organ systems and fetal involvement [Citation80,Citation81]. The standard work-up includes blood pressure determination, urine analysis for protein, a platelet count, determination of liver enzymes in peripheral blood, and a blood smear to detect schistocytes [Citation64,Citation79]. The prognostic performance of these tests in determining which patients will develop PE, require preterm delivery (PTD), or have maternal and/or neonatal morbidity is poor [Citation82–97]. Consequently, many patients with signs and/or symptoms associated with PE are hospitalized for observation. Those diagnosed with PE in preterm gestation often undergo long-term hospitalization or frequent monitoring as outpatients [Citation98,Citation99]. Thus, the lack of adequate biomarkers to predict disease progression and adverse outcomes in patients with suspected PE [Citation82,Citation84,Citation100] results in substantial financial burden to the health care system (i.e. frequent visits, hospitalizations, intensive laboratory surveillance, serial ultrasound examination and antepartum testing) [Citation101–105].
To address the need for biomarkers with high predictive value, we previously reported the results of a retrospective study which indicated that plasma concentrations of angiogenic/anti-angiogenic factors [placental growth factor (PlGF), soluble vascular endothelial growth factor receptors (sVEGFR) -1, soluble endoglin (sEng)] have prognostic value in patients with “suspected PE” at preterm gestations who present to the obstetrical triage area [Citation106]. We found that, based on the results of these analytes, it is possible to stratify patients into those at high, moderate and low risk of requiring PTD within 2 weeks [Citation106].
The purpose of this study was to determine whether the results of the retrospective study could be replicated prospectively. Namely, to examine whether maternal plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in identifying patients presenting to the obstetrical triage area with the diagnosis “rule out PE” and at risk for PTD due to PE or develop other complications within 2 weeks of presentation.
Materials and methods
Study design
This prospective cohort study included singleton pregnancies who presented to the obstetrical triage unit of Hutzel Women’s Hospital, Detroit, MI, for the diagnosis of “suspected PE” from July 2010 to March 2011. Inclusion criteria were: (1) singleton pregnancy; (2) no known major fetal anomaly; (3) gestational age 20–36 6/7 weeks; and (4) signs/symptoms of PE (elevated blood pressure, proteinuria, headache, blurred vision, epigastric or right upper quadrant pain and edema). All participants provided written informed consent and donated a blood sample for research purposes.
Based on our prior study [Citation106], we estimated that approximately 40% of patients presenting with suspected PE prior to 37 weeks of gestation would have an abnormal biomarker ratio using thresholds selected based on ROC curve analysis. Accordingly, we estimated that to have 80% power to detect a 2-fold difference in the rate of preterm delivery due to severe PE (between patients with abnormal biomarker ratio compared to those with normal biomarker ratio) with a 5% limit on Type I error probability, 85 patients would be required. Considering patients to be excluded for loss to follow-up (10%), we aimed to enroll 93 patients into this study.
Demographic data, medical, surgical and obstetrical history were recorded. Delivery outcomes were reviewed and used to classify patients into two groups: patients who remained stable until delivery at ≥37 weeks (Group I) and patients who developed severe PE requiring PTD (Group II). Maternal and neonatal complications within two weeks after presentation to the triage area were recorded as well. The collection and use of samples for research purposes was conducted under protocols approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS).
Clinical definitions
PE was defined as the new onset of hypertension and proteinuria that developed after 20 weeks of gestation. Hypertension was defined as systolic ≥140 or diastolic blood pressure ≥90 mm Hg, measured on two occasions, 4 h to 1 week apart. Proteinuria was defined as a urine protein of ≥300 mg in a 24-h urine collection, or two random urine specimens obtained 4 h to 1 week apart containing ≥1+ by dipstick [Citation64].
“Maternal morbidity” was considered to be present if one or more of the following complications was observed: (1) eclampsia; (2) Hemolysis elevated liver enzymes low platelet count syndrome; (3) pulmonary edema; (4) oliguria (<400 mL/day); (5) renal insufficiency (creatinine > 1.2 mg/dL); (6) abruptio placentae (defined as clinical vaginal bleeding and uterine tenderness with or without placental pathologic findings); (7) elevated liver enzymes or thrombocytopenia; (8) antepartum admission to the maternal intensive care unit (MICU); or (9) fetal death. “Adverse neonatal outcome” was defined as one or more of the following complications: (1) respiratory distress syndrome [Citation107,Citation108]; (2) intraventricular hemorrhage [Citation109]; (3) bronchopulmonary dysplasia [Citation110]; or (4) neonatal death.
Patients were managed by attending physicians blinded to the results of the analytes under study. The approach in our institution is that patients with mild PE diagnosed prior to 37 weeks of gestation are managed expectantly, – with weekly platelet counts and liver function tests in addition to non-stress tests. Patients diagnosed with severe PE are hospitalized to assess maternal/fetal conditions and the need for delivery. Individually, patients are managed at the discretion of the attending physician.
Sample collection and immunoassays
Venipuncture was performed and blood was collected into tubes containing EDTA. Samples were centrifuged and stored at −70 °C. Maternal plasma concentrations of PlGF, sVEGFR-1, sEng and sVEGFR-2 were determined by sensitive and specific immunoassays (R&D Systems, Minneapolis, MN) as previously described [Citation106]. The inter- and intra-assay coefficients of variation (CV) obtained were as follows: PlGF, 6.0 and 4.8%, respectively; sEng, 2.3 and 4.6%, respectively; sVEGFR-1, 1.4 and 3.9%, respectively; and sVEGFR-2, 2.0 and 4.0%, respectively. The sensitivity of the assays were as follows: PlGF, 9.52 pg/ml; sVEGFR-1, 16.97 pg/ml; sEng, 0.08 ng/ml; and sVEGFR-2, 19.01 pg/ml.
Statistical analysis
Normality of data was assessed by inspection of histograms and the Kolmogorov–Smirnov test. Continuous variables were compared using the Mann-Whitney U test or unpaired Student's t-test depending upon the distribution of the data. Contingency tables and χ2 tests were used to compare proportions. Methods used to construct maternal plasma angiogenic/anti-angiogenic factors and ratio concentration multiples of the median (MoM) have been previously reported [Citation106]. Briefly, quantile regression was used to calculate the median concentration of a particular analyte and the ratio conditional upon gestational age in 180 participants in a longitudinal study at Hutzel Women’s Hospital who had uncomplicated pregnancies and delivered at term. MoMs were calculated by dividing the observed analyte concentrations by the previously determined expected median ratio conditional on gestational age. Dichotomization thresholds were selected in a prior study [Citation106] based on inspection of receiver operating characteristic (ROC) curves.
Logistic regression was used to assess the magnitude of association between biomarkers and selected pregnancy outcomes. Multiple logistic regression models were constructed to account for potentially confounding factors selected based on clinical information (gestational age at presentation, average mean arterial blood pressure, chronic hypertension, combined parity and/or history of PE, and tobacco use). Potentially confounding factors were entered with respect to time-order into a full model. Model reduction was performed based on the plausibility of regression coefficients, association between independent and dependent variables, and the magnitude of change in the main effect parameter estimates; independent variables whose relationship with the dependent variable yielded a p-value >0.10 were excluded from the final models. Diagnostic performance metrics were calculated and correlated sample non-parametric statistical techniques were used to compare area under the ROC curves (AUC) [Citation111]. Differences in sensitivity at a fixed false positive rate were tested using McNemar’s tests, and exact p-values are reported.
Survival analysis and Cox proportional hazard models were utilized to examine the relationship between sampling-to-delivery interval and biomarker MoMs, adjusting for the above-mentioned potential confounders. Patients who delivered preterm due to causes other than PE had the interval between triage and delivery treated as a censored observation. Analysis was conducted with SPSS V.15 (SPSS Inc., Chicago, IL) and SAS version 9·3 (Carry, NC, U.S.A). A p value <0.05 was considered significant.
Results
During the study period, 122 visits to the obstetrical triage unit for the diagnosis of “suspected PE” met the inclusion criteria. After exclusion of repeated visits (n = 19), those who were lost to follow up (n = 8), and those who declined to participate (n = 10), 85 patients met the inclusion criteria. Among them, 37 remained stable until term (Group I) and 48 developed severe PE requiring PTD (Group II).
Demographic, clinical characteristics, pregnancy outcome and plasma concentrations of angiogenic/anti-angiogenic factors
Demographic/clinical characteristics, maternal morbidity and adverse neonatal outcomes are presented by group in . Patients who developed severe PE and subsequently required a PTD had a higher mean systolic and diastolic blood pressure and a higher frequency of maternal morbidity and/or adverse neonatal outcomes than those who delivered at term (each p < 0.001).
Table 1. Demographic/clinical characteristics, maternal morbidity and adverse neonatal outcome by study groups.
The median MoM plasma concentrations of angiogenic/anti-angiogenic factors differed significantly by patient groups (each p < 0.005; ). A plasma concentration of PlGF/sVEGFR-1 of ≤ 0.05 MoM and that of PlGF/sEng ≤0.07 MoM had the highest likelihood ratio for a positive test (8.2). In contrast, a plasma concentration of PlGF ≤0.4 MoM had the lowest likelihood ratio for a negative test, 0.13 for the identification of patients who developed severe PE requiring a PTD ().
Figure 1. Median maternal plasma Multiple of Median (MoM) concentrations of soluble vacular endothelial growth factor receptor (sVEGFR)-1, soluble endoglin (sEng), sVEGFR-2, placental growth factor (PlGF), PlGF/sVEGFR-1 ratio and PlGF/sEng ratio in patients who subsequently developed severe PE requiring preterm delivery and those who remained stable until term [sVEGFR-1: median 4.46 MoM, interquartile range (IQR) 2.5–9.8 MoM vs. 1.5 MoM, IQR 0.9–2.4 MoM; p < 0.001; sEng: median 3.3 MoM, IQR 2.1–5.6 MoM vs. 1.2 MoM, IQR 0.9–1.7 MoM; p < 0.001; sVEGFR-2: median 0.69 MoM, IQR 0.5–0.9 MoM vs. 0.9 MoM, IQR 0.7–1.1 MoM; p < 0.001; PlGF: median 0.15 MoM, IQR 0.08–0.23 MoM vs. 0.47 MoM, IQR 0.3–1.1 MoM; p < 0.001; PlGF/sVEGFR-1: median 0.03 MoM, IQR 0.008–0.07 MoM vs. 0.4 MoM, IQR 0.2-0.9 MoM; p < 0.001; PlGF/sEng: median 0.04 MoM, IQR 0.01–0.09 MoM vs. 0.4 MoM, IQR 0.2–0.9 MoM; p < 0.001].
![Figure 1. Median maternal plasma Multiple of Median (MoM) concentrations of soluble vacular endothelial growth factor receptor (sVEGFR)-1, soluble endoglin (sEng), sVEGFR-2, placental growth factor (PlGF), PlGF/sVEGFR-1 ratio and PlGF/sEng ratio in patients who subsequently developed severe PE requiring preterm delivery and those who remained stable until term [sVEGFR-1: median 4.46 MoM, interquartile range (IQR) 2.5–9.8 MoM vs. 1.5 MoM, IQR 0.9–2.4 MoM; p < 0.001; sEng: median 3.3 MoM, IQR 2.1–5.6 MoM vs. 1.2 MoM, IQR 0.9–1.7 MoM; p < 0.001; sVEGFR-2: median 0.69 MoM, IQR 0.5–0.9 MoM vs. 0.9 MoM, IQR 0.7–1.1 MoM; p < 0.001; PlGF: median 0.15 MoM, IQR 0.08–0.23 MoM vs. 0.47 MoM, IQR 0.3–1.1 MoM; p < 0.001; PlGF/sVEGFR-1: median 0.03 MoM, IQR 0.008–0.07 MoM vs. 0.4 MoM, IQR 0.2-0.9 MoM; p < 0.001; PlGF/sEng: median 0.04 MoM, IQR 0.01–0.09 MoM vs. 0.4 MoM, IQR 0.2–0.9 MoM; p < 0.001].](/cms/asset/cdc14140-34ab-4fed-8387-5fc8ee5f6441/ijmf_a_806905_f0001_b.jpg)
Table 2. Diagnostic performance of plasma concentrations of angiogenic/anti-angiogenic factors for the identification of patients who subsequently developed severe preeclampsia requiring preterm delivery.
Plasma concentrations of PlGF/sVEGFR-1 ≤0.05 MoM and PlGF/sEng ≤0.07 MoM were significantly associated with indicated PTD due to PE, maternal morbidity, composite adverse neonatal outcome, and composite maternal morbidity and/or neonatal outcome after adjustment for potential confounders (). The ratio of angiogenic/anti-angiogenic factors performed better than the mean arterial pressure or laboratory tests in identifying patients who required delivery within 2 weeks when the analysis was restricted to patients who presented prior to 34 weeks of gestation (; n = 43) compared to analysis of the full cohort (; n = 85). Therefore, subsequent analysis was restricted to patients who presented before 34 weeks of gestation.
Figure 2. Area under the Receiver-Operating Characteristic (ROC) curves for angiogenic/anti-angiogenic factor ratio Multiples of the Median (MoM) compared to mean arterial pressure and combined standard tests (mean arterial pressure, serum creatinine, serum uric acid, aspartate aminotransferase, platelet count) for the identification of patients who delivered within 2 weeks (A) Restricted to patients who presented before 34 weeks of gestation; and (B) Included all patients in the cohort.
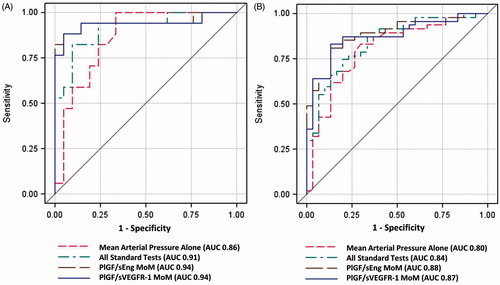
Table 3. Likelihood (unadjusted and adjusted) of each outcome according to angiogenic/antiangiogenic factors ratio.
Findings restricted to patients presenting prior to 34 weeks of gestation
Among patients who presented to the obstetrical triage area before 34 weeks, 18 (42%) delivered due to PE and 13 (30.2%) developed adverse maternal/neonatal outcomes within 2 weeks (). A ratio of PlGF/sVEGFR-1 ≤0.035 MoM and a ratio of PlGF/sEng of ≤0.05 MoM achieved a sensitivity of 89% (16/18), a specificity of 96% (24/25) and a likelihood ratio for a positive and negative test of 22 and 0.12, respectively, in identifying patients who required delivery within 2 weeks (). displays the magnitude of association between biomarker MoMs and selected outcomes. The ratios of PlGF/sVEGFR-1 ≤0.035 MoM and PlGF/sEng ≤0.05 MoM were significantly associated with indicated PTD within 2 weeks (aOR 53 and 58, respectively) and composite maternal morbidity and/or adverse neonatal outcome within 2 weeks (aOR 19.5) after adjustment for potential confounders.
Table 4. Demographic/clinical characteristics and adverse maternal and neonatal outcomes of the patients who presented to the triage area at <34 weeks according to interval to delivery within and more than 2 weeks.
Table 5. Diagnostic performance of plasma concentrations of angiogenic/anti-angiogenic factors among patients who presented at <34 weeks of gestation for the identification of patients who subsequently delivered within 2 weeks.
Table 6. Likelihood (unadjusted and adjusted) of selected pregnancy outcomes according to angiogenic/antiangiogenic factor ratio in patients who presented to the triage area at <34 weeks of gestation.
The biomarkers under study achieved a greater AUC (0.94) compared to combined standard clinical and laboratory tests (AUC 0.91) for the identification of patients who required PTD within 2 weeks. displays the sensitivity for the identification of patients who required delivery within 2 weeks or those who developed composite adverse maternal and/or neonatal outcomes within 2 weeks by pre-specified false-positive rate (3%, 5% and 10%) for biomarker MoMs, blood pressure and standard laboratory tests for PE. The addition of the PlGF/sVEGFR-1 ratio to standard tests significantly improved the sensitivity at a fixed false-positive rate of 3% (p = 0.004) and marginally improved sensitivity at a fixed false-positive rate of 5% (p = 0.06) for the identification of patients who would be delivered due to PE within 2 weeks (). Therefore, angiogenic/anti-angiogenic factor MoMs outperformed the standard tests used in the triage area for the prediction of these adverse outcomes, given that they had a higher sensitivity than standard laboratory tests.
Figure 3. Addition of the PlGF/sVEGFR-1 ratio to standard tests significantly improved the sensitivity at a fixed false-positive rate of 3% (p = 0.004) and marginally improved sensitivity at a fixed false-positive rate of 5% (p = 0.06) for the identification of patients who would be delivered due to preeclampsia within 2 weeks.
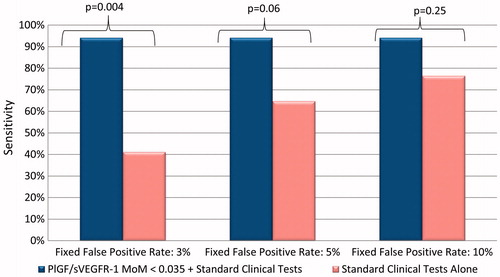
Table 7. Sensitivities of each outcome according to angiogenic/antiangiogenic factors ratio in patients who presented to the triage area at <34 weeks of gestation while fixing different false positive rates (FP).
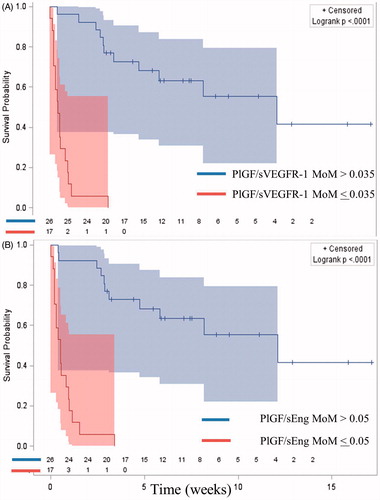
Time-to-event analysis confirmed that a low maternal PlGF/sVEGFR-1 ratio (≤0.035 MoM) or PlGF/sEng ratio (≤0.05 MoM) was associated with a shorter interval-to-delivery after adjusting for potential confounders – gestational age at presentation, average mean arterial blood pressure, chronic hypertension, combined parity and history of PE, and tobacco use [hazard ratio = 25 (95% CI 6.4–104.0) and 29.4 (95% CI 6.9–125.1); respectively; ].
Figure 4. Kaplan–Meier survival curves by angiogenic/anti-angiogenic ratio Multiple of Median (MoM) cut-offs of patients who delivered prior to 37 weeks of gestation (right censored). Patients who delivered preterm due to causes other than PE had the interval between blood draw and delivery treated as a censored observation. (A = PlGF/sVEGFR-1; B = PlGF/sEng).
displays the disposition (hospitalization versus discharge home) of the patient after visiting the triage area, rate of PTD and adverse perinatal outcomes according to the previously proposed grading of severity criteria based on plasma MoM concentrations of the PlGF/sVEGFR-1 ratio and the PlGF/sEng ratio [Citation106]. The cut-off values for patients at high-risk were based on ROC curves for the identification of patients who delivered within 2 weeks and those for patients at low-risk were based on ROC curves for the identification of patients who remained stable until term [Citation106]. Almost 40% of patients presenting prior to 34 weeks had analyte ratio MoM concentrations classified as Zone 3 (high-risk), while 35–42% were characterized as Zone 1 (low-risk) and 20–25% were considered Zone 2 (moderate-risk). Thirty-three (77%) patients who presented to the triage area with “suspected PE” at less than 34 weeks were hospitalized. Among them, one-third (11/33) were classified as Zone 1 (low-risk). Nine of these eleven patients did not have hypertension in the severe range, and five complained of headache in the triage area. A single patient within Zone 1 (6.7%) delivered within 2 weeks and had an abnormal liver function test. This patient had chronic hypertension and was referred to the triage area at 31 4/7 weeks because of an elevated blood pressure (161/86 mmHg).
Table 8. Disposition of the patient after visiting the triage area (hospitalization versus discharge home), rate of preterm delivery and adverse perinatal outcomes according to the previously proposed grading of severity criteria based on plasma MoM concentrations of the PlGF/sVEGFR-1 ratio and the PlGF/sEng ratio.
In contrast, among patients classified as high-risk (Zone 3) by either a PlGF/sVEGFR-1 ratio ≤0.035 MoM or a PlGF/sEng ratio ≤0.05 MoM, the rate of PTD before 34 weeks was 94% (16/17) and 100% (17/17), respectively; the rate of PTD within 2 weeks was 94% (16/17) for each ratio, and the rate of composite adverse maternal and/or neonatal outcome within 2 weeks of visiting the triage area was 70% (12/17) for each ratio ().
Discussion
Principal findings of the study
1) Patients who presented to the obstetrical triage area with “suspected PE” and subsequently required a PTD had a significantly lower mean plasma MoM concentration of angiogenic/anti-angiogenic ratio than those who remained stable until term; 2) low plasma concentrations of the PlGF/sVEGFR-1 ratio (≤0.05 MoM) or the PlGF/sEng ratio (≤0.07 MoM) were significantly associated with indicated PTD and composite maternal morbidity and/or adverse neonatal outcome in patients presenting at <37 weeks of gestation; however, the ratio of angiogenic/anti-angiogenic factors performed better than standard laboratory tests in identifying patients who required delivery within 2 weeks when the analysis was restricted to patients who presented prior to 34 weeks of gestation; and 3) a PlGF/sVEGFR-1 ratio ≤0.035 MoM and a PlGF/sEng ratio ≤0.05 MoM were significantly associated with composite maternal morbidity and/or adverse neonatal outcome, and a shorter interval-to-delivery than those above these cutoffs among patients who presented <34 weeks of gestation.
Meaning of the findings
The results of this study add further support to the concept that it is possible to classify patients presenting to the obstetrical triage area before 34 weeks of gestation with a suspected diagnosis of PE, based upon the results of PlGF, sVEGFR-1 and sEng [Citation106,Citation112]. We had previously proposed a three-tier classification of patients based on the results of these analytes and their ratios [Citation106]. This classification yielded encouraging results, but needed replication. This is important because data-driven techniques for prediction generally perform well on the data set used for the development of a particular algorithm, but they generally underperform when applied to an independent sample. This is a key clinical challenge because algorithms are developed for application to new patients, not in patients from whom the models were derived [Citation113]. The current study was designed to test the value of the proposed classification system constructed based on retrospective data. This method classified patients into three groups: those at high, moderate and low risk for indicated PTD and/or adverse maternal or neonatal outcome.
The results of the current study independently confirm that our classification system has prognostic value in identifying patients at risk for PTD or adverse maternal/neonatal outcome. Among patients presenting prior to 34 weeks of gestation, those classified as “high risk” (Zone 3) had a 94% probability of PTD and 70% developed maternal and/or neonatal complications within 2 weeks. On the other hand, patients classified as “low risk” (Zone 1) were unlikely to deliver within 2 weeks (6.7%) or develop maternal and/or neonatal complications (6.7%). We propose that such patients can be discharged home after the managing physicians have performed a thorough evaluation and considered the likelihood of compliance with a regimen for monitoring and follow-up. For example, in this cohort, 9 of 11 patients who presented to the triage area before 34 weeks of gestation had maternal plasma concentrations of angiogenic/anti-angiogenic factors in Zone 1, and did not have blood pressure in the severe range or abnormal laboratory tests, but were hospitalized. All delivered more than 2 weeks (range 20–111 days) after the initial presentation.
The management of patients at intermediate risk (Zone 2) remains a challenge: 25–27% delivered prior to 34 weeks of gestation, although few (9–12%) developed maternal complications. Additional biomarkers will be required to improve the prognostic assessment of these patients. Alternatively, serial testing may prove to be informative.
Recently, a model to predict maternal complications in patients admitted with the diagnosis of PE has been proposed [Citation114]. The current study had a different objective since we aimed to identify patients who required PTD and those who developed maternal and/or neonatal complications utilizing information in the triage area prior to admission.
Strengths and limitations of the study
The strengths of this study are its prospective nature and the independent replication of the previous findings [Citation106]. Other than our first retrospective study of angiogenic/anti-angiogenic factors in the obstetrical triage area [Citation106], three studies reported an association between maternal plasma angiogenic/anti-angiogenic factor concentrations in women who presented to the obstetrical triage area with suspected PE and adverse maternal and neonatal outcomes [Citation112,Citation115,Citation116]. However, indicated preterm birth was included in the composite maternal and/or neonatal complications in these studies. We reported this outcome separately from other complications since this is an observational study and institutional management policies may influence this outcome (i.e. policy that required delivery of all patients with severe PE at 34 weeks of gestation). Moreover, we have gone beyond reporting an association between the results of biomarker determination and outcome by proposing an algorithm for follow up and management.
The limitations of this study include the lack of statistically significant differences in AUCs observed in this study when comparing the performance of standard clinical parameters and the additional inclusion of angiogenic/anti-angiogenic factors. However, it is important to note that comparisons of the AUCs are insensitive measures of biomarker utility [Citation117]. In other clinical fields (such as cardiovascular disease), the limitations of ROC curves as a tool for model evaluation have been recently highlighted, and the comparison of the AUC is considered an insensitive measure of improvement in model accuracy [Citation117–119]. Second, this study utilized the conventional ELISA assay method rather than a rapid automated assay system whose results could be available for clinical decision-making in the obstetrical triage area. However, previous studies have demonstrated that measures obtained by rapid point-of-care and conventional ELISA assay systems are highly correlated [Citation120–123]. Third, this is an observational study, and further investigation is required to determine whether implementation of management policies based upon the risk assessment would prove to be cost-effective and achieve the clinical goals.
Conclusion
Maternal plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value for patients who present with suspected PE before 34 weeks of gestation. We propose that these biomarkers allow prospective stratification of patients whose illness will progress sufficiently to require a preterm delivery or develop adverse maternal and/or neonatal outcomes.
Declaration of interest
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
Notes
1Presented at the 58th Annual Meeting of the Society for Gynecologic Investigation, March 21–24, 2012, San Diego, CA.
References
- Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol 1988;12:302–23
- Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med 2009;22:633–5
- Romero R. Prenatal medicine: the child is the father of the man. Prenat Neonat Med 1996;1:8–11
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201
- Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–7
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506
- Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol 2004;24:565–70
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:5–15
- Roberts JM. Objective evidence of endothelial dysfunction in preeclampsia. Am J Kidney Dis 1999;33:992–7
- Chaiworapongsa T, Romero R, Yoshimatsu J, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med 2002;12:19–27
- Petrozella L, Mahendroo M, Timmons B, et al. Endothelial microparticles and the antiangiogenic state in preeclampsia and the postpartum period. Am J Obstet Gynecol 2012;207:140–6
- Cunningham FG, Pritchard JA. Hematologic considerations of pregnancy-induced hypertension. Semin Perinatol 1978;2:29–38
- Weenink GH, Treffers PE, Vijn P, et al. Antithrombin III levels in preeclampsia correlate with maternal and fetal morbidity. Am J Obstet Gynecol 1984;148:1092–7
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, et al. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002;11:362–7
- de Boer K, ten Cate JW, Sturk A, et al. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol 1989;160:95–100
- Erez O, Romero R, Kim SS, et al. Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: a mechanism for the intersection of coagulation and inflammation. J Matern Fetal Neonatal Med 2008;21:345–55
- Dekker G. Prothrombotic mechanisms in preeclampsia. Thromb Res 2005;115:17–21
- Erez O, Romero R, Hoppensteadt D, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med 2008;21:855–69
- Ahmed A, Whittle MJ, Khaliq A. Differential expression of placenta growth factor (PlGF) and vascular endothelial growth factor (VEGF) in abnormal placentation. J Soc Gynecol Investig 1997;4:246 A
- Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 2002;160:1405–23
- Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–63
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–58
- Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–51
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83
- Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–7
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004;145:4838–45
- Ahmad S, Ahmed A. Antiangiogenic effect of soluble vascular endothelial growth factor receptor-1 in placental angiogenesis. Endothelium 2005;12:89–95
- Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18
- Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–9
- Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005
- Vatten LJ, Eskild A, Nilsen TI, et al. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol 2007;196:239–6
- Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23
- Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–87
- Stepan H. Angiogenic factors and pre-eclampsia: an early marker is needed. Clin Sci (Lond) 2009;116:231–2
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–38
- Chaiworapongsa T, Romero R, Kusanovic JP, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol 2010;35:155–62
- Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med 2011;39:641–52
- Weed S, Bastek JA, Anton L, et al. Examining the correlation between placental and serum placenta growth factor in preeclampsia. Am J Obstet Gynecol 2012;207:140–6
- Weissgerber TL, Roberts JM, Jeyabalan A, et al. Haptoglobin phenotype, angiogenic factors, and preeclampsia risk. Am J Obstet Gynecol 2012;206:358
- Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med 2012;25:498–507
- Martin JN, Jr, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol 1999;180:1373–84
- Romero R, Mazor M, Lockwood CJ, et al. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am J Perinatol 1989;6:32–8
- Romero R, Vizoso J, Emamian M, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol 1988;5:146–51
- Belfort M, Van VT, White GL, et al. Low maternal middle cerebral artery Doppler resistance indices can predict future development of preeclampsia. Ultrasound Obstet Gynecol 2012;40:406--11
- Kobayashi T, Terao T. Preeclampsia as chronic disseminated intravascular coagulation. Study of two parameters: thrombin-antithrombin III complex and D-dimers. Gynecol Obstet Invest 1987;24:170–8
- Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens 2010;4:68–78
- Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol 2011;23:440–7
- Parrish MR, Laye MR, Wood T, et al. Impedance cardiography facilitates differentiation of severe and superimposed preeclampsia from other hypertensive disorders. Hypertens Pregnancy 2012;31:327--40
- Riskin-Mashiah S, Belfort MA, Saade GR, Herd AJ. Side-to-side differences in transcranial Doppler parameters in normotensive and preeclamptic pregnant women. Am J Obstet Gynecol 2004;190:194–8
- Barton JR, Sibai BM. Gastrointestinal complications of pre-eclampsia. Semin Perinatol 2009;33:179–88
- Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol 1982;142:159–67
- MacKenna J, Dover NL, Brame RG. Preeclampsia associated with hemolysis, elevated liver enzymes, and low platelets–an obstetric emergency? Obstet Gynecol 1983;62:751–4
- Martin JN, Jr, Blake PG, Perry KG, Jr, et al. The natural history of HELLP syndrome: patterns of disease progression and regression. Am J Obstet Gynecol 1991;164:1500–9
- de Boer K, Buller HR, ten Cate JW, Treffers PE. Coagulation studies in the syndrome of haemolysis, elevated liver enzymes and low platelets. Br J Obstet Gynaecol 1991;98:42–7
- Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–8
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500
- Oehm E, Hetzel A, Els T, et al. Cerebral hemodynamics and autoregulation in reversible posterior leukoencephalopathy syndrome caused by pre-/eclampsia. Cerebrovasc Dis 2006;22:204–8
- Melchiorre K, Sutherland GR, Baltabaeva A, et al. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011;57:85–93
- Reuwer AQ, Reuwer PJ, van der Post JA, et al. Prolactin fragmentation by trophoblastic matrix metalloproteinases as a possible contributor to peripartum cardiomyopathy and pre-eclampsia. Med Hypotheses 2010;74:348–52
- Escher G, Mohaupt M. Role of aldosterone availability in preeclampsia. Mol Aspects Med 2007;28:245–54
- Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol 2011;25:313–27
- Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol 2011;159:77–82
- Drewlo S, Czikk M, Baczyk D, et al. Glial cell missing-1 mediates over-expression of tissue inhibitor of metalloproteinase-4 in severe pre-eclamptic placental villi. Hum Reprod 2011;26:1025–34
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;77:67–75
- Hirashima C, Ohkuchi A, Takahashi K, et al. Gestational hypertension as a subclinical preeclampsia in view of serum levels of angiogenesis-related factors. Hypertens Res 2011;34:212–7
- Liu CM, Cheng PJ, Chang SD. Maternal complications and perinatal outcomes associated with gestational hypertension and severe preeclampsia in Taiwanese women. J Formos Med Assoc 2008;107:129–38
- Walker RL, Hemmelgarn B, Quan H. Incidence of gestational hypertension in the Calgary Health Region from 1995 to 2004. Can J Cardiol 2009;25:e284–e287
- Homer CS, Brown MA, Mangos G, Davis GK. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens 2008;26:295–302
- donald-Wallis C, Lawlor DA, Heron J, et al. Relationships of risk factors for pre-eclampsia with patterns of occurrence of isolated gestational proteinuria during normal term pregnancy. PLoS One 2011;6:e22115
- Morikawa M, Yamada T, Minakami H. Outcome of pregnancy in patients with isolated proteinuria. Curr Opin Obstet Gynecol 2009;21:491–5
- Holston AM, Qian C, Yu KF, et al. Circulating angiogenic factors in gestational proteinuria without hypertension. Am J Obstet Gynecol 2009;200:392–10
- Ohkuchi A, Hirashima C, Matsubara S, et al. Serum sFlt1:PlGF ratio, PlGF, and soluble endoglin levels in gestational proteinuria. Hypertens Pregn 2009;28:95–108
- Morikawa M, Yamada T, Yamada T, et al. Pregnancy outcome of women who developed proteinuria in the absence of hypertension after mid-gestation. J Perinat Med 2008;36:419–24
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol 2009;200:481–7
- Aarnoudse JG, Houthoff HJ, Weits J, et al. A syndrome of liver damage and intravascular coagulation in the last trimester of normotensive pregnancy. A clinical and histopathological study. Br J Obstet Gynaecol 1986;93:145–55
- Goodlin RC. Severe pre-eclampsia: another great imitator. Am J Obstet Gynecol 1976;125:747–53
- Bailey DJ, Walton SM. Routine investigations might be useful in pre-eclampsia, but not in gestational hypertension. Aust N Z J Obstet Gynaecol 2005;45:144–7
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998;105:1177–84
- Milne F, Redman C, Walker J, et al. Assessing the onset of pre-eclampsia in the hospital day unit: summary of the pre-eclampsia guideline (PRECOG II). Br Med J 2009;339:b3129
- Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol 2011;205:191–8
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 2003;102:181–92
- Anumba DO, Lincoln K, Robson SC. Predictive value of clinical and laboratory indices at first assessment in women referred with suspected gestational hypertension. Hypertens Pregnancy 2010;29:163–79
- Thangaratinam S, Ismail KM, Sharp S, et al. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG 2006;113:369–78
- Menzies J, Magee LA, MacNab YC, et al. Current CHS and NHBPEP criteria for severe preeclampsia do not uniformly predict adverse maternal or perinatal outcomes. Hypertens Pregnancy 2007;26:447–62
- Bell SC, Halligan AW, Martin A, et al. The role of observer error in antenatal dipstick proteinuria analysis. Br J Obstet Gynaecol 1999;106:1177–80
- Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension 2011;58:704–8
- van der Tuuk K, Koopmans CM, Groen H, et al. Prediction of progression to a high risk situation in women with gestational hypertension or mild pre-eclampsia at term. Aust N Z J Obstet Gynaecol 2011;51:339–46
- Thornton CE, Makris A, Ogle RF, et al. Role of proteinuria in defining pre-eclampsia: clinical outcomes for women and babies. Clin Exp Pharmacol Physiol 2010;37:466–70
- Paula LG, da Costa BE, Poli-de-Figueiredo CE, Antonello IC. Does uric acid provide information about maternal condition and fetal outcome in pregnant women with hypertension? Hypertens Pregn 2008;27:413–20
- Martin JN, Jr, May WL, Magann EF, et al. Early risk assessment of severe preeclampsia: admission battery of symptoms and laboratory tests to predict likelihood of subsequent significant maternal morbidity. Am J Obstet Gynecol 1999;180:1407–14
- Haddad B, Barton JR, Livingston JC, et al. Risk factors for adverse maternal outcomes among women with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol 2000;183:444–8
- Cavkaytar S, Ugurlu EN, Karaer A, et al. Are clinical symptoms more predictive than laboratory parameters for adverse maternal outcome in HELLP syndrome? Acta Obstet Gynecol Scand 2007;86:648–51
- Witlin AG, Saade GR, Mattar F, Sibai BM. Risk factors for abruptio placentae and eclampsia: analysis of 445 consecutively managed women with severe preeclampsia and eclampsia. Am J Obstet Gynecol 1999;180:1322–9
- Ganzevoort W, Rep A, Bonsel GJ, et al. Dynamics and incidence patterns of maternal complications in early-onset hypertension of pregnancy. BJOG 2007;114:741–50
- Oney T, Meyer-Sabellek W. Variability of arterial blood pressure in normal and hypertensive pregnancy. J Hypertens Suppl 1990;8:S77–S81
- Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: the need for a more pathophysiological approach. Obstet Gynecol 2010;115:365–75
- Nisell H, Palm K, Wolff K. Prediction of maternal and fetal complications in preeclampsia. Acta Obstet Gynecol Scand 2000;79:19–23
- Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol 2007;196:514–9
- Odendaal HJ, Pattinson RC, du TR. Fetal and neonatal outcome in patients with severe pre-eclampsia before 34 weeks. S Afr Med J 1987;71:555–8
- Ganzevoort W, Rep A, de Vries JI, et al. Prediction of maternal complications and adverse infant outcome at admission for temporizing management of early-onset severe hypertensive disorders of pregnancy. Am J Obstet Gynecol 2006;195:495–503
- Gazmararian JA, Petersen R, Jamieson DJ, et al. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol 2002;100:94–100
- Bacak SJ, Callaghan WM, Dietz PM, Crouse C. Pregnancy-associated hospitalizations in the United States, 1999-2000. Am J Obstet Gynecol 2005;192:592–7
- Callaghan WM, MacKay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol 2008;199:133–8
- Liu S, Heaman M, Sauve R, et al. An analysis of antenatal hospitalization in Canada, 1991–2003. Matern Child Health J 2007;11:181–7
- Brooten D, Kaye J, Poutasse SM, et al. Frequency, timing, and diagnoses of antenatal hospitalizations in women with high-risk pregnancies. J Perinatol 1998;18:372–6
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med 2011;24:1187–207
- Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–56
- Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol 2005;25:251–7
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012;125:911–9
- Pencina MJ, D'Agostino RB Sr. Thoroughly modern risk prediction? Sci Transl Med 2012;4:131fs10
- von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet 2011;377:219–27
- Sibiude J, Guibourdenche J, Dionne MD, et al. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS One 2012;7:e50208
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med 2012;25:2651–7
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–35
- Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–16
- Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008;54:17–23
- Molvarec A, Szarka A, Walentin S, et al. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res 2010;33:892–8
- Ohkuchi A, Hirashima C, Suzuki H, et al. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res 2010;33:422–7
- Schiettecatte J, Russcher H, Anckaert E, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 2010;43:768–70
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010;202:161
