ABSTRACT
Gamma-glutamyl transferase (GGT) is a clinical marker of biliary disease, but is also of importance in anti-oxidant metabolic pathways and, consequently, is a potential biomarker of oxidative stress in COPD. Serum GGT is increased in alpha-1 antitrypsin deficiency (AATD) but this could reflect a hepatic, systemic or pulmonary origin. We aimed to investigate the relationship between serum GGT, lung disease, liver disease and mortality in subjects with AATD. Serum GGT was measured at the baseline assessment in 334 PiZ subjects from the UK AATD registry, and related to static lung function, chronic bronchitis, sputum purulence, history of acute exacerbations, smoking status, mortality, alcohol consumption, cirrhosis and serum markers of liver disease. GGT correlated with airflow obstruction and was associated with chronic bronchitis. GGT levels were higher in current smokers compared with ex-smokers and never smokers, and in non-survivors compared with survivors. Although GGT related to alcohol consumption and established liver disease, it was independently related to FEV1, mortality, smoking history and male gender. In conclusion, although serum GGT reflects the presence of liver disease it is independently associated with airflow obstruction and mortality. Further studies are needed to establish the role of GGT in oxidative lung injury, and its use as a potential biomarker in chronic inflammatory lung disease.
INTRODUCTION
AATD predisposes to chronic obstructive pulmonary disease (COPD)(Citation1) and also to liver disease, including neonatal cholestasis (Citation2), clinical evidence of cirrhosis (Citation3,4) particularly in early life, and primary hepatocellular carcinoma (Citation5). The pathogenesis of these conditions is heterogeneous but inflammation is understood to be a common component(Citation6). COPD is associated with neutrophilic inflammation (Citation7) and oxidant stress (Citation8) and the levels of inflammatory biomarkers, such as the acute phase reactant C-Reactive Protein (CRP), predict outcome (Citation9, 10). Biomarkers are recognised to be important in predicting future outcome in several chronic diseases (Citation11, 12), but their role in alpha-1-antitrypsin deficiency (AATD) is less well characterised.
Serum gamma glutamyl transferase (GGT) is present in high concentrations in the biliary tree and is conventionally used in clinical practice as a marker of liver disease (Citation13). Transient elevation of GGT occurs in all infants with AATD, but only 11% of infants have neonatal jaundice and only 6% have clinical evidence of liver disease (Citation2). The abnormalities of liver function generally settle in adult survivors. However, a recent clinical study of adult subjects from the UK registry for AATD, that excluded subjects with cirrhosis, found that serum GGT was increased above the normal range in 27% (Citation14). These findings suggest that the origin of serum GGT in some subjects with AATD may be non-hepatic. Furthermore, since GGT is found in several organs (Citation15), including the lung (Citation16), it is unlikely to be a specific marker of biliary or liver disease alone.
Lung tissue is also a source of GGT under normal physiological conditions (Citation16), and is the only enzyme capable of breaking the γ-linkage in glutathione (GSH), the main antioxidant in the lung (Citation17). Animal models have shown that the concentration and activity of GGT increases in alveolar epithelial cells in response to oxidative stress (Citation18) and that GGT-deficiency is associated with the development of severe bronchiolar cellular injury and pulmonary oedema (Citation19).
This observational study explored the possibility that serum GGT would be related to inflammation associated with COPD in subjects with AATD independently of an anticipated, primary association with liver disease. Therefore, the level of serum GGT was assessed in 334 patients with the PiZ phenotype of AATD, and related to the presence of factors known to influence the measurement as well as the severity of lung disease, liver disease, and subsequent mortality.
MATERIALS AND METHODS
Subject selection
All subjects with the PiZ phenotype were identified from the database of the ADAPT (Antitrypsin Deficiency Assessment and Programme for Treatment) UK registry, and the first individuals recruited with baseline serum measurements that included GGT at the time of assessment were selected (n = 344). ADAPT enrols all referred subjects and those identified via family screening with the PiZ, PiSZ and rare mutations irrespective of the presence of lung or liver disease. Subjects are assessed annually while in the stable state (i.e., free from any exacerbation in the preceding 6 weeks).
Subject characterisation
GGT was measured by spectrophotometric assay (reference range <40 IU/L for females and <50 IU/L for males, 2% coefficient of variation) using an Instrumentation Laboratory IL-900, (Instrumentation Laboratory Ltd., Warrington, UK). Measurements were recorded at baseline in all patients and had been repeated over the subsequent 3 years in 128 of the subjects. The presence of liver disease was characterised by evidence of a previous clinical diagnosis of liver disease, and by assessing routine liver function tests (serum levels of aspartate transaminase [AST], alkaline phosphatase [ALP] and bilirubin) for subclinical disease. Subjects with impairment of liver function tests were investigated further with hepatic ultrasound imaging and, in some cases, liver biopsy.
Lung function measurements were obtained as described previously (Citation20) and the severity of COPD was staged according to the National Institute for Clinical Excellence (NICE) criteria (Citation21).
Subjects with chronic bronchitis (Citation22) were identified from clinical history, and sputum was observed in the stable clinical state to determine the degree of purulence (Citation23) using the BronkoTest sputum colour chart (BronkoTest, Middlesex, UK). A record of the number of exacerbations requiring a change in therapy over the previous year was obtained by direct questioning, along with details relating to smoking status and the number of pack-years of cigarette smoking. Information on average weekly alcohol intake was collected by direct questioning and converted to conventional weekly units.
Relationship between GGT and clinical phenotype
GGT was related to alcohol consumption by comparing serum GGT levels between 3 subject groups defined by reported alcohol consumption: Group 1, no alcohol consumption; Group 2, alcohol consumption within UK government guidelines and Group 3, alcohol consumption in excess of government guidelines (>14 units per week for females and >21 units per week for males (Citation24)).
COPD severity, defined by FEV1 (%predicted), FEV1/FVC ratio and NICE disease stage was related to serum GGT level.
Relationship between GGT and mortality
Causes of death were ascertained from death certification, hospital medical records and by contacting the family practitioners. Baseline serum GGT levels were also compared between subjects who subsequently died of non-hepatic causes and survivors.
Statistical analysis
Data were analysed using SPSS 12.0.1 for Windows (SPSS Inc, Chicago, IL, U.S.A.). The relationship between serum GGT and continuous variables was determined by Spearman's coefficient, and differences between GGT and categories of ordinal data determined by the Jonckheere-Terpstra test. The Kruskal–Wallis test was used to determine differences in serum GGT for non-ordinal categorical variables with more than 2 groups, and the Mann–Whitney test was used to assess the difference in serum GGT for variables with 2 groups.
Although several factors were shown to relate to GGT levels, we were unable to perform multiple logistic regression analysis as some of the categorical variables had more than 2 groups. We therefore used a univariate analysis of variance test, to determine which variables remained independently associated with GGT after correction for all other associated factors entered into the model. (This test also takes multiple related factors into account and identifies those independently related to GGT, and hence is distinct from the ANOVA test, which does not.) GGT underwent log transformation in order to obtain the necessary parametric distribution for this analysis. p < 0.05 was considered significant. The study was approved by the South Birmingham Research and Ethics Committee and all patients gave written informed consent.
RESULTS
The mean age of subjects was 50.0 years (SE ± 0.6) and there was a predominance of male subjects (61%). The overall mean GGT for the cohort was 43.6 IU/L (SE ± 2.0) and there was no change (p = 0.328) in GGT from baseline (39.9, SE ±2.9) to 3 years (43.0, SE ±3.0). Twenty-six percent of subjects had a value above the normal range of baseline, and males had a greater (p < 0.001) mean serum GGT (51.9, SE ± 2.7) than females (30.7, SE ± 2.6). There was no identifiable relationship between age and GGT (r = 0.088, p = 0.105).
Relationship between GGT and COPD
Serum GGT was greater (p = 0.001) in the 272 subjects with established COPD (mean = 45.4, SE ± 2.4) than the 72 without airflow obstruction (36.8, SE ± 3.8), and was negatively correlated with post-bronchodilator FEV1% predicted (r = −0.148, p = 0.006), and with post-bronchodilator FEV1/FVC (r = −0.127, p = 0.018) (). There was no relationship between serum GGT and transfer factor corrected for alveolar volume (DLCO/VA) expressed as% predicted (r = −0.040, p = 0.5).
Figure 1. Relationship of GGT to COPD severity according to NICE [21]. The mean GGT (± SEM) is shown related to increasing severity in the stage of COPD. The significance of the relationship (p) is shown using the Jonckheere-Terpstra test.
![Figure 1. Relationship of GGT to COPD severity according to NICE [21]. The mean GGT (± SEM) is shown related to increasing severity in the stage of COPD. The significance of the relationship (p) is shown using the Jonckheere-Terpstra test.](/cms/asset/d8604775-465f-44c9-a2ee-629f2569b014/icop_a_463682_f0001_b.gif)
GGT was higher (p = 0.029) in subjects with a history of chronic bronchitis (45.6, SE +/- 3.1, n = 137) compared to those without (42.2, SE ± 2.7, n = 207), and was elevated in ex-smokers (43.9, SE ± 2.4; p = 0.009, n = 237) and current smokers (48.8, SE ± 5.3; p = 0.003, n = 37) compared with subjects who had never smoked (39.6, SE ± 5.1, n = 70). There was a positive correlation with the number of pack-years of cigarette smoking (r = 0.162, p = 0.007), but no relationship with sputum purulence (p = 0.2) or the number of exacerbations over the previous 12 months (p = 0.4).
Relationship between GGT and liver disease
There were no significant differences in demographic and lung function data between subjects with a clinical history of liver disease and those without (). Individuals with a proven diagnosis of cirrhosis (n = 5) had significantly elevated GGT that was approximately 3 times the upper limit of the reference range (116, SE ± 30.8; p = 0.004). However, there was no difference in GGT levels between subjects with a history of jaundice (39.9, SE ± 5.4, n = 38, p = 0.196) or those with a previous diagnosis of hepatitis (62.5, SE ±18.1, n = 12, p = 0.434) compared to those with no such history (44.1, SE ± 2.2, n = 289). Bilirubin, AST and ALP correlated with GGT (r = −0.185, p = 0.001; r = 0.301, p < 0.001; r = 0.289, p < 0.001 respectively) but were abnormal in only 4.4%, 6.2% and 0.3%, respectively, of subjects who did not have a clinical diagnosis of cirrhosis.
Table 1. Demographic, smoking, alcohol and pulmonary function data for each liver disease category
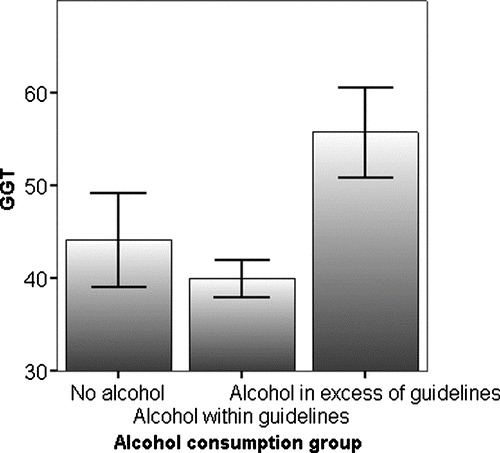
GGT was positively related to reported estimates of alcohol consumption (r = 0.249, p < 0.001) but subjects who consumed alcohol within government guidelines (40.0, SE ± 2.0, n = 188), had a similar (p = 0.108) serum GGT to subjects who did not consume alcohol (44.2, SE ± 5.1, n = 103). Nevertheless, GGT was above the gender related reference range in 43% of patients who exceeded the daily recommended alcohol intake. The average value in this group (55.8, SE ± 4.9, n = 51) was greater than that for both non-drinkers (p < 0.001) or those who drank within recommended guidelines (p < 0.001) ().
Relationship between GGT and mortality
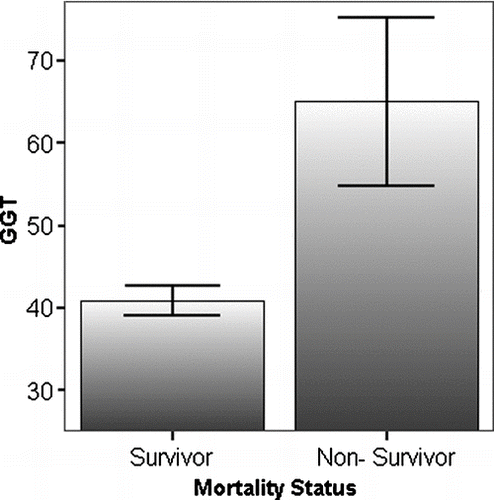
Mortality status could not be determined for 15 of the 344 subjects and these patients were excluded from the mortality analysis. Thirty-nine deaths were reported at the time of analysis in the remainder of the subjects. The cause of death was COPD in 21 subjects, post-lung transplant complications in 3, malignancy in 4, pulmonary embolus in 2, cardiovascular disease in 2, liver disease in 3 and cerebral vasculitis, endocarditis, intracranial bleed and pancreatitis in 1 case each. The baseline GGT in the non-survivors was greater than in survivors (65.0, SE ± 10.2 v 40.8, SE±1.8, p = 0.001), and this difference was unaltered by removal of the 3 patients who died of liver related illness (64.8, SE ± 11.0; p = 0.001), as shown in .
Factors independently related to GGT
FEV1 (p = 0.044), mortality (p = 0.041), gender (p < 0.001), smoking (p = 0.007), AST (p < 0.001), ALP (p < 0.001) and a clinical diagnosis of liver cirrhosis (p = 0.023) were independently associated with serum GGT, following correction for all other factors associated with GGT. The presence of liver cirrhosis and elevation of ALP were not independent predictors of GGT levels within the normal range.
Relationship between lung and liver disease
There was no significant correlation between other serum markers of liver disease and FEV1 (r = 0.085, p = 0.119 for bilirubin; r = 0.035, p = 0.524 for AST; r = −0.014, p = 0.524 for ALP) or KCO (r = 0.014, p = 0.80; r = −0.027, p = 0.622; r = 0.020, p = 0.714, respectively).
DISCUSSION
The current observational study explored the clinical relationships to GGT for subjects with AATD and in particular, identified the prevalence of elevated levels of serum GGT, and explored whether GGT specifically reflected liver disease or was a also a potential marker of inflammation reflected in oxidant stress associated with COPD. The data indicate that the prevalence of an elevated level of serum GGT in patients with AATD (26%) was more than double the prevalence in healthy volunteers (11%)(Citation25). GGT was closely related to the clinical presence of liver cirrhosis, and the serum levels of AST, ALP and bilirubin, as expected from the usual use of serum GGT measurement as an indicator of biliary and liver disease. However, the data also indicate that serum GGT was related to the severity of airflow obstruction and respiratory mortality. The relationship between GGT and COPD was independent of other factors, including clinical and biochemical evidence of liver disease, and smoking history.
A previous study in an unselected population of 127 Swedish infants with a PiZ phenotype also identified a high prevalence of raised serum GGT. All subjects between the ages of 2–4 months were found to have increased levels, but the proportion was shown to decline with increasing age (Citation26). In the current study, the level of GGT correlated with the level of other serum markers that are usually associated with liver pathology. Consequently, it is likely that the demonstrated elevation of GGT in these few subjects primarily reflects liver dysfunction. The concordant relationship that exists between serum GGT and other ‘liver function tests’ in hepatic disease is well established, although details of the pathogenesis of liver disease in AATD remain unclear (Citation6).
It is likely that alcohol consumption contributes to liver dysfunction in AATD and a relationship between GGT and excessive alcohol consumption was demonstrated in the current study (and has also been described in otherwise healthy subjects (Citation27)). However, the retention of polymeric Z protein in the hepatocellular endoplasmic reticulum is also likely to be of primary pathogenic importance and is understood to be pro-inflammatory (Citation6). Whether serum GGT levels reflect oxidant stress associated with hepatic inflammation or a direct cytopathological effect of Z polymers is speculative.
Notwithstanding the association that was demonstrated between GGT and liver disease, a significant independent relationship was also identified between post-bronchodilator FEV1 FEV1/FVC and GGT that was independent of both clinical evidence of cirrhosis and other biochemical markers of liver disease. There was no identifiable relationship between GGT and KCO and these findings together would suggest that GGT is related to airways disease rather than emphysema (Citation28). A previous study in COPD not related to AATD has also demonstrated a relationship between GGT and chronic bronchitis (Citation29) suggesting that our observation is not related to parallel deterioration of subclinical liver disease. Furthermore, the prevalence of airways disease in AATD has been shown to be greater than previously recognised(Citation30) and markers of inflammation, including serum CRP, relate to airways disease scores, but not to emphysema severity (Citation31).
Consequently, it is likely that serum GGT is derived from both hepatic and pulmonary origins in this cohort of patients. It is possible that those subjects with the most severe lung disease also have a greater degree of sub-clinical liver disease, but the absence of a relationship between FEV1 and the other biochemical markers of liver disease does not support this explanation. Furthermore, lung function parameters were similar between subjects who had clinical evidence of liver disease and those who did not (), and importantly FEV1 remained independently related to GGT even after correction for bilirubin, AST and ALP.
GGT is involved in regeneration of intracellular glutathione and may therefore be important in maintaining antioxidant defences. Inflammatory cells, in particular neutrophils, are a potent source of oxidants. The neutrophil is central to the pathophysiology of COPD (Citation32), releasing reactive oxygen species and proteinases, and the number of airway neutrophils relates to the severity of airway obstruction in COPD (Citation33). Consequently, the relationship between GGT and the functional and clinical features of COPD that were demonstrated in the current study is consistent with an increased oxidant burden and mucus hypersecretion (Citation34) that is associated with greater neutrophilic infiltration. In addition, the relationship between GGT and smoking status would support the contention that the level of GGT reflects oxidative stress, because the high level of oxidants that are present in cigarette smoke would likely be associated with greater oxidative stress in current smokers and, therefore, higher serum GGT levels. GGT may therefore reflect local oxidative stress in the lung, as well as systemic oxidative stress perhaps reflecting the increased need for cysteine to sustain increased glutathione and protein synthesis associated with other co-morbidities such as cardiovascular disease and diabetes described by others (Citation35, 36). Nevertheless our population is young and co-morbidities were rare suggesting the lung is likely to be the central focus for oxidative stress.
We also demonstrated that serum GGT was independently related to mortality in AATD, and this is consistent with a recent study performed in a general population (Citation37). Although it is possible that this relationship is partly a reflection of the severity of liver disease, most of our patients died of lung disease suggesting that serum GGT levels specifically reflect pulmonary pathophysiological features that increase mortality in these patients. Furthermore, the relationship between GGT and mortality remained unchanged even when subjects with clinical and biochemical evidence of liver cirrhosis were excluded from the analysis.
As with CRP, many factors have been reported to be related to serum GGT, and although no study to date has been able to capture all data on all reported associations, we believe that we have explored the effects of the majority of these issues as fully as possible within the confines of this observational study which suggests that more work is warranted to explore the possibility that GGT could be a novel biomarker of lung disease. The current population have a low incidence of confounding co-morbidities and generally take few medications known to occasionally affect GGT due to their young age. Furthermore, no subjects are assessed at ADAPT if they are currently taking antibiotics which can also influence GGT levels. Further prospective studies are therefore needed which record all factors known to be associated with GGT to explore its value as an indicator of lung disease, both in AATD and also in usual COPD, where the prevalence of liver disease is comparable to the normal population.
In conclusion, although GGT is conventionally considered a sensitive marker of liver disease and is recommended as a simple tool for hepatic monitoring in patients with AATD (Citation38), we have observed that serum GGT is also associated with the severity of lung disease and subsequent respiratory mortality. Consequently, this relationship should be considered when interpreting the results of GGT measurement in patients with AATD. It is possible that GGT may represent a novel biomarker for respiratory disease, although more definitive work is clearly required.
ACKNOWLEDGMENTS
Thanks to past and present medical, nursing and administrative staff at ADAPT for collecting data and to Peter Nightingale for statistical advice. The study was sponsored by an unrestricted grant from Talecris Biotherapeutics, North Carolina, USA. The sponsor had no involvement in the study design, collection, analysis and interpretation of data, writing reports or the decision to submit the manuscript.
Declaration of interests
JH has received lecturing fees from GSK and Astra Zeneca. RAS has received fees for consultancy as part of an advisory board from GSK, Boehringer, Roche, Schering Plough and MSD. Lecture fees have been paid to RAS by Talecris and GSK. RAS has received industry sponsored non-commercial grants from AZ, Altana and Talecris, to cover research costs and staff salaries. DP has received a grant from the European Union for £ 79853.57 to conduct research. He has also received approximately US$4,000 as lecture fees and US$10,000 towards subsistence and travel to international conferences from various pharmaceutical companies. He has received approximately US $20,000 in consultancy fees.
REFERENCES
- Laurell C, Eriksson S. The electrophoretic alpha1-globulin pattern of serum in alpha-1-antitrypsin deficiency. Scand J Clin Lab Invest 1963; 15:132–140.
- Sveger T. The natural history of liver disease in alpha 1-antitrypsin deficient children. Acta Paediatr Scand 1988 Nov; 77(6):847–851.
- Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med 1969 Jun; 73(6):934–939.
- Cox DW, Smyth S. Risk for liver disease in adults with alpha 1-antitrypsin deficiency. Am J Med 1983 Feb; 74(2):221–227.
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med 1986 Mar 20; 314(12):736–739.
- Lomas DA, Parfrey H. Alpha1-antitrypsin deficiency. 4: Molecular pathophysiology. Thorax 2004 Jun; 59(6):529–535.
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest 2002 May; 121(5 Suppl):151S–5S.
- Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation 2005 Feb; 29(1):23–32.
- Shaaban R, Kony S, Driss F, Leynaert B, Soussan D, Pin I, Change in C-reactive protein levels and FEV1 decline: a longitudinal population-based study. Respir Med. 2006 Dec; 100(12):2112–2120.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004 Jul; 59(7):574–580.
- Abraham J, Campbell CY, Cheema A, Gluckman TJ, Blumenthal RS, Danyi P. C-reactive protein in cardiovascular risk assessment: a review of the evidence. J Cardiometabol Syndr. 2007 Spring; 2(2):119–123.
- Ndumele CE, Pradhan AD, Ridker PM. Interrelationships between inflammation, C-reactive protein, and insulin resistance. J Cardiometabol Syndr 2006 Summer; 1(3):190–196.
- Sheehan M, Haythorn P. Predictive values of various liver function tests with respect to the diagnosis of liver disease. Clin Biochem 1979 Dec; 12(6):262–263.
- Stockley RA, Griffiths D, Holme J, Stockley EK. Liver function in alpha-1 antitrypsin deficiency (AATD). American Thoracic Society International Conference, San Diego, CA, 2006.
- Nemesanszky E, Lott JA. Gamma-glutamyltransferase and its isoenzymes: progress and problems. Clin Chem 1985 Jun; 31(6):797–803.
- Wetmore LA, Gerard C, Drazen JM. Human lung expresses unique gamma-glutamyl transpeptidase transcripts. Proc Natl Acad Sci USA 1993 Aug 15; 90(16):7461–7465.
- Haddad JJ. Glutathione depletion is associated with augmenting a proinflammatory signal: evidence for an antioxidant/pro-oxidant mechanism regulating cytokines in the alveolar epithelium. Cytokines Cell Mol Ther 2000 Dec; 6(4):177–187.
- Kugelman A, Choy HA, Liu R, Shi MM, Gozal E, Forman HJ. Gamma-Glutamyl transpeptidase is increased by oxidative stress in rat alveolar L2 epithelial cells. Am J Respir Cell Mol Biol 1994 Nov; 11(5):586–592.
- Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol 2002 Oct; 283(4):L766–776.
- Dowson LJ, Guest PJ, Hill SL, Holder RL, Stockley RA. High-resolution computed tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health status. Eur Respir J 2001 Jun; 17(6):1097–1104.
- National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Chronic obstructive pulmonary disease. Thorax 2004 Feb; 59(Suppl 1):1–232.
- Committee on the Aetiology of Chronic Bronchitis. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their. Lancet 1965 Apr 10; 1(7389):775–779.
- Stockley RA, Bayley D, Hill SL, Hill AT, Crooks S, Campbell EJ. Assessment of airway neutrophils by sputum colour: correlation with airways inflammation. Thorax 2001 May; 56(5):366–372.
- Department of Health. Alcohol Needs Assessment Research Project (ANARP): The 2004 national alcohol needs assessment for England. London: Department of Health, 2005.
- Bo S, Gambino R, Durazzo M, Guidi S, Tiozzo E, Ghione F, Associations between gamma-glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population-based cohort: a possible implication for oxidative stress. World J Gastroenterol. 2005 Dec 7; 11(45):7109–7117.
- Piitulainen E, Carlson J, Ohlsson K, Sveger T. Alpha1-antitrypsin deficiency in 26-year-old subjects: lung, liver, and protease/protease inhibitor studies. Chest 2005 Oct; 128(4):2076– 2081.
- Rosalki SB, Rau D, Lehmann D, Prentice M. Gamma-glutamyl transpeptidase in chronic alcoholism. Lancet 1970 Nov 28; 2(7683):1139.
- Panzner P, Lafitte JJ, Tsicopoulos A, Hamid Q, Tulic MK. Marked up-regulation of T lymphocytes and expression of interleukin-9 in bronchial biopsies from patients With chronic bronchitis with obstruction. Chest 2003 Nov; 124(5):1909–1915.
- Barton AD, Powers JL, Lourenco RV. Gamma glutamyl transpeptidase in chronic obstructive pulmonary disease. Proc Soc Exp Biol Med 1974 May; 146(1):99–103.
- Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2007 Dec 15; 176(12):1215–1221.
- Parr DG, Reynolds JH, Guest PJ, Bayley D, Sullivan AS, Stockley RA. The relationship between inflammation and COPD phenotype in Alpha-1 antitrypsin deficiency (AATD). American Journal of Respir Crit Care Med 2007; 175:A990.
- Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999 Nov; 160(5 Pt 2):S49–52.
- Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 1998 Oct; 158(4):1277–1285.
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury. Airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001 Nov 15; 164(10 Pt 2):S76–80.
- Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol 1995 Oct 1; 142(7):699–708.
- Lee DH, Jacobs DR, Jr., Gross M, Kiefe CI, Roseman J, Lewis CE, Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem 2003 Aug; 49(8):1358–1366.
- Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vascul Biol 2007 Jan; 27(1):127–133.
- American Thoracic Society/European Respiratory Society Statement: Standards for the Diagnosis and Management of Individuals with Alpha-1 Antitrypsin Deficiency. Am J Respir Crit Care Med 2003 Oct 1; 168(7):818–900.