Abstract
Objective: Studying the biology of the human placenta represents a major experimental challenge. Although conventional cell culture techniques have been used to study different types of placenta-derived cells, current in vitro models have limitations in recapitulating organ-specific structure and key physiological functions of the placenta. Here we demonstrate that it is possible to leverage microfluidic and microfabrication technologies to develop a microengineered biomimetic model that replicates the architecture and function of the placenta.
Materials and methods: A “Placenta-on-a-Chip” microdevice was created by using a set of soft elastomer-based microfabrication techniques known as soft lithography. This microsystem consisted of two polydimethylsiloxane (PDMS) microfluidic channels separated by a thin extracellular matrix (ECM) membrane. To reproduce the placental barrier in this model, human trophoblasts (JEG-3) and human umbilical vein endothelial cells (HUVECs) were seeded onto the opposite sides of the ECM membrane and cultured under dynamic flow conditions to form confluent epithelial and endothelial layers in close apposition. We tested the physiological function of the microengineered placental barrier by measuring glucose transport across the trophoblast-endothelial interface over time. The permeability of the barrier study was analyzed and compared to that obtained from acellular devices and additional control groups that contained epithelial or endothelial layers alone.
Results: Our microfluidic cell culture system provided a tightly controlled fluidic environment conducive to the proliferation and maintenance of JEG-3 trophoblasts and HUVECs on the ECM scaffold. Prolonged culture in this model produced confluent cellular monolayers on the intervening membrane that together formed the placental barrier. This in vivo-like microarchitecture was also critical for creating a physiologically relevant effective barrier to glucose transport. Quantitative investigation of barrier function was conducted by calculating permeability coefficients and metabolic rates in varying conditions of barrier structure. The rates of glucose transport and metabolism were consistent with previously reported in vivo observations.
Conclusion: The “Placenta-on-a-Chip” microdevice described herein provides new opportunities to simulate and analyze critical physiological responses of the placental barrier. This system may be used to address the major limitations of existing placenta model systems and serve to enable research platforms for reproductive biology and medicine.
Introduction
The placenta is key to successful reproduction. Its most important function is the exchange of endogenous and exogenous substances, which enables adequate supply of oxygen and nutrients, excretion of fetal metabolic waste [Citation1–6], and protection against potentially harmful agents, such as xenobiotics [Citation7–11], bacteria [Citation12–17], viruses [Citation18–26] and parasites [Citation26–32]. Transfer of substances between the intervillous space and fetal capillaries takes place across a multilayered structure often called the “placental barrier”, which is composed of trophoblasts, connective tissue, basal lamina and the fetal endothelium [Citation1–6,Citation33–37]. The four major mechanisms responsible for this critical process are: (1) passive diffusion; (2) facilitated diffusion; (3) active transport; and (4) endocytosis/exocytosis [Citation38–40]. Moreover, biochemical and physical factors influence the dynamics of placental transfer, including utero-placental and umbilical blood flow, barrier thickness, placental metabolism, concentration gradients, and transporter expression/activity in the placenta [Citation41,Citation42].
Previous studies on placental transport have used a wide range of experimental systems including in vivo animal models [Citation43–60], ex vivo placental perfusion systems [Citation61–65] and in vitro cell cultures [Citation10,Citation66–73]. In some cases, placental transfer has been studied in humans for frequently-used therapeutic agents such as antibiotics and hormones [Citation7–10,Citation74–78]. However, such studies are difficult to perform, time-consuming and always carry the risk of exposure to the fetus. With the increasing availability of human tissue for laboratory studies, alternatives to animal models have been developed that rely on intact human placental tissue [Citation79]. Although these new types of model systems provide advantages in recapitulating human-relevant physiology, the lack of standardization in this strategy often leads to conflicting results due to lab-to-lab variability [Citation79]. Similarly, other ex vivo approaches, such as placental perfusion models [Citation61–63,Citation65,Citation79–84], have limited ability to dissect the process of placental transfer and to reveal mechanistic insights into its biological underpinnings at physiologically-relevant length scales. Cell culture systems have been successfully used in improving the understanding of placental transfer and metabolism [Citation66–69,Citation72,Citation85–87]. However, they largely fail to reconstitute the physiological structure and microenvironment that profoundly influence transport processes, raising questions regarding their adequacy as an experimental platform in the study of this placental function [Citation79].
Herein, we propose a new bioengineering approach to model placental transport that combines microfluidics and microfabrication technologies with the culture of placenta-derived human cells to recapitulate the organ-specific architecture and physiological microenvironment critical to placental barrier function. Specifically, we developed a “Placenta-on-a-Chip” microdevice that enabled the compartmentalized perfusion co-culture of human trophoblasts (JEG-3) and human umbilical vein endothelial cells (HUVECs) on a thin extracellular matrix (ECM) membrane to create a physiological placental barrier in vitro. In this report, we employ novel microengineering techniques required for the fabrication of this biomimetic system, as well as for the prolonged maintenance of microfluidic co-culture in the microdevice. We also demonstrate the physiological function and relevance of this model by analyzing glucose transport across a microengineered placental barrier. This approach creates new opportunities to address major limitations of existing placenta models and holds great potential as a novel enabling platform for the study of placental barrier function.
Materials and methods
Cell culture
JEG-3 (ATCC, Manassas, VA) was selected among various trophoblast cell lines [Citation79]. The cells were cultured in DMEM (Dulbecco’s Modified Eagle Medium, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells with passages below 15 were used in microfluidic cell culture experiments.
Green fluorescent protein (GFP)-expressing HUVECs (Angio-Proteomie, Boston, MA) were cultured in EBMTM-2 basal medium (Lonza, Walkersville, MD) supplemented with the Endothelial Cell Growth Medium-2 (EGM™-2) MV Bullet Kit (Lonza). Cells with passages below 10 were used in microfluidic cell culture experiments. Both cell lines were maintained at 37 °C in a humidified incubator under 5% CO2 in air.
Device design and fabrication
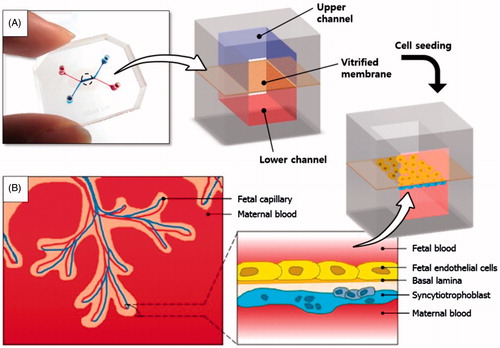
To create the placenta-on-a-chip microdevice, two poly(dimethylsiloxane) (PDMS) slabs containing microchannel features were bonded together with a vitrified collagen membrane sandwiched in between (). This design allowed for the independent control of fluid flows in the upper and lower channels, which correspond to the fetal capillary compartment and intervillous space, respectively ().
Figure 1. A placenta-on-a-chip microdevice: (A) The microengineered device is composed of the upper (blue) and lower (red) PDMS chambers separated by a vitrified collagen membrane. (B) Endothelial cells and trophoblasts are co-cultured in close apposition on the opposite sides of the intervening membrane to form a microengineered placental barrier in the placenta-on-a-chip device.
PDMS microchannels were generated using soft-lithography [Citation88]. Briefly, a negative photoresist (PR) (SU-8 2075, MicroChem Corp., Westborough, MA) was spin-coated on a clean silicon wafer (Taewon Scientific, Seoul, Republic of Korea) and baked first at 65 °C for 7 min and then at 95 °C for 40 min. Subsequently, the photoresist layer was brought into contact with a mask film containing channel patterns and exposed to ultraviolet (UV) light. Following this step, the wafer was baked again at 65 °C for 5 min and at 95 °C for 18 min before being placed in a developer solution to dissolve the unexposed photoresist. Finally, the wafer was thoroughly rinsed with copious amounts of the developer solution and isopropyl alcohol to generate a mold containing the positive relief of the desired microchannel patterns ().
Figure 2. Fabrication of the placenta-on-a-chip: (A) Production of a microchannel master using photolithography. (B) Soft lithography-based replica molding of PDMS microchannels and the formation of the vitrified collagen membrane. The upper PDMS slab is permanently bonded to the lower slab containing a microchannel covered with the vitrified membrane. (C) Sequential seeding of JEG-3 cells and HUVECs in the multilayered microfluidic device.
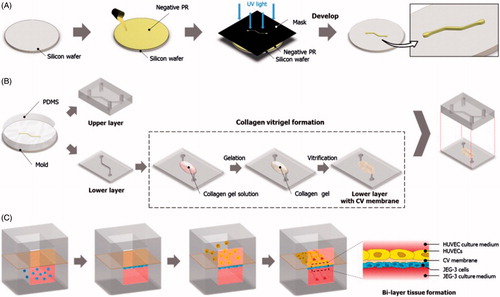
For the fabrication of PDMS channels (), PDMS prepolymer was mixed with a curing agent at a weight ratio of 10:1 (prepolymer:curing agent) and placed in a vacuum chamber to remove trapped air. After degassing, the PDMS mixture was poured onto the channel mold and incubated at 65 °C for 4 h. When the PDMS mixture was fully cured, it was detached from the mold and cut into a desired shape and size to generate an upper PDMS slab engraved with a microchannel (). This replica molding process was repeated to produce a lower PDMS layer. The cross-sectional size of the microchannel in each layer was approximately 500 μm × 200 μm (width × height).
For the formation of the vitrified collagen membrane (), collagen type I (BD Biosciences, San Jose, CA) was mixed with distilled water and 10 × DMEM (Dulbecco’s Modified Eagle Medium, Sigma–Aldrich Corp., St. Louis, MO) at a final concentration of 2.43 mg ml−1. The pH of the solution was adjusted to 7.4 using 1 N Sodium hydroxide (NaOH). The gel solution was gently dispensed on the central part of the lower microchannel and then incubated at 37 °C for 40 min for gelation. Subsequently, the collagen gel was dried overnight at room temperature to produce a vitrified collagen membrane that remained attached to the lower PDMS microchannel.
As the final step of device fabrication, the PDMS surfaces of the upper and lower channels were treated with plasma using a plasma cleaner (Harrick Plasma, Ithaca, NY) and bonded together. During this step, care was taken to ensure the alignment of the upper and lower microchannels. Incubation of the bonded channels at 65 °C for 30 min produced a permanently sealed microfluidic device that allowed for independent fluidic access to the upper and lower microchannels.
Microfluidic cell seeding and culture
Prior to cell seeding, silicon tubing (Tygon tube with an inner diameter of 1/32 inch) was inserted into the inlet and outlet ports of both channels to facilitate the handling of cells and fluids. The inlet tubing was used to connect the upper and lower microchannels to two separate reservoirs for cell culture media. The fully assembled device was sterilized by UV irradiation immediately before cell seeding.
To promote cell attachment and growth, the upper and lower surfaces of the vitrified membrane were coated with 40 μg ml−1 of fibronectin (BD Biosciences) and 1.5% gelatin (Sigma-Aldrich Corp.), respectively. Following ECM coating, trophoblasts (JEG-3 cells) (ATCC, Manassas, VA) suspended in a complete growth medium at 5 × 106 cells ml−1 were gently injected into the lower channel using a BD Falcon 1 mL syringe (BD Biosciences) and allowed to attach to the lower membrane surface (). The device remained inverted for the entire duration of this procedure. Once the trophoblasts established firm attachment, green fluorescent protein (GFP)-expressing HUVECs (Angio-Proteomie) were introduced into the upper microchannel and incubated for two hours to enable their adhesion to the upper side of the vitrified membrane (). For microfluidic culture, JEG-3 cells and HUVECs were perfused with their respective media using a syringe pump (Braintree Scientific, Inc., Braintree, MA) connected to the outlets that withdrew at a constant volumetric flow rate of 30 μl h−1.
Monitoring and analysis of cell growth
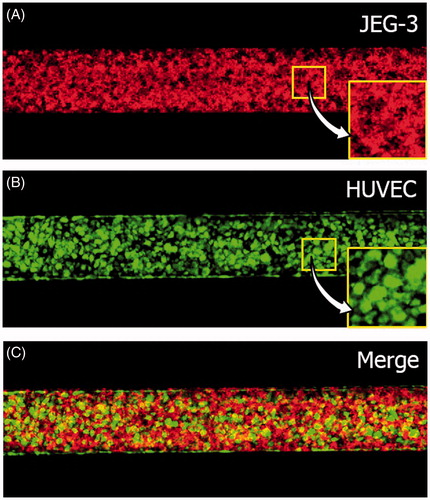
Formation and structural integrity of the placental barrier in our co-culture model were assessed using confocal microscopy of the confluent monolayers of JEG-3 cells and GFP-HUVECs on the opposite sides of the membrane. For fluorescence imaging, JEG-3 cells were labeled with CellTracker Red CMTPX (Invitrogen, Life Technologies, Carlsbad, CA) prior to cell seeding. Fluorescence micrographs of the cells were acquired after three days of culture and the resultant confluences were analyzed with an image-processing algorithm developed using MATLAB® (Natick, MA).
Measurement of glucose transfer across the placental barrier
The cells in our microfluidic device were cultured for at least three days in order to ensure the maturation of the placental barrier. To recapitulate physiological gradients of glucose across the maternal-fetal interface, DMEM supplemented with a higher concentration (4.5 g ml−1) of glucose was supplied to the lower trophoblast culture chamber, while EBMTM-2 containing a lower glucose concentration (1.1 g ml−1) was introduced into the upper endothelial channel. Continuous flow of the culture media was maintained at 30 μl h−1 for 68 h, and perfused media were collected from the outlets of the upper and lower microchannels separately at the end of each experiment. The concentration of glucose in the collected samples was measured using a glucose analyzer (YSI Life Sciences, Yellow Springs, OH), and the results were analyzed in comparison to those obtained from the following three control groups: (i) an acellular model; (ii) a mono-culture model with JEG-3 cells alone; and (iii) a mono-culture model with HUVECs alone.
Analysis of glucose transfer
The rate of glucose transfer in our model was evaluated using a previously described method [Citation89]. Briefly, the percent rate of transfer (%TR) was calculated as follows:
(1)
where ΔQU and ΔQL refer to the increase in glucose concentration in the upper and lower channels, respectively. Glucose permeability coefficients (GP) were calculated using the following equation,
(2)
where ΔQ is the amount of glucose transferred to the upper channel, Δt is the time elapsed, A is the surface area of the placental barrier, and CL is the glucose concentration in the lower channel. In our analysis, CL was assumed to be constant due to flow-induced continuous replenishment of glucose in the lower channel. The glucose permeability coefficient of an unsupported cell monolayer (GPUM) (i.e. without the vitrified collagen membrane) was calculated using the equation derived by Utoguchi et al. [Citation90],
(3)
where GPSM and GPCV refer to the glucose permeability coefficients of a cell monolayer supported by the membrane and the collagen vitrified membrane alone, respectively. Similarly, the glucose permeability coefficient of the bi-layer (GPBL) barrier consisting of JEG-3 and HUVEC monolayers was determined by the following equation,
(4)
where GPJM and GPHM are the permeability coefficients of the unsupported JEG-3 and HUVEC monolayers. This GPBL,derived refers to a theoretically-derived glucose permeability coefficient of the barrier formed by the physical super-position of the JEG-3 and HUVEC monolayers, and the vitrified collagen membrane without considering their functional interactions.
Statistical analysis
Experimental data were obtained from at least six independent experiments. Statistical significance was determined using ANOVA followed by Tukey’s post hoc tests.
Results
Cell growth on the vitrified collagen membrane
Our microfluidic culture conditions provided an environment conducive to proliferation and maturation of JEG-3 cells and HUVECs in the co-culture model. As illustrated in the fluorescence micrographs in , three days of perfusion culture in the device produced highly confluent monolayers of JEG-3 cells and HUVECs on either side of the membrane that resembled the microarchitecture of the placental barrier in vivo. Our imaging analysis revealed that the JEG-3 cells and HUVECs in our microdevice reached a confluency of 99.3% and 99.4%, respectively. Although the vitrified membrane underwent rapid rehydration upon injection of ECM coating solutions into the channels, it remained intact without undesirable structural deformation for the entire duration of culture and supported cell attachment and growth. We did not observe cross-contamination between the epithelial and endothelial populations due to cellular migration across the membrane.
Glucose transfer across the placental barrier
To evaluate the transport of glucose from the maternal to the fetal side in our model, we first measured changes in glucose concentration in the outflow collected from the upper (fetal) channel. The amount of glucose transferred to the fetal compartment was found to be the smallest in the co-culture model, illustrating the ability of the bi-layer microarchitecture to limit molecular transport across the barrier (). Glucose transfer was higher in the devices with JEG-3 cells or HUVECs alone, but the HUVECs monoculture model exhibited a greater magnitude of concentration increase. This observation suggests that the trophoblast epithelium serves as the main barrier to glucose transport. The absence of cellular components in the acellular model resulted in a substantially increased transfer across the membrane and concomitant elevation of glucose concentration in the fetal endothelial compartment.
Figure 4. Changes in the amounts of glucose: (A) Glucose increases in the fetal compartment; (B) Reduction in glucose concentration in the maternal chamber. Boxes denote the interquartile range, with the red horizontal line representing the median. Whiskers extend from the box to the extreme values unless there were outliers (+ red signs).
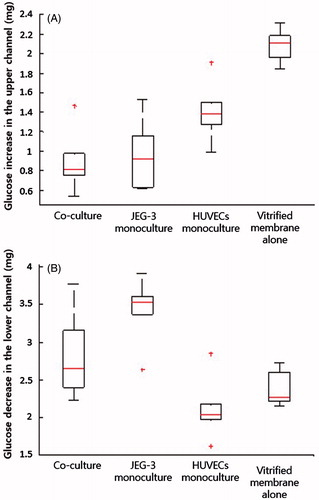
To examine more closely the placental barrier function in our model, we also evaluated reduction of the glucose concentration in the lower maternal compartment (). Interestingly, the magnitude of decrease was the largest when JEG-3 cells were cultured alone, and our co-culture configuration led to a greater reduction in glucose concentration compared to the HUVECs monoculture and acellular devices. It was also noted that the endothelial layer in the HUVECs monoculture model induced a greater depletion of glucose in the lower channel. The differences in glucose concentration in the upper and lower channels imply that the transport of glucose across the barrier in this model is governed by mechanisms other than concentration gradient-induced passive diffusion, and that the cells serve as a critical determinant of glucose transfer. Moreover, glucose transporters expressed by placental cells may contribute to the mechanisms responsible for glucose transfer in our device.
Quantitative analysis of glucose transfer
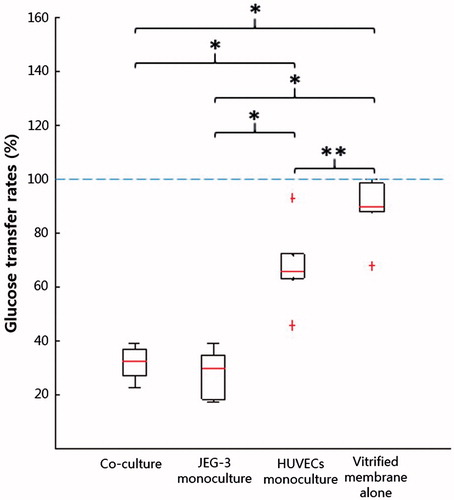
Glucose passed through the vitrified collagen membrane without much hindrance, as shown by the relatively high transfer rates (67.8–99.5%) (). However, when cells were incorporated into the device, the transfer rate decreased substantially. The HUVECs monoculture model yielded a transfer rate of 45.5–93%, and this was further reduced to 17.3–39.1% with the addition of trophoblasts, confirming their predominant role in placental barrier function.
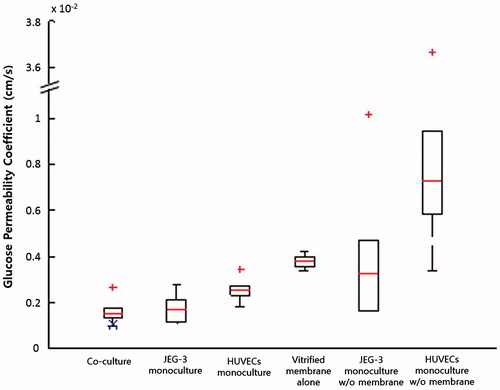
Evaluation of glucose permeability coefficients (GPs) showed that the GP for a monolayer of HUVECs was significantly greater than that of JEG-3 cells, indicating that the endothelial cells cultured in our model were more permeable to glucose than trophoblasts (). The GPBL,derived calculated using the equation (Equation4(4) ) was 1.02 × 10−4 cm s−1, and this value was in the range of experimentally obtained GPs for the bi-layer (BL) model. The GPBL,derived was evaluated without considering the association of the epithelial barrier with the endothelium, the results suggest that glucose transfer may not be affected by functional interactions between trophoblasts and endothelial cells.
Figure 6. Glucose permeability coefficients. GPBL,derived calculated using Equation (Equation4(4) ) is marked with a blue X in the co-culture group.
Discussion
We developed a “Placenta-on-a-Chip” device inhabited by living human cells which recapitulate the critical fetal-maternal interface in the villous tree of the human placenta. Trophoblasts and HUVECs were co-cultured in a compartmentalized three-dimensional PDMS microsystem consisting of the upper and lower cell culture chambers separated by a thin vitrified collagen membrane. The resultant bi-layer tissue resembled a physiological placental barrier, which is often challenging to generate while using standard two-dimensional cell culture methods. We also demonstrated that continuous perfusion of the cells in our microfluidic device supported cell growth and long-term viability.
We analyzed glucose transfer characteristics across the microengineered placental barrier. Glucose was chosen as a model molecule due to (i) its importance in normal pregnancy [Citation40,Citation91–93], gestational diabetes [Citation40,Citation93–97], and overt diabetes [Citation93,Citation98] and (ii) its permeability across the placental barrier in spite of placental metabolism. The transfer rates measured in our model ranged from 22.6 to 33.9%, indicating metabolic consumption (66.1∼77.4%). These results are consistent with previously reported placental metabolic rates in the lamb [Citation99]. However, direct comparison was not possible due to a paucity of such data in the literature. Based on these results, we conclude that our model mimics not only structural characteristics of the human placental barrier, but also some aspects of physiology.
Despite its capabilities and advantages, our system has limitations. From the perspective of model development, although our microfluidic culture system provides a greater physiological cell culture environment than conventional static cultures, the levels of shear stress generated in our model are substantially lower than those in fetal capillaries. This is mainly due to the large size of the cell culture chambers and could be overcome simply by reducing the dimensions of the fetal compartment and/or increasing the rate of fluid flow on the fetal side. We employed the JEG-3 cell line and HUVECs to represent trophoblast and endothelial cells, respectively. Future studies using primary human trophoblasts [100] and fetal capillary endothelial cells need to be performed.
Our placenta-on-a-chip model has potential to serve as a low-cost experimental platform with a broad range of applications. For example, the capabilities afforded by this microsystem provide new opportunities to quantitatively and mechanistically investigate placental transfer of various substances under varying conditions and to eventually improve our fundamental understanding of complex human placental physiology. This biomimetic model may also enable the quantitative analysis of placental transport of small molecules and biologics for the development and screening of new therapeutic modalities. Moreover, the microengineering approach demonstrated in this study could be leveraged to recapitulate key pathological features of different placental disorders to develop new types of in vitro human disease models. As exemplified by the recent launching of the Human Placenta Project by NICHD/NIH [Citation101], there has been a growing interest in the application of microengineered physiological systems to the study of placental biology. Our placenta-on-a-chip model represents exciting progress in this area and lays the groundwork for future studies aiming to explore the potential of organs-on-chips technology for reproductive biology and medicine.
Declaration of interest
The authors report no conflicts of interest. This work was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS; and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C, and by the Seoul National University Bundang Hospital Research Fund (Grant No. 03-2013-001) and the National Research Foundation of Korea (2012M3A7B4035286 and 2013R1A2A2A04013379). We also acknowledge support from the National Medical Center and Asan Medical Center in Seoul, Republic of Korea. D. H. is a recipient of the NIH Director's New innovator Award (1DP2HL127720-01).
References
- Fox H. Pathology of the placenta. Clin Obstet Gynaecol;13:501–19
- Kraus FT, Redline RW, Gersell DJ, et al. Placental pathology. 1st ed. Washington, DC: American Registry of Pathology; 2004
- Fox H, Sebire NJ. Pathology of the placenta. 3rd ed. Philadelphia (PA): Elsevier; 2007
- Kay HH, Nelson MD, Wang Y. The placenta from development to disease. 1st ed. New Jersey: Blackwell; 2011
- Benirschke K, Burton GJ, Baergen RN. Pathology of the human placenta. 6th ed. Berlin: Springer; 2012
- Burton GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta 2012;33:S23–7
- Hakkola J, Pelkonen O, Pasanen M, Raunio H. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol 1998;28:35–72
- Myllynen P, Pasanen M, Vahakangas K. The fate and effects of xenobiotics in human placenta. Expert Opin Drug Metab Toxicol 2007;3:331–46
- Polachek H, Holcberg G, Polachek J, et al. Carrier-mediated uptake of Levofloxacin by BeWo cells, a human trophoblast cell line. Arch Gynecol Obstet 2010;281:833–8
- Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos 2010;38:1623–35
- Grafmuller S, Manser P, Krug HF, et al. Determination of the transport rate of xenobiotics and nanomaterials across the placenta using the ex vivo human placental perfusion model. J Vis Exp 2013;76:e50401
- Benirschke K. Syphilis – the placenta and the fetus. Am J Dis Child 1974;128:142–3
- Barber EM, Fazzari M, Pollard JW. Th1 cytokines are essential for placental immunity to Listeria monocytogenes. Infect Immun 2005;73:6322–31
- Lecuit M. Understanding how Listeria monocytogenes targets and crosses host barriers. Clin Microbiol Infect 2005;11:430–6
- Seveau S, Pizarro-Cerda J, Cossart P. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect 2007;9:1167–75
- Qi Z, Zhao H, Zhang Q, et al. Acquisition of maternal antibodies both from the placenta and by lactation protects mouse offspring from Yersinia pestis challenge. Clin Vaccine Immunol 2012;19:1746–50
- Zeldovich VB, Clausen CH, Bradford E, et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog 2013;9:e1003821
- Burton GJ, Watson AL. The structure of the human placenta: implications for initiating and defending against virus infections. Rev Med Virol 1997;7:219–28
- Halwachs-Baumann G. The congenital cytomegalovirus infection: virus-host interaction for defense and transmission. Curr Pharm Biotechnol 2006;7:303–12
- Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol 2009;61:196–212
- Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 2010;185:1248–57
- Aldo PB, Mulla MJ, Romero R, et al. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol 2010;64:27–37
- Moodley S, Bobat R. Expression of HLA-G1 at the placental interface of HIV-1 infected pregnant women and vertical transmission of HIV. Placenta 2011;32:778–82
- Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol 2011;65:110–17
- Pereira L, Petitt M, Tabata T. Cytomegalovirus infection and antibody protection of the developing placenta. Clin Infect Dis 2013;57:S174–7
- Moro L, Bardaji A, Nhampossa T, et al. Malaria and HIV infection in mozambican pregnant women are associated with reduced transfer of antimalarial antibodies to their newborns. J Infect Dis 2015;211:1004–14
- Staalsoe T, Shulman CE, Bulmer JN, et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 2004 24;363:283–9
- Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology 2007;134:1877–81
- Castillo C, Lopez-Munoz R, Duaso J, et al. Role of matrix metalloproteinases 2 and 9 in ex vivo Trypanosoma cruzi infection of human placental chorionic villi. Placenta 2012;33:991–7
- Diaz-Lujan C, Triquell MF, Schijman A, et al. Differential susceptibility of isolated human trophoblasts to infection by Trypanosoma cruzi. Placenta 2012;33:264–70
- Bedu-Addo G, Meese S, Mockenhaupt FP. An ATP2B4 polymorphism protects against malaria in pregnancy. J Infect Dis 2013;207:1600–3
- Liempi A, Castillo C, Duaso J, et al. Trypanosoma cruzi induces trophoblast differentiation: a potential local antiparasitic mechanism of the human placenta? Placenta 2014;35:1035–42
- Nagashige M, Ushigome F, Koyabu N, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta 2003;24:951–8
- Myren M, Mose T, Mathiesen L, Knudsen LE. The human placenta – an alternative for studying foetal exposure. Toxicol In Vitro 2007;21:1332–40
- Pijnenborg R, Brosen I, Romero R. Placenta bed disorders. Cambridge, UK: Cambridge University Press; 2010
- Levkovitz R, Zaretsky U, Gordon Z, et al. In vitro simulation of placental transport: part I. Biological model of the placental barrier. Placenta 2013;34:699–707
- Levkovitz R, Zaretsky U, Jaffa AJ, et al. In vitro simulation of placental transport: part II. Glucose transfer across the placental barrier model. Placenta 2013;34:708–15
- Knipp GT, Audus KL, Soares MJ. Nutrient transport across the placenta. Adv Drug Deliv Rev 1999;38:41–58
- Sibley CP, Brownbill P, Dilworth M, Glazier JD. Review: adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta 2010;31:S70–4
- Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Preg 2012;2012:179827
- Carter AM. Evolution of factors affecting placental oxygen transfer. Placenta 2009;30 A:S19–25
- Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 2013;4:101–15
- Rosso P. Maternal-fetal exchange during protein malnutrition in the rat. Placental transfer of alpha-amino isobutyric acid. J Nutr 1977;107:2002–5
- Rosso P. Maternal-fetal exchange during protein malnutrition in the rat. Placental transfer of glucose and a nonmetabolizable glucose analog. J Nutr 1977;107:20006–10
- Saintonge J, Rosso P. Placental blood flow and transfer of nutrient analogs in large, average, and small guinea pig littermates. Pediatr Res 1981;15:152–6
- Ahokas RA, Lahaye EB, Anderson GD, Lipshitz J. Effect of maternal dietary restriction on fetal growth and placental transfer of alpha-amino isobutyric acid in rats. J Nutr 1981;111:2052–8
- Jansson T, Persson E. Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatr Res 1990;28:203–8
- Varma DR, Ramakrishnan R. Effects of protein-calorie malnutrition on transplacental kinetics of aminoisobutyric acid in rats. Placenta 1991;12:277–84
- Dwyer CM, Madgwick AJ, Crook AR, Stickland NC. The effect of maternal undernutrition on the growth and development of the guinea pig placenta. J Dev Physiol 1992;18:295–302
- Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol 1996;271:C295–303
- Glazier JD, Sibley CP, Carter AM. Effect of fetal growth restriction on system A amino acid transporter activity in the maternal facing plasma membrane of rat syncytiotrophoblast. Pediatr Res 1996;40:325–9
- Reid GJ, Lane RH, Flozak AS, Simmons RA. Placental expression of glucose transporter proteins 1 and 3 in growth-restricted fetal rats. Am J Obstet Gynecol 1999;180:1017–23
- Lesage J, Hahn D, Leonhardt M, et al. Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. J Endocrinol 2002;174:37–43
- Jansson N, Pettersson J, Haafiz A, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 2006;576:935–46
- Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, et al. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.). Placenta 2007;28:783–93
- Coan PM, Angiolini E, Sandovici I, et al. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol 2008;586:4567–76
- Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol 2008;32:225–30
- Coan PM, Vaughan OR, Sekita Y, et al. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 2010;588:527–38
- Sferruzzi-Perri AN, Vaughan OR, Coan PM, et al. Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 2011;152:3202–12
- Belkacemi L, Jelks A, Chen CH, et al. Altered placental development in undernourished rats: role of maternal glucocorticoids. Reprod Biol Endocrinol 2011;9:105–15
- Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol 1972;114:822–8
- Polliotti BM, Holmes R, Cornish JD, et al. Long-term dual perfusion of isolated human placental lobules with improved oxygenation for infectious diseases research. Placenta 1996;17:57–68
- Heikkila A, Myllynen P, Keski-Nisula L, et al. Gene transfer to human placenta ex vivo: a novel application of dual perfusion of human placental cotyledon. Am J Obstet Gynecol 2002;186:1046–51
- Fokina VM, Patrikeeva SL, Zharikova OL, et al. Transplacental transfer and metabolism of buprenorphine in preterm human placenta. Am J Perinatol 2011;28:25–32
- Woo CS, Partanen H, Myllynen P, et al. Fate of the teratogenic and carcinogenic ochratoxin A in human perfused placenta. Toxicol Lett 2012;208:92–9
- Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res 1968;28:1231–6
- Pattillo RA, Gey GO, Delfs E, et al. The hormone-synthesizing trophoblastic cell in vitro: a model for cancer research and placental hormone synthesis. Ann N Y Acad Sci 1971;172:288–98
- Kohler PO, Bridson WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab 1971;32:683–7
- Azizkhan JC, Speeg KV, Jr Stromberg K, Goode D. Stimulation of human chorionic gonadotropin by JAr line choriocarcinoma after inhibition of DNA synthesis. Cancer Res 1979;39:1952–9
- Wice B, Menton D, Geuze H, Schwartz AL. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res 1990;186:306–16
- Miller RK, Genbacev O, Turner MA, et al. Human placental explants in culture: approaches and assessments. Placenta 2005;26:439–48
- Wolfe MW. Culture and transfection of human choriocarcinoma cells. Methods Mol Med 2006;121:229–39
- Orendi K, Kivity V, Sammar M, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011;32:S49–54
- Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet 1995;28:235–69
- Ushigome F, Takanaga H, Matsuo H, et al. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur J Pharmacol 2000;408:1–10
- Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 2004;43:487–514
- Evseenko D, Paxton JW, Keelan JA. Active transport across the human placenta: impact on drug efficacy and toxicity. Expert Opin Drug Metab Toxicol 2006;2:51–69
- Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Assessing drug transport across the human placental barrier: from in vivo and in vitro measurements to the ex vivo perfusion method and in silico techniques. Curr Pharm Biotechnol 2011;12:804–13
- Myllynen P, Vahakangas K. Placental transfer and metabolism: an overview of the experimental models utilizing human placental tissue. Toxicol In Vitro 2013;27:507–12
- Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, et al. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol 2006;195:1081–5
- Nanovskaya TN, Nekhayeva IA, Hankins GD, Ahmed MS. Transfer of methadone across the dually perfused preterm human placental lobule. Am J Obstet Gynecol 2008;198:126 e1–4
- Ceccaldi PF, Ferreira C, Gavard L, et al. Placental transfer of enfuvirtide in the ex vivo human placenta perfusion model. Am J Obstet Gynecol 2008;198:433 e1–2
- Smith JA, Gaikwad A, Mosley S, et al. Utilization of an ex vivo human placental perfusion model to predict potential fetal exposure to carboplatin during pregnancy. Am J Obstet Gynecol 2014;210:275 e1–9
- Mandelbrot L, Duro D, Belissa E, Peytavin G. Placental transfer of rilpivirine in an ex vivo human cotyledon perfusion model. Antimicrob Agents Chemother 2015;59:2901–3
- Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol 1997;273:C1596–604
- Ampasavate C, Chandorkar GA, Vande Velde DG, et al. Transport and metabolism of opioid peptides across BeWo cells, an in vitro model of the placental barrier. Int J Pharm 2002;233:85–98
- Tobin KA, Johnsen GM, Staff AC, Duttaroy AK. Long-chain polyunsaturated fatty acid transport across human placental choriocarcinoma (BeWo) cells. Placenta 2009;30:41–7
- Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci 1998;28:153–84
- Vinot C, Gavard L, Treluyer JM, et al. Placental transfer of maraviroc in an ex vivo human cotyledon perfusion model and influence of ABC transporter expression. Antimicrob Agents Chemother 2013;57:1415–20
- Utoguchi N, Magnusson M, Audus KL. Carrier-mediated transport of monocarboxylic acids in BeWo cell monolayers as a model of the human trophoblast. J Pharm Sci 1999;88:1288–92
- Bax BE, Bloxam DL. Energy metabolism and glycolysis in human placental trophoblast cells during differentiation. Biochim Biophys Acta 1997;1319:283–92
- Hay WW Jr. Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc 2006;117:321–39 (discussion 339–40)
- Brett KE, Ferraro ZM, Yockell-Lelievre J, et al. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 2014;15:16153–85
- Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab 1999;84:695–701
- Jansson T, Ekstrand Y, Wennergren M, Powell TL. Placental glucose transport in gestational diabetes mellitus. Am J Obstet Gynecol 2001;184:111–16
- Colomiere M, Permezel M, Riley C, et al. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol 2009;160:567–78
- Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci 2011;18:20–7
- Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol 1999;180:163–8
- Simmons M, Battaglia F, Meschia G. Placental transfer of glucose. J Dev Physiol 1979;1:227–43
- Kliman HJ, Nestler JE, Sermasi E, et al. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 1986;118:1567–82
- NICHD. The Human Placenta Project 2015. Available from: http://www.nichd.nih.gov/research/HPP/Pages/default.aspx [last accessed 20 Feb 2015]