Abstract
Objective: To assess the performance of the universal newborn hearing screen in England. Design: Retrospective analysis of population screening records. Study sample: A total of 4 645 823 children born 1 April 2004 to 31 March 2013. Results: 97.5% of the eligible population complete screening by 4/5 weeks of age and 98.9% complete screening by three months of age. The refer rate for the 12/13 birth cohort is 2.6%. The percentage of screen positive (i.e. referred) babies commencing follow up by four weeks of age and six months of age is 82.5% and 95.8% respectively. The yield of bilateral PCHL from the screen is around 1/1000. For bilateral PCHL in the 12/13 birth cohort the median age is nine days at screen completion, 30 days at entry into follow up, 49 days at confirmation, 50 days at referral to early intervention, and 82 days at hearing-aid fitting. Conclusion: The performance of the newborn hearing screening programme has improved continuously. The yield of bilateral PCHL from the screen is about 1/1000 as expected. The age of identification and management is well within the first six months of life, although there remains scope for further improvement with respect to timely entry into follow up.

| Abbreviations | ||
| AABR | = | Automated auditory brainstem response |
| ANSD | = | Auditory neuropathy spectrum disorder |
| AOAE | = | Automated otoacoustic emission |
| CI | = | Confidence interval |
| eSP | = | e-Screener Plus. The information system used to manage and report on the newborn hearing screening programme in England |
| GA | = | Gestational age |
| KPI | = | Key performance indicator |
| NHSP | = | Newborn hearing screening programme (England) |
| NICU/SCBU | = | Neonatal intensive care unit/Special care baby unit |
| PCHL | = | Permanent childhood hearing loss of ≥40 dB averaged over 0.5, 1, 2, and 4 kHz in one or both ears |
| PPV | = | Positive predictive value |
The Newborn Hearing Screening Programme for England (NHSP-England) was introduced across the country in a phased and nationally organized process between 2002 and 2006 with full implementation achieved in March 2006. The evidence base for the programme was presented by CitationDavis et al (1997). An evaluation of the first phase of the programme was commissioned by the Department of Health and carried out by Bamford and colleagues (CitationBamford et al, 2005; CitationYoung et al, 2004, Citation2005; CitationCrockett et al, 2005, Citation2006; CitationUus et al, 2005, Citation2006; CitationUus & Bamford, 2006). The vision for the programme is one of improving outcomes for every child through a high quality hearing screening programme, safe and effective assessments, and family centred early intervention. The aim of the programme is to screen eligible babies within the first few weeks of life, and by three months of age at the latest, and to identify cases of bilateral congenital moderate to profound PCHL by six months of age. This paper reports the performance of the programme for the first seven annual birth cohorts since full implementation throughout England in March 2006. Data were compiled on 18 February 2014 at which time the youngest child in the cohort was aged 11.6 months.
Newborn Hearing Screening Programme (England) (NHSP)
Organization
The National Screening Committee for the UK (UKNSC) is responsible all screening programmes in the UK. For English programmes the UKNSC is responsible for detailed specification including setting and monitoring standards, developing protocols and procedures, specifying and procuring equipment and information systems, advising on incident management and quality assurance. National screening programmes are commissioned by NHS England (formerly the Commissioning Board) via their local area teams to a nationally agreed specification and delivered by local health providers.
For newborn hearing screening there are currently 113 local programmes that cover all births in England (approximately 660 000 per annum). The size of local programmes varies from 2500–25 000 births per annum. The newborn hearing screening programme (NHSP) receives all birth notifications and a record is automatically created in the national information system for every baby. Newborn hearing screening is part of the National Health Service provision and, along with paediatric audiology services, is free at the point of use. A very small proportion of births take place in private hospitals (< 0.5%); these babies are eligible for screening by the national programme but some are screened privately. All screening tests and outcomes that are part of NHSP are recorded in the national information system (e-Screener Plus: eSP). This includes audiological follow up outcomes for babies referred on the screen and for babies who pass the screen but are referred for targeted surveillance (CitationWood et al, 2013) because of the presence of risk factors.
Target condition
In this paper hearing loss is categorized as mild, moderate, severe, or profound on the basis of hearing threshold in dB HL averaged over the frequencies 0.5, 1, 2, and 4 kHz for the better hearing ear as follows:
Mild: 21–39 dB
Moderate: 40–69 dB
Severe: 70–95 dB
Profound: > 95 dB
This categorization is widely used in the literature (CitationDavis et al, 1997). It is slightly different from that of the British Society of Audiology (CitationBSA, 1988). The World Health Organization has recently defined an alternative classification (CitationWHO, 2013).
While many North American screening programmes include babies with permanent mild and unilateral hearing loss in their target group, NHSP does not: it aims to identify all children with a moderate-profound PCHL in the better hearing ear. As a by-product, the screen will also identify a number of babies who have unilateral and in some cases mild permanent hearing loss, as well as temporary hearing loss.
Screening protocol
Screening is carried out using standard techniques and protocols that have been developed for use within NHSP. Screening equipment is specified, evaluated, and procured nationally. There are separate protocols for well babies and babies that have been cared for in NICU/SCBU for 48 hours or more. Detailed protocols and pathways are available at http://hearing.screening.nhs.uk/.
Well babies follow a two-technology protocol; the first stage is a transient automated otoacoustic emission (AOAE) screen, with a maximum of two attempts, followed by an automated auditory brainstem response (AABR) screen if required. A pass in both ears using AOAE, or a pass in both ears on AABR constitutes an overall screen pass. Babies cared for in NICU or SCBU for more than 48 hours undergo an otoacoustic emission screen and an automated auditory brainstem response (AABR). Babies that fail the AABR screen in one or both ears are considered to be screen referrals and are referred for audiological assessment. Babies that pass the AABR screen in both ears and fail the AOAE screen in both receive a follow up at eight months (“targeted follow up”). This protocol will detect auditory neuropathy spectrum disorder (ANSD) as target cases in the NICU population but not in the well-baby population.
Babies with microtia/atresia in one or both ears and babies that recover from neonatal meningitis are considered to be automatic screen referrals. They do not undergo screening tests but are referred directly for audiological assessment.
There are two delivery models for newborn hearing screening for well babies. The majority of programmes operate a hospital-based programme; babies are screened in hospital before discharge by dedicated hearing screeners or in outpatient screening clinics for those babies that do not complete screening before discharge. A minority (∼25%) operate a community-based model; well babies are screened at home by Health Visitors or Health Care Assistants at age 10 days or later. Babies screened under the NICU protocol are screened in hospital before discharge.
Quality standards and key performance indicators
NHSP has developed extensive quality standards that cover the performance of the screening programme and services along the care pathway for children identified with PCHL. These standards can be found at http://hearing.screening.nhs.uk/. Two of these quality standards are designated as key performance indicators (KPIs). These are:
KPI 1: The proportion of babies eligible for newborn hearing screening for whom the screening process is complete by four weeks corrected age (hospital programmes - well babies, NICU babies) or by five weeks corrected age (community programmes - well babies). Target is ≥ 95%. This is referred to as coverage by 4/5 weeks of age.
KPI 2: The percentage of referred babies receiving an audiological assessment within four weeks of the decision that referral for assessment is required or by 44 weeks gestational age (GA). Target is ≥ 90%.
The rationale for the selection of these two measures is that early completion of screening and high coverage must be coupled with prompt referral and assessment of screen positives in order to achieve a high ascertainment of target cases at an early age. Early assessment also means that (1) audiological assessment with electrophysiological tests such as the auditory brainstem response, and habilitative procedures such as real ear measurements for hearing-aid fittings are easier in the first few months of life and can more readily be carried out during periods of natural sleep, and (2) the earlier that parents and services know about the child's hearing loss, the earlier decisions can be made about management, including fitting of appropriate hearing aids, referral for cochlear implant, choice of communication methodology, and provision of family support. In order to realize these advantages, screening and assessment must be viewed as the initial steps in a seamless family- and child-centred service with a multidisciplinary focus.
Results
Coverage
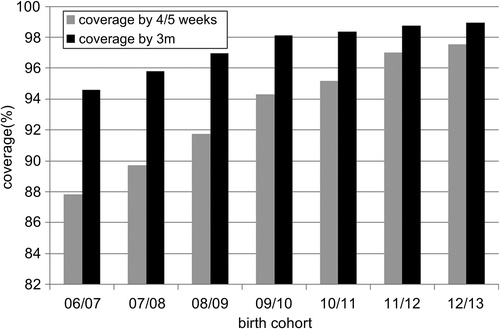
shows the screen coverage by 4/5 weeks of age and by three months of age for England. Coverage by three months of age has been high from the start of the programme in 2006 and for the most recent birth cohort reaches 98.95%. Coverage by 4/5 weeks of age has steadily improved from just below 88% for the 06/07 birth cohort to 97.54% for the 12/13 birth cohort. There are a small number of screen declines (typically < 0.1%), and a small number of families who do not respond to the screening invitation in spite of the best efforts of services.
Refer rates
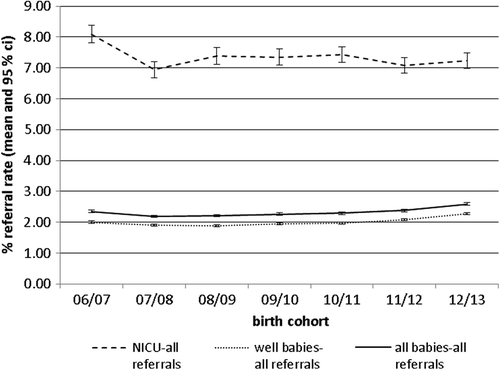
shows the total refer rate for well babies and NICU babies by year of birth cohort. Total refer rate includes babies who refer on one or both ears and babies referred directly to audiology (e.g. with microtia/atresia or who have recovered from neonatal meningitis).
Refer rates are within the programme quality standard target of 3% for well babies and 9% for NICU. More recently the refer rate for well babies is showing an upward trend. This is currently under investigation; a possible explanation is that screening is being carried out earlier due to changes in maternity discharge practice as programmes are striving to improve coverage by 4/5 weeks of age.
Follow up
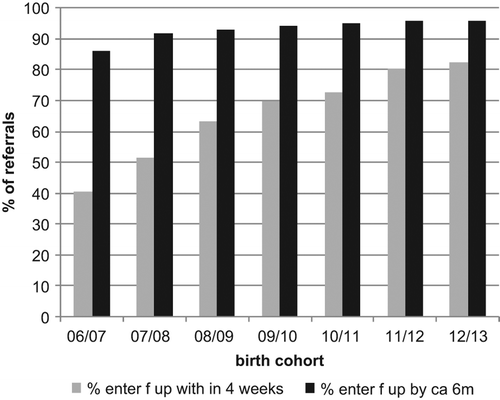
shows the proportion of referred babies attending a follow up appointment within four weeks of the referral decision or by 44 weeks GA. The purpose of the latter condition is to allow some leeway for audiological assessment to be delayed for premature babies to allow them to reach full term. Performance early in the programme was initially poor but has improved considerably over the time period and reached 82.5 % for the 12/13 birth cohort. It has not yet reached the quality standard target of 90%. also shows the proportion of referred babies that have attended an audiological assessment by six months of age. This has improved gradually and reaches 95.8% for the 12/13 birth cohort.
Yield
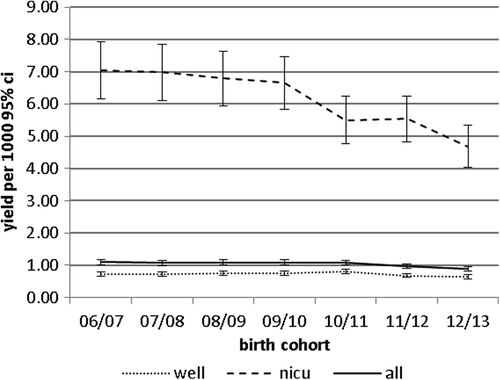
shows the yield of bilateral PCHL from the screen. This is the number of babies identified in the screen refer group per 1000 of the population screened. Overall the rate is just under 1 per 1000. The yield for NICU babies is showing a downward trend particularly for the 12/13 birth cohort. The reason for this is unclear at present (see Discussion).
Positive predictive value
Positive predictive value (PPV) is the probability that an individual has the target condition, given a positive screening test result. (CitationRaffle & Gray, 2009). It is calculated by dividing the true screen positives by the total screen positives and is conventionally expressed as a number between 0 and 1. For ease of interpretation we have expressed PPV as the percentage of screen positives that have the target condition. shows a matrix of PPV values for different screening outcomes (all referrals, bilateral referrals, and unilateral referrals), for all PCHL and separately for bilateral and unilateral PCHL for three population groups (all babies, NICU and well). Values are calculated using data for all births between 1 April 2006 and 31 March 2013.
Table 1. PPV (95% CI) by screen outcome for PCHL for births 1 April 2004 to 31 March 2013 (values are percentages).
Age at identification of bilateral PCHL
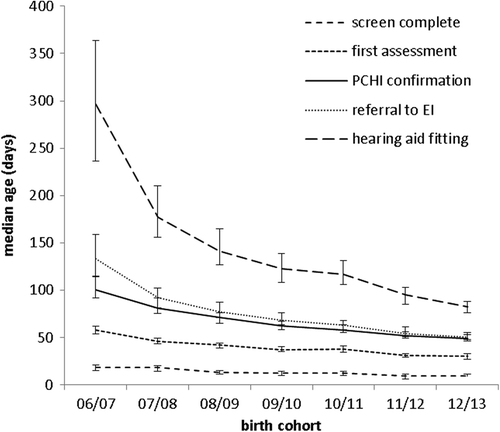
shows, for children with bilateral PCHL who referred from newborn screening, the median age (in days) at screening completion, first assessment, confirmation of PCHL, referral to early intervention services, and hearing-aid fitting. This demonstrates a reduction since the screen started on all measures but particularly in the last three.
Discussion
Coverage
Coverage is a key performance indicator for screening programmes and is monitored closely. Coverage is high. In hospital programmes the majority of babies are screened before discharge and outpatient clinics are provided for babies discharged from hospital before screening can be completed. In community programmes babies are screened at home. Screening is free and widely accepted. Screening services use multiple strategies to maximize coverage by adopting family-friendly practices, implementing initiatives to organize screening appointments for babies that do not complete the screen in hospital, and enlisting the support and input of primary care professionals for “hard to reach” families.
Refer rate
The quality standard for the refer rate for NHSP is ≤ 3% overall and this is further specified as ≤ 1% bilateral refer and ≤ 2% unilateral refer. The refer rate reported here is within our quality target. There is an upward trend in referral rate in well babies and this is currently being monitored and investigated. Possible reasons for this are reduction in age at screening linked to maternity discharge patterns. The final refer rate in any newborn hearing screening programme is crucially dependent on the number of stages and type of screening test used, on the number of test attempts permitted and accepted within each stage, and on the age at screening. Few screening programmes include full details of these aspects in their reports. Many North American programmes report a routine outpatient rescreen prior to referral to audiology which will reduce the refer rate. The American Academy of Audiology specifies an overall refer rate of < 4% (CitationJCIH, 2007). The refer rate reported here is lower than that but substantially higher than the 0.3% reported by the Netherlands programme (Citationvan der Ploeg et al, 2012) using an identical screening protocol. However the majority of babies in the Netherlands are screened at home at or after four days of age: age at screen is known to have a significant effect on refer rates (CitationThornton et al, 2003; CitationHergils, 2007).
Follow up
Early entry into follow-up within four weeks of screen completion/ by 44 weeks GA has improved steadily since implementation, but has not yet reached the quality target of 90%. This aspect requires on-going vigilance and effort with initiatives to identify and disseminate good practice (CitationWood, 2012) and to encourage the worst performing programmes to improve to the level of the best performing programmes. Early entry into follow-up requires good teamwork and communication between the screening and the audiology teams, good communication with parents, and a smooth process that (ideally) allows direct booking of follow-up audiology appointments by screeners at the point of screen completion. Much work has been undertaken to embed such flexible practices as routine within paediatric audiology services.
Although not yet reaching the quality target performance, these follow up rates are much better than those in the USA where some programmes report that just over half referrals receive follow up diagnostic testing and only a third with PCHI are documented as receiving intervention by six months of age (CitationASHA, 2008; CitationGaffney et al, 2010). In contrast programmes in Australia and New Zealand generally report follow up rates approaching 100% (CitationBarker et al, 2012)
Yield
CitationFortnum and Davis (1997) reported the results of a retrospective ascertainment of all cases of permanent bilateral hearing loss of moderate or greater degree in children born in the UK's Trent health region between 1985 and 1993. Based on the birth cohorts for 1985–1990, the prevalence of moderate-to-profound congenital bilateral PCHL was 1.12 per 1000 live births (95% CI 1.01–1.23). The yield reported here is similar. There appears to be a trend to a reduced prevalence in the NICU population in recent years but it remains to be determined if this represents a genuine reduction. Alternatively some cases of hearing loss in the screen refer group are either not manifest or not identified by the immediate post screen audiological assessment. The overall prevalence of around one per thousand is widely reported in the developed world (CitationHyde, 2005; Citationvan der Ploeg et al, 2012).
There is much interest in the increase in prevalence of PCHL through childhood. CitationFortnum et al (2001) reported an observed prevalence rising from 0.91 per 1000 (95% per cent CI 0.85–0.98) for the three-year-old cohort to 1.65 per 1000 (95% CI 1.62–1.68) for the nine-year-old cohort, where it levelled off. They then included an adjustment for under-reporting based on data for the sub group that had received cochlear implants. This adjustment increased the estimate to 1.07 at age three years rising to 2.05 at age nine years. They concluded that there are more late-onset and progressive permanent childhood hearing loss than previously suspected. To date there are no longitudinal population studies that confirm the extent of this increase in prevalence in the UK population. For Australian children CitationChing et al (2006) reported a prevalence of 1.04/1000 at three years of age increasing to 1.57/1000 at age 16 years. Preliminary data from the screening programme in England show that the prevalence of bilateral PCHL for the 06/07 birth cohort has increased from the 1.09/1000 identified by the screen to 1.36/1000 by the age of seven years. This is consistent with the observed prevalence of Fortnum et al. However there remains the possibility of incomplete ascertainment of later identified cases.
Positive predictive value (PPV)
Overall the PPV for all babies for all PCHL is fairly low at 6.7% (1 in 15). For bilateral referrals this increases to 16.0% (1 in 6). For babies screened under the NICU protocol the PPV for all referrals for all PCHL is 10.9% (1 in 9). For unilateral referrals the PPV for bilateral PCHL is 0.8% (1 in 126). Not all newborn hearing screening programmes follow up unilateral referrals. The primary rationale for doing so in this programme is primarily as a means of increasing the sensitivity for bilateral PCHL given the possibility of a false negative screening test result. Arguably there is also benefit in identifying unilateral PCHL early in life to enable monitoring for progression of hearing loss and/or episodes of otitis media with effusion. However the benefits and disbenefits of some aspects of management (e.g. early hearing-aid fitting) remain uncertain.
Age at identification of PCHL, referral to early intervention and hearing-aid fitting
CitationFortnum and Davis (1997) reported median ages at referral, confirmation, and fitting of hearing aids, respectively, as 10.4, 18.1, and 26.3 months for births in the period 1985–93. Although this was 20 years ago it was not untypical of (and in fact probably better than) most services in England prior to the introduction of universal newborn hearing screening. These ages are also fairly typical in western developed countries: CitationHarrison and Roush (1996) reported that in the USA, prior to widespread newborn hearing screening, the age at identification and intervention often exceeded two years. CitationWake et al (2005) showed strikingly similar results to those of Fortnum at al for children born January 1991 to July 1993 in Victoria, Australia with median age at confirmation, hearing-aid fitting and referral to early intervention services as 18.5, 19.5, and 23 months respectively.
The data presented here show that for those identified with bilateral PCHL the median age at screen completion, entry into follow up, confirmation, referral to early intervention, and hearing-aid fitting have reduced over the time period and are 9, 30, 49, 50, 82 days respectively for the 12/13 birth cohort. This represents an enormous improvement that can be attributed to universal newborn hearing screening.
The data for the 06/07 birth cohort shows that although referral to early intervention was achieved for the majority within the first six months, the process of assessment, confirmation and referral extended over the first six months. Since then the age at which these events occur has reduced; referral to early intervention now occurs very shortly after confirmation and provision of amplification much sooner thereafter and well within the first three months. During this period there has been much attention to the quality and timeliness of paediatric audiology diagnostic and habilitation services. In 2006 many services were largely relying on air-conduction click ABR for the assessment of screen referrals. Since then there has been extensive development of guidelines and training; services now routinely use air- and bone-conduction frequency-specific stimuli in ABR assessment and the quality of testing and interpretation is much improved (CitationLightfoot et al, 2012; CitationStevens et al, 2013; CitationSutton & Lightfoot, 2013). This has contributed to improved confidence and reliability in early audiological assessment and identification of PCHL.
Conclusion
There has been a continuous improvement in the performance of the screening programme over the first seven years. The programme now achieves coverage of 98.9%, refer rate of 2.59%, and 82% entry into follow up within four weeks. The yield of bilateral PCHL from the screen is around 1/1000 as expected. For those identified with bilateral PCHL the median age at screen completion, entry into follow up, confirmation, referral to early intervention, and hearing-aid fitting have reduced over the time period and are 9, 30, 49, 50, 82 days respectively for the 12/13 birth cohort. The screening programme exceeds its major aim of screening within three months and identification of bilateral PCHL by six months.
These achievements have been produced by a nationally organized programme with clear protocols, procedures, and standards. There remains an increase in prevalence with age, although the exact extent of this increase in prevalence remains uncertain and professionals and parents need to remain alert to this possibility and reactive systems must be in place to deal with such cases.
Declaration of interest: The authors report no declaration of interest. The authors alone are responsible for the content and writing of this paper.
References
- ASHA. 2008. Loss to follow up in Early Hearing Detection and Intervention (Technical report). www.asha.org/policy.
- Bamford J., Ankjell H., Crockett R., Marteau T., McCracken W. et al. 2005. Evaluation of the newborn hearing screening programme in England: Studies, results, and recommendations. Report to Department of Health and National Screening Committee.
- Barker M.J., Hughes E.J. & Wake M. 2012. NICU-only versus universal screening for newborn hearing loss: Population audit. Journal of Paediatrics and Child Health, 49, E74–E79.
- British Society of Audiology. 1988. Descriptors for pure tone audiograms. Br J Audiol, 22, 123.
- Ching T.Y.C., Oong R. & van Wanrooy E. 2006. The ages of intervention in regions with and without universal newborn hearing screening and prevalence of childhood hearing impairment in Australia. Aus New Zeal J Audiol, 28, 137–150.
- Crockett R., Baker H., Uus K., Bamford J. & Marteau T.M. 2005. Maternal anxiety and satisfaction following infant hearing screening: A comparison of the health visitor distraction test and newborn hearing screening. J Med Screen, 12 (2), 78–82.
- Crockett R., Wright A.J., Uus K., Bamford J. & Marteau T.M. 2006. Maternal anxiety following newborn hearing screening: The moderating role of knowledge. J Med Screen, 13 (1), 20–5.
- Davis A., Bamford J., Wilson I., Ramkalawan T., Forsaw M. et al. 1997. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technol Assessment, 1 (10).
- El-Rafei A., Parker D. & Bamford J.M. 1996. Otoacoustic emissions versus ABR screening: The effect of external and middle-ear abnormalities in a group of SCBU neonates. Br J Audiol, 30, 3–8.
- Fortnum H. & Davis A.C. 1997. Epidemiology of permanent childhood hearing impairment in Trent region, 1985–1993. Br J Audiol, 31, 409–46.
- Fortnum H.M., Summerfield A.Q., Marshall D.H., Davis A.C. & Bamford J.M. 2001. Prevalence of permanent childhood hearing impairment in the UK and implications for universal neonatal hearing screening: Questionnaire based ascertainment study. BMJ, 323, 536–9.
- Gaffney M., Green D.R. & Gaffney C. 2010. Newborn hearing screening and follow up: Are children receiving recommended services? Public Health Reports, 125 (2), 199–207.
- Harrison M. & Roush J. 1996. Age of suspicion, identification and intervention for infants and young children with hearing loss: A national study. Ear Hear, 17, 55–62.
- Hergils L. 2007. Analysis of measurements from the first Swedish universal neonatal hearing screening programme. Int J Audiol, 46, 680–685.
- Hyde M.L. 2005. Newborn hearing screening programmes: Overview. J Otolaryngol, 34, Suppl. 2, S70–S78.
- Joint Committee on Infant Hearing (JCIH). Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 120, 898–921.
- Korver A.M.H., Konings S., Dekker F.W., Beers M., Wever C.C. et al. 2010. Newborn hearing screening vs. later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. J Am Med Assoc, 304, 1701–1708.
- Lightfoot G. Sutton G.S. & Wood S.A. 2012. Post newborn hearing screening ABR: Quality assurance and the role of peer review. ENT and Audiology News, 20, 73–79.
- Newborn hearing screening care pathways. 2013. http://healthguides.mapofmedicine.com/choices/map/newborn_hearing_screening1.html
- NHS newborn hearing screening programme quality standards. http://hearing.screening.nhs.uk/standardsandprotocols
- Raffle A.E. & Gray J.A.M. 2009. Screening: Evidence and Practice. Oxford University Press.
- Stevens J.C., Sutton G.J. & Wood S.A. (eds.). 2013. Guidelines for the Early Audiological Assessment and Management of Babies Referred from the Newborn Hearing Screening Programme (v3.1). http://hearing.screening.nhs.uk/audiology
- Sutton G.J. & Lightfoot G. (eds.). 2013. Guidance for Auditory Brainstem Response Testing in Babies (v2.1). http://hearing.screening.nhs.uk/audiology
- Thornton A.R.D., Kimm L. & Kennedy C.R. 2003. Methodological factors involved in neonatal screening using transient-evoked otoacoustic emissions and automated auditory brainstem response testing. Hear Res, 182, 65–76.
- UKNSC. UK National Screening Committee. http://www.screening.nhs.uk/about
- Uus K. & Bamford J. 2006. Effectiveness of population-based newborn hearing screening in England: Ages of interventions and profile of cases. Pediatrics, 117(5), e887–93.
- Uus K., Bamford J. & Taylor R. 2006. An analysis of the costs of implementing the national newborn hearing screening programme in England. J Med Screen, 13(1), 14–9.
- Uus K., Bamford J., Young A. & McCracken W. 2005. Readiness of paediatric audiology services for newborn hearing screening: Findings and implications from the programme in England. Int J Audiol, 44(12), 712–20.
- Van der Ploeg C.P.B., Uilenburg N.N., Kauffman-de Boer M.A. & Oudesluys-Murphy A.M. 2012. Newborn hearing screening in youth health care in the Netherlands: National results of implementation and follow up. Int J Audiol, 51, 584–590.
- Wood S.A. 2012. How can we improve follow up for newborn hearing screening referrals? Oral presentation at the 6th International Conference Beyond Newborn Hearing - Infant and Childhood Hearing in Science and Clinical Practice, Como, Italy.
- Wood S.A., Davis A.C. & Sutton G.J. 2013. Effectiveness of targeted surveillance to identify moderate to profound permanent childhood hearing impairment in babies with risk factors who pass newborn screening. Int J Audiol, 52, 394–399.
- World Health Organization. 2013. Classification of hearing impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/
- Wake M., Poulakis Z., Hughes E.K., Carey-Sargeant C. & Rickards F.W. 2005. Hearing impairment: A population study of age at diagnosis, severity, and language outcomes at 7–8 years. Arch Dis Child, 90, 238–244.
- Young A., McCracken W., Tattersall H. & Bamford J. 2005. Interprofessional working in the context of newborn hearing screening: Education and social services compare challenges. J Interprof Care, 19(4), 386–95.
- Young A., Tattersall H., Uus K., Bamford J. & McCracken W. 2004. To what extent do the characteristics of the object of evaluation influence the choice of epistemological framework? The case of universal newborn hearing screening. Qual Health Res, 14(6), 866–74.