ABSTRACT
Patients with ventilatory failure due to chronic obstructive pulmonary disease (COPD) are increasingly managed with long-term non-invasive positive pressure ventilation (NIPPV) and this may improve survival. NIPPV can frequently be interrupted but there are few data detailing the short-term effects and none on the longer-term consequences of treatment withdrawal. Ten patients withdrew from NIPPV for 1 week and were randomised to restart NIPPV or to continued withdrawal for up to 6 months. Outcomes assessed included daytime blood gases, nocturnal ventilation, lung function, exercise capacity and health status. After 1 week of withdrawal PaO2, PaCO2, nocturnal oximetry, lung function and exercise capacity did not change, but mean nocturnal transcutaneous CO2 (6.3 (1) vs. 7.6 (1.1) kPa p = 0.04) and daytime blood gas bicarbonate (30.3 (4.5) vs. 31.2 (3.9) mmol/L p = 0.04) rose. During a 6-month period of withdrawal of nocturnal NIPPV, daytime PaCO2 (6 (1.1) vs. 7.5 (1.3) kPa p = 0.002) increased and health status (total St George's Respiratory Questionnaire score 55.5 (6.3) vs. 65.6 (10) p = 0.006) worsened. Three out of five patients met a priori criteria to restart NIPPV in the continued withdrawal group. Short interruptions to domiciliary NIPPV used to manage chronic ventilatory failure as a consequence of COPD do not cause a rapid clinical deterioration but nocturnal ventilation worsens and daytime bicarbonate levels increase following 1 week's cessation. Thereafter, daytime PaCO2 rises and health status worsens, supporting the role of long-term NIPPV in the management of such patients.
INTRODUCTION
Long-term nocturnal non-invasive positive pressure ventilation (NIPPV) used to manage chronic ventilatory failure associated with chronic obstructive pulmonary disease (COPD) is not of proven benefit (Citation1) but a recent randomised controlled trial suggests a possible survival advantage (Citation2). Increasing numbers of patients have been started on this treatment (Citation3) and a third of all long-term NIPPV in Europe is provided for this indication (Citation4). Up to 19% of COPD patients discharged from respiratory intensive care units are reported to require long-term NIPPV (Citation5). COPD patients with severe hypercapnia and nocturnal hypoventilation are thought to be the most likely to respond to treatment (Citation6) and current consensus statements conclude domiciliary NIPPV may be considered in such individuals (Citation7).
Interruptions to domiciliary NIPPV may occur for a variety of reasons. Upper respiratory tract infections can make mask delivered ventilation intolerable for short periods and patients may need to suspend treatment to allow facial pressure areas to heal. Mechanical failure of the ventilator or equipment has been reported in 39% of patients (Citation8). Few data are available on the consequences of interruptions to treatment. The effects of withdrawing NIPPV from patients treated for ventilatory failure associated with restrictive thoracic disease (RTD) have been investigated (Citation9, 10). Deterioration in nocturnal ventilation was noted but there were no changes in lung function or daytime arterial blood gases (ABG). In a further study withdrawal of long-term NIPPV for six days from a population with varied diagnoses, including COPD, found no significant changes in daytime ABG's but effects on nocturnal ventilation were not reported (Citation11).
The aims of this study were two-fold. The first was to examine the impact of withdrawing NIPPV for 1 week in a population of COPD patients established on NIPPV to manage severe, chronic ventilatory failure to assess if interruptions to therapy cause a rapid decline in ventilatory status, an important consideration for centres supporting COPD patients receiving domiciliary NIPPV. The second was to perform a randomised controlled trial examining the effects of prolonged withdrawal of treatment.
METHODS
Subjects were recruited between 1st July 2005 and 30th September 2006 from patients under the care of a tertiary referral centre that specialises in the provision of domiciliary NIPPV. Data were collected prospectively. Ethical approval for the protocol was granted by the local research ethics committee and the research complied with the principles laid down in the Declaration of Helsinki. Written consent was obtained from all participants. Randomisation was performed independently of the research team, using sealed opaque envelopes. Blinding of participants was not possible as all were experienced NIPPV users and would be aware if sham NIPPV was used. The trial was assigned ISRCTN00075564.
Protocol
Participants were identified using the centre's computer database. Patients were contacted by post and telephoned to invite them to attend a screening outpatient appointment.
Screening entry criteria
Diagnosis of COPD: FEV1 (forced expiratory volume in 1 second) <50% predicted, FEV1/FVC (forced vital capacity) ratio <70%, TLC (total lung capacity) >80% predicted.
Smoking history >20 pack years.
Prior to commencing NIPPV chronic hypercapnic ventilatory failure with a normal pH (7.35–7.45) and daytime PaCO2 >7.5 kPa or nocturnal transcutaneous CO2 (PtcCO2) >9 kPa.
Treated with NIPPV for at least 3 months and compliance of a minimum of 4 hours/day.
Screening exclusion criteria
Age over 80 years.
Other significant respiratory disease (interstitial lung disease, asthma, bronchiectasis, neuromuscular or restrictive chest wall disorders)
Documented Left Ventricular dysfunction with an Ejection Fraction <40%
Following screening, patients continued domiciliary NIPPV. Four weeks later, at trial entry, clinical stability was confirmed using the following criteria; no increase in breathlessness, cough or sputum in the last 4 weeks, PaCO2 within ± 1 kPa of screening value and no change (>15% and 200 mls) in FEV1 from screening value. Patients meeting these criteria were recruited and admitted to hospital for 48 hours to monitor withdrawal from NIPPV. Baseline outcome measures were collected prior to the first night of withdrawal.
Table 1. Historical ABG and lung function data prior to NIPPV (mean (SD))
Nasal airflow measurement, chest and abdominal plethysmography and oxygen saturation monitoring (Embla, ResMed, Poway, CA) were performed on the first night of withdrawal to exclude significant obstructive sleep apnoea (OSA). Data were recorded by an integrated system (Embla, ResMed, Poway, CA) and scored manually by trained sleep medicine registered polysomnographers. Apnoeas were defined as a cessation of airflow for 10 seconds or more and hypopnoeas as a reduction in airflow of over 50% for 10 seconds or a 30% reduction in airflow associated with a 4% oxygen desaturation. The apnoea/hypopnoea index (AHI) was calculated on the basis of the total recording time, typically 2300–0700 hrs. Those with an AHI over 10 were excluded. At this point subjects were prospectively randomised to a continued withdrawal (NIPPV-) or planned re-introduction to NIPPV (NIPPV+) group following the scheduled one week cessation of treatment.
A week after cessation of NIPPV subjects were admitted again for nocturnal monitoring of pulse oximetry (SpO2), PtcCO2 and assessment of outcome measures. NIPPV was restarted in the NIPPV+ group at identical settings to those previously used. The compliance of the NIPPV+ group was monitored by the ventilators’ internal meters that record duration of use.
Patients in both groups were reviewed at 3, 5, 9, 13, 17, 21 and 25 weeks and outcome data collected. No other changes to treatment, including long-term oxygen therapy (LTOT), were made during the trial period unless required for acute exacerbations of COPD.
Outcomes
The primary endpoint was withdrawal failure defined by any one of the following criteria:
Daytime PaCO2 >9 kPa
Mean nocturnal PtcCO2 >10 kPa
Respiratory acidosis (pH <7.35)
Intolerable symptoms, including morning headaches, breathlessness and drowsiness.
Subjects meeting any of these criteria were instructed to recommence NIPPV and follow-up continued.
Secondary outcomes
Arterial blood gas analyses were performed following 20 minutes rest, self ventilating and during working hours (0900–1700). Patients on LTOT had ABG sampling performed on their usual oxygen flow rate. ABG samples were tested using an automated blood gas analyser (GEM Premier 3000, Instrumentation Laboratory, Lexington, MA). The overnight SpO2 (3900 Datex-Ohmeda, Louisville, CO) and PtcCO2 (TCM3 Radiometer, Copenhagen) were recorded using finger probes and a heated electrode attached to the forearm, respectively. PtcCO2 was calibrated to a known CO2 concentration. Both signals were sampled once a second and analysed using an automated system “Download 2000” (Stowood Scientific instruments, Oxford, UK), which calculates mean values for the period monitored, typically 23.00 Hrs to 07.00 Hrs.
Health status was assessed with a disease specific questionnaire, the St Georges Respiratory Questionnaire (SGRQ) (Citation12). Spirometry was performed using a rotary turbine pneumotachograph (Micromedical, Kent, UK). Maximum inspiratory (MIP) and expiratory (MEP) pressures were measured using a manometer (Micromedical, Kent, UK). Nasal clips were worn. Technique was visually assessed and the best of three measurements recorded. An incremental shuttle walking test assessed exercise capacity (Citation13).
Statistical analysis
Statistical analysis was performed using statistical software (SPSS 15.0, SPSS, Chicago, IL). Data were assessed to establish normal distribution using the Shapiro–Wilk test. For comparison of characteristics between groups and of baseline with follow-up values, unpaired and paired t tests or the non-parametric Mann–Whitney U-test and Wilcoxon rank test were used wherever appropriate. To analyse the effect of withdrawal of NIPPV, if patients met withdrawal failure criteria and restarted treatment, their last values prior to restarting NIPPV were carried forward. A p-value of <0.05 was regarded as statistically significant.
RESULTS
Eighty-seven patients met entry and exclusion criteria and were invited to join the study. Nineteen gave consent to be screened of whom 9 were excluded and 10 were randomised. Of those excluded: 4 were diagnosed with OSA as a confounding cause of ventilatory failure on withdrawal of NIPPV, two had recurrent exacerbations preventing entry, one had poor compliance with NIPPV, one withdrew consent and one subject suffered a severe exacerbation and died between screening and trial entry.
One-week withdrawal of NIPPV
Ten (3 female) subjects were withdrawn from NIPPV for 1 week. Prior to withdrawal, patients received pressure control ventilation (NIPPY 2, B&D Electromedical, Stratford, UK) with a mean inspiratory pressure (IPAP) 30 (6) cmH2O, expiratory pressure (EPAP) 4 (1) cmH2O and back-up respiratory rate 16 (2) breaths per minute. All had used NIPPV for over one year with a mean compliance of 7.4 (1.7) hours/day. The subjects’ historical ABG and lung function data prior to commencing NIPPV are displayed in . Values at trial entry are shown in . No significant changes in ABG, spirometric, mouth pressure, exercise tolerance or SGRQ values took place between screening and trial entry. The mean AHI of the group was 4.6 (2.8).
Table 2. Values at trial entry
No subjects met the primary end-point of withdrawal failure during the first week. Comparisons between secondary outcomes are displayed in showing a statistically significant rise in mean nocturnal PtcCO2 and daytime ABG bicarbonate after one week of withdrawal.
Table 3. Comparison in outcome variables between 1st and 7th day of withdrawal (n = 10)
Continued withdrawal of NIPPV
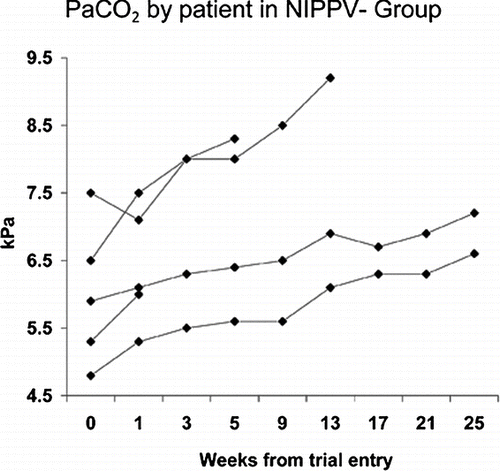
There were no statistically significant differences between groups at randomisation in blood gas tensions, lung function or exercise capacity. Two subjects in the NIPPV-Group continued LTOT and three in the NIPPV+ Group. Patients 1–5 as presented in comprised the NIPPV- Group. Three subjects in the NIPPV− Group met the primary endpoint of withdrawal failure during follow-up; one because of intolerable breathlessness (after 21 days), one developed respiratory acidosis (day 50) and one a daytime PaCO2 over 9 kPa (day 103). None of the NIPPV+ Group met withdrawal failure criteria and demonstrated good compliance with mean NIPPV usage of 7.6 (1.6) hours/day. The two patients in the NIPPV – Group who did not meet withdrawal failure criteria chose to restart NIPPV at the end of the trial.
There was a significant rise in daytime arterial PaCO2 from 6 (1.1) to 7.5 (1.3) kPa (p = 0.002) during follow-up in the NIPPV- Group. All patients in the NIPPV- Group displayed a rise in PaCO2 over time in comparison to those in the NIPPV + Group ( and ). There were no statistically significant differences between the groups in daytime PaCO2 () following 6 months withdrawal. The values of PaO2 declined during follow-up in the NIPPV- Group but this did not meet statistical significance.
There were no significant changes or trends in spirometric, mouth pressure or exercise capacity data within or between the groups. There were no significant differences between the groups in all SGRQ components at baseline or changes in any SGRQ components in the NIPPV+ Group at the end of 6 months treatment. In the NIPPV- Group there was a statistically significant increase in the total (55.5 (6.3) vs. 65.6 (10) p = 0.006) and impact SGRQ score (44.6 (8.6) vs. 57.8 (10) p = 0.01) after 6 months. Comparison between groups shows higher impact (43 (7.1) vs. 57.8 (10) p = 0.03) and symptom (46.4 (13.8) vs. 65.4 (10.5) p = 0.04) scores in the NIPPV- Group at the end of the trial. There was a trend to increased total SGRQ score in the NIPPV- Group but this did not reach statistical significance (65.6 (10) vs. 55 (8.6) p = 0.1).
DISCUSSION
The short-term withdrawal of domiciliary NIPPV initiated to manage chronic ventilatory failure in COPD did not lead to significant changes in lung function, exercise capacity, PaO2 or PaCO2 but nocturnal ventilation did worsen and metabolic compensation with a rise in daytime bicarbonate occurred. This is the first randomised controlled study to assess the longer-term impact of withdrawing NIPPV. Deterioration in disease specific health status and ventilation, as reflected by daytime PaCO2, was observed. Our results show that short interruptions to domiciliary NIPPV in stable well characterised COPD patients are not associated with a rapid decline in ventilatory status but longer term withdrawal is associated with clinical deterioration.
Safety of short-term withdrawal
One other study has examined the short-term effects of withdrawing nocturnal NIPPV in COPD patients (Citation11). Eleven subjects with mixed diagnoses, including 6 COPD patients, were studied. In agreement with the current findings no statistically significant changes in pH, PaCO2 and PaO2 or lung function values were observed in the group after 6 days’ withdrawal of therapy. In addition to previous findings the effects of short-term withdrawal of NIPPV on nocturnal ventilation were assessed. We documented no change in mean or minimum nocturnal SpO2 but these measurements may have been influenced by the high proportion of subjects receiving LTOT.
A significant rise in mean PtcCO2 was observed suggesting worsening of nocturnal ventilation. This was combined with a compensatory rise in diurnal plasma bicarbonate levels as would be expected (Citation14). Given the low number of COPD patients included to date in withdrawal studies of NIPPV it is difficult to draw firm conclusions. Greater numbers may have revealed a statistically significant difference in diurnal PaCO2 but the clinical effect of a small (0.3 kPa) mean rise in daytime PaCO2 is unclear. In our well characterised and stable population a weeks cessation of domiciliary NIPPV was not associated with a rapid decline in ventilatory status but four patients (21%) excluded had undiagnosed OSA. This may have led to a more rapid deterioration and COPD patients may often have concomitant OSA (Citation15). Therefore caution is warranted in the general application of these results.
Longer-term withdrawal
All subjects withdrawn from NIPPV experienced a progressive rise in PaCO2 whilst in those treated with NIPPV no discernable trend was evident. An increase in PaCO2 by 0.7 kPa over a year has been associated with increased mortality in COPD patients receiving LTOT (Citation16). Supporting nocturnal ventilation with NIPPV in the control subjects appears to prevent a progressive decline in daytime ventilation and is in keeping with reports of reduced hospital admissions (Citation17) and improved prognosis (Citation2, Citation18). In the study the subjects received “high-intensity” NIPPV with a mean inspiratory pressure of 30 cm H2O prior to withdrawal and in the control arm. This appeared to improve ventilatory parameters from those prior to NIPPV in agreement with other published reports using high-intensity NIPPV (Citation19). High ventilatory pressures during treatment may be required to increase ventilation, reduce plasma bicarbonate and maintain central drive to breathe allowing ventilatory homeostasis to be achieved (Citation14).
The decline in health status with the withdrawal of NIPPV is mirrored by improvements seen when NIPPV is initiated (Citation20, 21), although not all studies have reported such findings (Citation2). Conclusions are difficult to reach because of the potential for placebo effect in an unblinded study. It is of interest to note that few patients chose to enter the trial (19/87) suggesting, regardless of any perceived burden of nocturnal ventilation, benefit may be experienced by patients. At the end of the study the two patients who remained off NIPPV elected to restart treatment.
Limitations
This study recruited few participants as a consequence of the single centre design, rigorous inclusion and exclusion criteria, and lack of volunteers to have NIPPV withdrawn. This has limited the power of the study to draw firm conclusions but the results will act as a proof of concept and inform power calculations for future research. The randomisation produced poorly matched groups, but it is likely that no bias in favour of NIPPV was introduced as younger patients with better ABG parameters were randomised to withdraw from therapy. The trial design using withdrawal of treatment as the intervention raises some methodological issues; changes seen may be due to a detrimental effect of NIPPV, such as respiratory muscle atrophy, which becomes apparent on withdrawal of treatment.
This interpretation seems less likely as the effect should be greatest immediately after withdrawal with gradual recovery and the data show a gradual and progressive deterioration. Our patients show a rise in PaCO2 on withdrawal but they may be returning to their pre-treatment condition. There is a paucity of evidence to support the long term use of NIPPV in COPD (1) and although improvements in ABGs (Citation21) and quality of life (Citation20, 21) are reported the effects on exacerbation, hospital admission and uncorrected mortality rates remain to be established. The subjects recruited had relatively high BMIs (mean 31 kg/m2) with some in the range where obesity hypoventilation may have contributed to the development of ventilatory failure, although OSA was excluded. BMI may have had an effect on the response to NIPPV and its withdrawal in this population.
In summary, short interruptions to nocturnal ventilatory support used to manage ventilatory failure associated with COPD do not appear to have significant clinical impact. Longer-term withdrawal was associated with a rise in daytime PaCO2 that was preceded by worsening nocturnal ventilation and a rise in plasma bicarbonate. This suggests nocturnal hypoventilation may play a role in the development of daytime ventilatory failure in COPD. The importance of the assessment and titration of NIPPV, and possibly the application of high-intensity NIPPV, to improve nocturnal ventilation and lower diurnal bicarbonate levels should be born in mind in the design of future studies examining long-term ventilatory support in COPD.
Declaration of interest
The authors have no conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wijkstra PJ, Lacasse Y, Guyatt GH, Goldstein R, Struik F. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No. CD002878.
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, O’Donoghue FJ, Barnes DJ, Grunstein RR. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomized controlled trial. Thorax 2009; 64:561–566.
- Janssens JP, Derivaz S, Breitenstein E, Muralt B, Fitting J-W, Chevrolet J-C, Rochat T. Changing patterns in long-term noninvasive ventilation. Chest 2003; 123:67–79.
- Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Fauroux FB, Ribert D, Schoenhofer B, Simonds AK, Wedzicha JA. Patterns of home mechanical ventilation use in Europe. Results from the Eurovent survey. Eur Respir J 2005; 25:1025–1031.
- Cuvelier A, Viacroze C, Benichou J, Molano LC, Hellot M-F, Benhamou D, Muir J-F. Dependency on mask ventilation after acute respiratory failure in the intermediate care unit. Eur Respir J 2005; 26:289–297.
- Wijkstra PJ. NIPPV in stable patients with COPD. Respir Med 2003; 97:1086–1093.
- Anonymous. Clinical indications for non-invasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Srinvasan S, Doty SM, White TR, Segura VH, Jansen MT, Davidson Ward SL, Keens TG. Frequency, causes and outcomes of home mechanical ventilator failure. Chest 1998; 114:1363–1367.
- Hill NS, Eveloff SE, Carlilse CC, Goff SG. Efficacy of nocturnal nasal ventilation in patients with restrictive thoracic disease. Am Rev Respir Dis 1992; 145:365–371.
- Jiminez JFM, Escuin JS, Vicente CD, Valle MH, Otero FF. Nasal intermittent positive pressure ventilation: analysis of its withdrawal. Chest 1995; 107:382–388.
- Karakurt S, Fanfulla F, Nava S. Is it safe for patients with chronic hypercapnic respiratory failure undergoing home noninvasive ventilation to discontinue ventilation briefly? Chest 2001; 119(5):1379–1386.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
- Singh SJ, Morgan, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47:1019–1024.
- Elliott MW. Domiciliary non-invasive ventilation in stable COPD? Thorax 2009; 64:553–556.
- Chaouat A, Weitenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of COPD and sleep apnoea syndrome. Am J Respir Crit Care Med 1995; 151:82–86.
- Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med 1998; 158:188–193.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58:867–871.
- Quinnell TG, Pilsworth S, Shneerson JM, Smith IE. Prolonged invasive ventilation following acute ventilatory failure in COPD: Weaning results, survival, and the role of noninvasive ventilation. Chest 2006; 129:133–139.
- Windisch W, Vogel M, Sorichter S, Hennings E, Bremer H, Hamm H, Matthys H, Virchow JC. Normocapnia during NIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir Med 2002; 96:572–579.
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, Ambrosino N. The Italian multicentre study on non-invasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:529–538.
- Meecham-Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538–544.