ABSTRACT
Recent advances in chronic obstructive pulmonary disease (COPD) treatment offer symptom relief, but disease modification remains an unmet goal of pharmacotherapy. Reducing the frequency and severity of COPD exacerbations may help slow disease progression and reduce the morbidity, mortality, and costs associated with these major events. Other desirable characteristics for a COPD treatment include a once-daily dosing schedule, an oral formulation, and a low frequency of systemic side effects. Phosphodiesterase 4 inhibitors have been in clinical development for some years and roflumilast is currently the most advanced of these agents. In this review, the preclinical evidence, clinical safety, and efficacy of roflumilast available in published reports are considered. The data reviewed here suggest that the clinical efficacy of roflumilast occurs through a mechanism unrelated to bronchodilation and may be due to the suppression of lung inflammation. Lung function improved with roflumilast treatment and in some studies, the reduction in exacerbations was substantial and statistically significant. Notably, this effect appeared to be greatest in the subgroup of patients with more severe disease and more severe exacerbations. The evaluation of roflumilast safety largely centers on gastrointestinal adverse events, with diarrhea, nausea, and weight loss occurring more frequently with the drug than placebo. If approved for general use, we expect roflumilast to find its role initially as a substitute for inhaled corticosteroids in the maintenance treatment of severe and very severe disease, particularly in patients who have frequent acute exacerbations, and perhaps as a supplementary drug when symptoms are not adequately controlled by current conventional COPD therapy.
INTRODUCTION
Our current understanding of the pathophysiology of chronic obstructive pulmonary disease (COPD) and its management are the subject of several comprehensive, consensus reviews included in the GOLD Guidelines (Citation1) and in Statements from the American Thoracic Society and European Respiratory Society (Citation2, 3). Despite recent advances in treatment that contribute to symptom relief, effective disease-modifying therapies are limited. Smoking cessation, at least early in the course of the disease, slows lung function loss and improves survival. Similarly, oxygen administration in hypoxic patients and volume reduction surgery in selected patients also improve survival. Survival benefits from other forms of therapy have been difficult to show. Two large, well-planned, well-conducted international trials, one with the anticholinergic drug, tiotropium and one with the combination of fluticasone and salmeterol, have shown trends toward a survival benefit (Citation4, 5). Other aspects of COPD pathophysiology, however, may also benefit from current therapy. Long-acting bronchodilators and inhaled corticosteroids (ICSs) slow the rate of lung function loss, although the effect is less than that achieved by smoking cessation early in the course of the disease and the clinical importance of the effects observed remains uncertain. In addition, several treatments can reduce exacerbation frequency. Thus, disease modification remains our most important unmet need in COPD (Citation6).
Acute exacerbations of COPD (AECOPDs) are associated with accelerated disease progression (Citation7–10). Although not yet established, it is reasonable to conclude that reducing the frequency and severity of AECOPDs would lead to a moderation of disease progression. Apart from that potential outcome, a reduction in AECOPDs is highly desirable in order to reduce the significant morbidity, mortality, and expense associated with these major events. The achievable reduction in exacerbations is limited with currently available COPD therapies: long-acting β2 agonists ((LABA)s; [3, 11, 12]), long-acting muscarinic antagonists ((LAMA)s; [3, 13]), ICSs (Citation13–16), and their combinations. A therapy that further reduces AECOPDs would be desirable.
Although the pathophysiological mechanisms of COPD disease progression and the AECOPDs that occur during its course are not fully understood, airway inflammation is present and plays an important role at all stages of the disease. Two important features of inflammation in established COPD include that it persists long after the inciting event (usually tobacco smoking) has ceased (Citation17, 18), suggesting that the inflammatory process is self-propagating. Second, airway inflammation in COPD responds poorly to corticosteroid administration (Citation19–21), presumably because it is associated with neutrophils, CD8+ T lymphocytes, and CD68+ macrophages, cells that are minimally inhibited by corticosteroids. Clearly the airway inflammation of COPD differs from that in asthma in many respects: cell types, inflammatory mediators, and response to treatments (Citation22–25). A therapy that would effectively address the inflammation present in COPD, both in its stable and acute stages, would be a major advance in COPD treatment.
To address these three issues, disease modification, reductions in the frequency and severity of acute exacerbations and effective suppression of COPD-type airway inflammation, new therapies and/or strategies are required. For purely practical reasons, other unmet needs include a once-daily oral dosing schedule and efficacy without risks of serious systemic side effects. In this regard, not a single new class of pharmacotherapy has been approved for COPD use in more than two decades.
Phosphodiesterase (PDE) 4 inhibitors have been in development for many years. Interest in this class of compound arose in the 1980s when it became clear that methylxanthines such as theophylline, which had been widely used for its bronchodilator action since the 1930s, were difficult to use and could be dangerous. As a consequence, safer substitutes were sought. About the same time it was discovered that: theophylline was a nonselective inhibitor of PDEs; there are multiple families of PDEs; one of these families, PDE4, is expressed in airway smooth muscle and in immune and pro-inflammatory cells (Citation26–29). Inhibitors of PDE4 were synthesized and appropriate candidates entered clinical development. Currently, the most advanced of these agents is roflumilast (Daxas), which has been submitted for the approval in Europe and USA as a maintenance treatment for COPD associated with chronic bronchitis in patients at risk of exacerbations. We review here the preclinical and clinical properties of roflumilast, its pharmacology, clinical efficacy, and safety as revealed in clinical trials.
PHARMACOLOGY OF PDE4 INHIBITORS
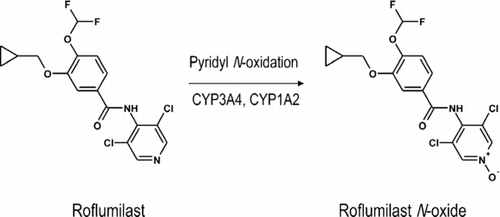
Cyclic nucleotide PDEs are a large family of enzymes that catalyze the inactivation of cyclic adenosine-3′,5′-monophosphate (cAMP) and/or cyclic guanosine-3′,5′-monophosphate (cGMP) to their respective nucleotide 5′-monophosphates (Citation30). Eleven families have been discovered with PDE4 representing the desired molecular target for roflumilast and related compounds (Citation30). In humans, PDE4 exists as a large number of variants that are encoded by four genes, PDE 4A, B, C, and D (Citation31). The enzymes have absolute specificity for cAMP, are expressed in immune and pro-inflammatory cells, and may, therefore, be of therapeutic potential as targets in COPD for small molecule inhibitors. Roflumilast and its primary metabolite, roflumilast N-oxide (), are very potent and competitive inhibitors of PDE4 (Citation32).
Both roflumilast and roflumilast N-oxide have IC50 values against PDE4 isolated from human neutrophils of 800 pM and 2 nM, respectively. They are highly selective for PDE4 and are essentially inactive against PDEs 1, 2, 3, 5, and 7 at concentrations up to 10 μM (Citation33). Roflumilast inhibits all PDE4 variants and this might contribute to its improved therapeutic ratio, relative to other development candidates such as cilomilast, which selectively inhibits PDE4D (see section “Safety Outcomes”). It is, however, approximately 10-fold less potent against PDE4C gene products (Citation33), a property shared with cilomilast (Citation34).
Pharmacodynamics of PDE4 inhibitors
The rationale for developing selective PDE4 inhibitors is based on three critical findings: PDE4 is a primary regulator of cAMP degradation in essentially all immune and pro-inflammatory cells; PDE4 inhibitors suppress a myriad of responses that are considered to be pro-inflammatory; and PDE4 inhibitors are efficacious in animal models of pulmonary inflammation (Citation26, 27, Citation35, 36).
With the exception of platelets, all immune and pro-inflammatory cells express PDE4 (Citation37). In most cases, these cells co-express multiple PDE4 variants derived from PDE4A, PDE4B, and PDE4D (Citation36). Currently, the isoforms that must be inhibited for the anti-inflammatory actions of PDE4 inhibitors to be realized largely are unknown. There is extensive in vitro data describing the inhibitory effect of roflumilast and PDE4 inhibitors on a variety of responses that, in vivo, are considered to be pro-inflammatory (Citation33, Citation38–42). Similarly, in preclinical animal models that reproduce specific facets of COPD pathophysiology, roflumilast is efficacious, suggesting that this drug might be disease modifying in human COPD (Citation43–46). PDE4 is also expressed in structural cells including fibroblasts, raising the possibility that targeting PDE4 could arrest the airway remodeling that is a defining characteristic of COPD and is thought to compromise long-term lung function.
The mechanism of action of roflumilast has not unequivocally been established. In animals, roflumilast does not protect against leukotriene D4- or 5-hydroxytryptamine-induced bronchoconstriction (Citation47, 48). Similarly, there is no evidence that PDE4 inhibitors evoke bronchodilation in patients with COPD (Citation49). Thus, beneficial effects on pro-inflammatory/immune cell function rather than on airway smooth muscle tone may account, in part, for the clinical efficacy of this compound in COPD. However, data supporting an anti-inflammatory effect of roflumilast in human subjects are limited. In a double-blind, cross-over, placebo-controlled study involving 38 patients with COPD who were minimally responsive to inhaled albuterol and whose mean post-bronchodilator FEV1 was 61% of predicted, roflumilast (500 μg/day for 4 weeks) reduced, at the end of the study, the absolute number of neutrophils, eosinophils, and lymphocytes in induced sputum by 36% (p < 0.0017), 50% (p < 0.0005), and 35% (p < 0.022), respectively, relative to placebo (Citation50). Significant reductions in eosinophil cationic protein, interleukin-8, neutrophil elastase, and α2-macroglobulin (a marker of microvascular leak) relative to placebo were also reported (Citation50). The ex vivo generation of TNFα induced by lipopolysaccharide (LPS) in whole blood (a biomarker of systemic inflammation) was reduced by 10.4% (p < 0.047). These actions of roflumilast on indices of inflammation were accompanied by a significant improvement in pre- and post-bronchodilator FEV1 (mean change 79.5 ml and 68.7 ml, respectively, compared to placebo; p < 0.0001). The concern with these data is that the statistical significance for most inflammatory endpoint measures was driven by the placebo. Thus, at the end of the placebo arm of the study, the absolute number of neutrophils and eosinophils was increased by ∼20–40% relative to baseline (Citation50). Similar effects were also seen for neutrophil elastase and α2-macroglobulin. Therefore, the anti-inflammatory activity of roflumilast may have been overestimated. The mechanism responsible for this rapid apparent “worsening” in inflammatory status after placebo is unclear and, while identified by the investigators, was not discussed. A possible explanation could be “regression to the mean,” because subjects were selected to have been “stable” and exacerbation-free for one month preceding enrollment. Although the mechanisms are not established, the available data are consistent with an anti-inflammatory effect of roflumilast.
Despite difficulties interpreting the aforementioned results, independent studies with roflumilast and evidence obtained with other PDE4 inhibitors (e.g. cilomilast, Bay 19–8004) support an anti-inflammatory mechanism of action for this class of compound in airway inflammatory diseases. Thus, roflumilast significantly inhibited the appearance of neutrophils in the bronchoalveolar lavage fluid of healthy subjects following segmental challenge with LPS (Citation51) and reduced LPS-induced TNFα generation ex vivo (Citation52). Similar anti-inflammatory effects have been reported with other PDE4 inhibitors (Citation49, Citation53).
Pharmacokinetics of roflumilast and roflumilast N-oxide
The absorption, distribution, metabolism, and excretion (clearance [CL]) of roflumilast have been studied in adults, adolescents, and children following oral administration (Citation54–56). In an open-label, randomized, two-period cross-over study involving 12 healthy, fasted, white adult subjects, the absorption of roflumilast (2 × 250 μg immediate release tablets) was rapid and complete after oral administration, with the time to achieve peak plasma concentration (Tmax) of approximately 1 hr (Citation55). Roflumilast given orally is highly bioavailable (F = 0.79), bound extensively (98.9%) to plasma proteins, achieves steady-state levels within 4 days of once-daily dosing, has an elimination half-life (t1/2) of 7–25 hr (mean ∼17 hr), and is subject to negligible first pass hepatic metabolism (Citation38, Citation54–57). Furthermore, after a single intravenous dose (120 μg) of roflumilast to healthy adult subjects, the CL and volume of distribution (Vd) were 13 L/hr and 2.92 L/kg, respectively, indicating pronounced distribution in tissues (Citation58).
A further study in 15 healthy subjects using an open-label, randomized, two-period, two-sequence crossover design established that oral administration (both single and repeat doses) of roflumilast (250 μg and 500 μg) provided dose-proportional systemic exposure with no difference between the single and repeat dose regimens. Similar dose proportionality data also were observed for roflumilast N-oxide (the primary metabolite—see below) indicating that both compounds display linear pharmacokinetics (Citation54).
In humans, after oral administration, the major metabolic pathway for the elimination of roflumilast is N-oxidation (). This process is catalyzed primarily by the mixed function oxidases, cytochrome (CYP) 3A4, and CYP1A2 to yield roflumilast N-oxide (). Significantly, this metabolite retains appreciable inhibitory activity against PDE4. The pharmacokinetics of roflumilast N-oxide generally are distinct from the parent compound. Thus, the Tmax is between 4 hr and 12 hr and the maximum observed plasma concentration (Cmax) is typically one- to two-fold higher (Citation54, Citation57, Citation59–61). Steady-state plasma levels of roflumilast N-oxide are usually achieved within 6 days of once-daily oral administration and the elimination t1/2 is approximately 27 hr, which is significantly prolonged relative to the parent compound. Finally, total systemic exposure estimated from the area under the concentration-time curve (AUC) exceeds that of roflumilast by approximately 10-fold (Citation58). Taken together, these data strongly suggest that the N-oxide accounts for about 90% of the biological action of roflumilast and produces a long-lasting, competitive inhibition of PDE4 over a 24-hr period such that roflumilast may be administered once daily. Roflumilast N-oxide is dealkylated (inactivated) primarily by CYP3A4 (with a small contribution by CYP2C19 and extrahepatic CYP1A1) (Citation57), glucuronidated and eliminated via the kidney (Citation59). Very low amounts of roflumilast and roflumilast N-oxide are excreted unchanged in urine.
Contraindications, effect of food and drug–drug interactions
No potential contraindications have, thus far, been identified. Although the metabolism of roflumilast is significantly arrested in patients with mild and moderate hepatic insufficiency leading to increased systemic exposure (AUC0–24 = 51% and 92% higher in patients meeting Child-Pugh A and Child-Pugh B criteria, respectively, when compared to healthy subjects), changes to the pharmacokinetics of roflumilast N-oxide are relatively modest (Citation62). Since the primary metabolite is believed to account for approximately 90% of the pharmacodynamic impact of roflumilast, the small pharmacokinetic changes reported are not believed to be clinically relevant. Thus, no dose adjustments are predicted to be required in patients with mild and moderate liver compromise (Citation62).
Similarly, although a high fat meal decreases Cmax and delays Tmax of roflumilast versus the fasted state, the same pharmacokinetic parameters are not changed for roflumilast N-oxide (Citation55). Thus, again, because the primary metabolite mediates most of the pharmacological effects of roflumilast, these data strongly suggest that the parent drug can be taken with or without food.
Patients with COPD typically have multiple co-morbidities for which they may receive other medications. The possibility that roflumilast and/or its N-oxide could interact unfavorably with drugs commonly used in COPD has, therefore, been evaluated. Neither roflumilast nor roflumilast N-oxide by themselves inhibit CYP3A4 or CYP1A2 (Citation40). Other data suggest a low potential for roflumilast to interact adversely with other drugs including montelukast, erythromycin, ketoconazole, budesonide, albuterol, midazolam (Citation40), and antacids containing magnesium hydroxide or aluminium hydroxide (Citation59–61, Citation63–67). This is important to determine because inducers of CYP3A4 and CYP1A2 have the potential to increase the CL of roflumilast thereby lowering its efficacy. Conversely, xenobiotics that are metabolized by the same enzyme(s) could compete with roflumilast, delay its inactivation and so increase systemic exposure, with the potential for adverse events. Thus far, only rifampicin, an antibiotic used to treat pulmonary tuberculosis, has been shown to significantly limit the efficacy of roflumilast due to its ability to induce xenobiotic-metabolizing enzymes that include CYP3A4, CYP2C19, and extrahepatic CYP1A2 (Citation57).
Polyaromatic hydrocarbons, which are constituents of tobacco smoke, are known to induce drug-metabolizing enzymes including CYP1A1 and CYP1A2 (Citation68–70). Although CYP1A2 contributes to the metabolism of roflumilast and, as such, may enhance the rate at which the N-oxide is produced in smokers (see above), the N-oxide itself is not a substrate for CYP1A2 (Citation40). Accordingly, no dose adjustments are likely to be required in patients with COPD who smoke tobacco.
CLINICAL EFFICACY
The clinical efficacy of roflumilast has been explored in six large prospective, randomized, double-blind, placebo-controlled studies that provide the majority of the efficacy and safety data that we now review (Table 1). A study by Rabe and colleagues (Citation71) was performed in 1,411 patients with moderate-to-severe COPD (mean post-bronchodilator FEV1 50% of predicted) and a documented absence of significant improvement of FEV1 (reversibility) following inhalation of albuterol 400 μg. Subjects were randomized to receive either 250 or 500 μg of roflumilast, or matching placebo, orally, once daily for 24 weeks. Two co-primary outcomes were specified: the change from baseline in post-bronchodilator FEV1 and the Saint George's Respiratory Questionnaire (SGRQ). The study was completed by 82% of subjects, withdrawals being somewhat higher in the active treatment arms. At the 24-week endpoint, the post-bronchodilator FEV1 dose-related increases over placebo were 74 ml and 97 ml in the roflumilast 250 and 500 μg groups, respectively (p < 0.0001), and both also significantly greater than baseline values. The pre-bronchodilator FEV1 and FVC similarly improved. The changes from baseline in SGRQ were −3.4 and −3.5 units for 250 and 500 μg of roflumilast, respectively, and both were statistically significant (p < 0.0001). However, when compared to placebo, which also improved, but to a lesser degree, neither of these improvements was statistically significant. Among secondary outcomes, fewer subjects in each of the active treatment arms experienced an AECOPD, the mean numbers of AECOPDs being 1.13, 1.03, and 0.75 per patient/year in the groups that received placebo, 250, and 500 μg of roflumilast, respectively. The results for both treatment groups for acute exacerbations were statistically significant compared to placebo (p = 0.0029, one-sided), the greatest effect being in the 500 μg roflumilast arm in which a 34% reduction was seen.

A 1-year study of similar design was performed with only the 500 μg dose of roflumilast and published by Calverley and colleagues (Citation72). The other main difference to the Rabe study was that enrollees were required to have more severe disease, with the actual mean post-bronchodilator FEV1 of enrollees reported as 41% of predicted. The primary efficacy variables were the change from baseline to endpoint in post-bronchodilator FEV1 (as in the Rabe study), and the number of moderate or severe exacerbations per patient per year, instead of SGRQ. Again, study dropouts were higher in the active treatment arm. The mean improvement with roflumilast in post-bronchodilator FEV1 from baseline compared with placebo was 39 ml (p < 0.001). The overall rate of moderate or severe exacerbations was not significantly different between roflumilast- and placebo-treated patients, 0.86 versus 0.92 exacerbations/patient/year. However, a post-hoc subgroup analysis showed that exacerbations were less frequent among GOLD stage IV subjects in the roflumilast group compared to the placebo-treated arm, 1.01 versus 1.59 exacerbations/patient/year, respectively (p = 0.024). The SGRQ, a secondary outcome, deteriorated similarly in both groups. In the subgroup with very severe disease, GOLD stage IV, there was a better, but still non-significant (p = 0.086), improvement in SGRQ in the roflumilast arm.
To expand on the findings above, an identical pair of studies (M2–124 and M2–125) in more select populations was performed and reported by Calverley and colleagues. The results were combined into a single publication (Citation73). Although similar in design to the previous studies (Citation71, 72), the Calverley 2009 report differed by specifying the pre-bronchodilator (rather than post-bronchodilator) FEV1 as a co-primary outcome. The rate of moderate or severe acute exacerbations was the other co-primary outcome. Entry criteria, which were designed to reproduce the subset that responded in the first two studies, included a post-bronchodilator FEV1 of 50% or less of predicted and a history of at least one acute exacerbation requiring corticosteroids or hospitalization in the previous year. Enrollees were allowed to continue use of a long-acting bronchodilator, but corticosteroids were withheld throughout the studies. A total of 3,091 subjects with a mean FEV1 of 36% of predicted were recruited and, after a 4-week run-in, randomized to receive either 500 μg of roflumilast or matching placebo, orally, once daily for 1 year. Subject withdrawals were marginally higher in the active treatment arm at the endpoint versus placebo (Citation35% vs. 31% in M2–124 and 32% vs. 31% in M2–125; ). Both primary outcomes were achieved (Table 2). The pre-bronchodilator FEV1 increased by a mean of 48 ml more in the treatment arm than placebo arm (p < 0.0001) (); and the rate of moderate or severe acute exacerbations was less in the roflumilast group than placebo arm in each of the individual studies, the pooled result being 1.14 versus 1.37 exacerbations/patient/year, respectively, a reduction of 17% (p = 0.0003). The time to first exacerbation in the roflumilast group was also significantly prolonged (p = 0.0185), but only in the pooled analysis. Of the secondary outcomes, only the Transition Dyspnea Index focal score was significantly improved (p = 0.0009) in the treatment compared to the placebo group, although the difference was less than the generally accepted clinically important difference.
Figure 2. Probability of treatment discontinuation in roflumilast and placebo groups in trials M2–124 (A) and M2–125 (B). Reproduced from Calverley PMA et al Lancet 2009;374:685–94 with permission. *Number of patients still at risk at the beginning of the respective week; number at risk might be different from the number completing the study because the protocol allowed patients to finish the study up to 7 days before the end of week 52.
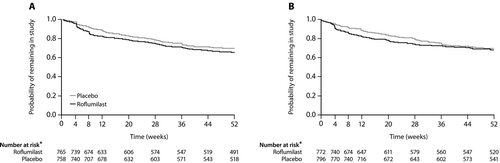
Figure 3. Prebronchodilator and postbronchodilator forced expiratory volumes in 1 s (FEV1) over 52 weeks in patients in roflumilast and placebo groups in trials M2–124 (A) and M2–125 (B), and changes in prebronchodilator and postbronchodilator FEV1 over 52 weeks in patients in roflumilast and placebo groups in trials M2–124 (C) and M2–125 (D). Error bars are SE. Reproduced from Calverley PMA et al Lancet 2009;374:685–94 with permission.
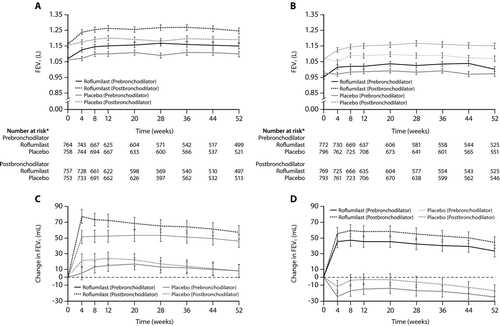
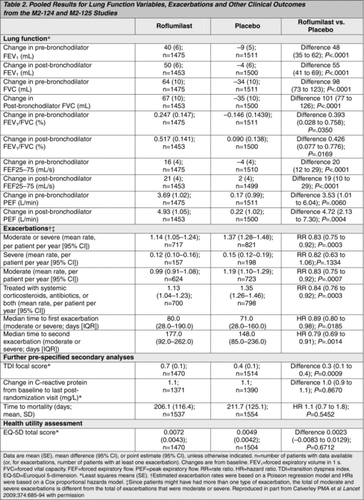
To summarize the efficacy results of these four Phase III studies, they each have two coprimary outcomes, one of which is an FEV1 outcome. Initially, it was the post-bronchodilator that was specified; later, the pre-bronchodilator FEV1. The latter is probably a more appropriate outcome for an anti-inflammatory agent that may have some bronchodilator effect. However, in either case, whether pre- or post-bronchodilator, the FEV1 improvement with roflumilast is statistically significant in each case, although modest at 40–100 ml. While this is less than the 100–150 ml often considered to be the minimum clinically important difference for FEV1 (Citation74), one notes that only patients with little or no response to inhaled albuterol were admitted to the trials, that LABAs were permitted during the studies, and that the amount of spirometric improvement that nevertheless occurred was similar to that achieved by the corticosteroid components when added to a LABA in both of the two COPD-approved combinations, Advair and Symbicort. The second coprimary endpoint was the health-related quality of life outcome, SGRQ, in the first pivotal study (Citation71). This only reached statistical significance when compared to the original baseline value, not when compared to placebo. However, the improvements in SGRQ, although modest, were also similar in magnitude to those reported in efficacy studies of ICS in COPD (Citation75). In each of the three most recent efficacy studies, the coprimary outcome was frequency of acute exacerbations. Significant reductions of 34% were observed for roflumilast 500 μg in the Rabe study (Citation71), in which it was not a primary outcome, and of 15% and 18% in each of the two studies reported by Calverley, in which it was a primary outcome (Citation73). In the earlier study published by Calverley et al. in 2007, no reduction in exacerbations was seen in the roflumilast-treated population as a whole (Citation72). However, the exacerbation rate in the placebo-treated population in this study was unexpectedly low (only 0.92 exacerbations/patient/year). Furthermore, post hoc analysis suggested that severe exacerbations, namely those requiring systemic corticosteroids, were significantly less common in the roflumilast-treated arm (18.4% vs. placebo; p = 0.029). Moreover, in the very severe subgroup of patients, GOLD stage IV, roflumilast-treated patients experienced 36% fewer exacerbations (p = 0.024). Thus, the exacerbation studies suggest that important reductions in both the frequency and severity of acute exacerbations can be achieved with the 500 μg dose of roflumilast, particularly in patients with more severe COPD, and that these reductions are similar to those that have been seen with other COPD treatments.
Two additional efficacy and safety studies have recently been reported (Citation76). These explored the effect of roflumilast in patients who were concomitantly treated with other long-acting bronchodilators, namely salmeterol and tiotropium. The protocols, which were similar to each other as well as to the previously discussed long-term roflumilast studies, enrolled patients with moderate-to-severe COPD, and called for a 4-week run-in period followed by random assignment of subjects to receive roflumilast 500 μg plus salmeterol inhalation b.i.d. or matching placebo plus salmeterol inhalation b.i.d. in one study. In the other, subjects received roflumilast 500 μg plus tiotropium inhalation once daily or placebo plus tiotropium inhalation once daily. In this study, subjects were also required to have a history of using 28 puffs or more per week of a short-acting bronchodilator and symptoms of chronic bronchitis. Mean post-bronchodilator FEV1 was 55% of predicted in the roflumilast plus salmeterol study and 56% in the roflumilast plus tiotropium study (moderate to severe COPD). The studies concluded after 24 weeks and the primary outcome was the change in pre-bronchodilator FEV1 from the original baseline. A total of 744 subjects completed the salmeterol study; 642 completed the tiotropium study. In the salmeterol study, roflumilast augmented the pre-bronchodilator FEV1 by a mean of 49 ml over subjects who received only salmeterol plus placebo; in the tiotropium study, the corresponding mean increase was 80 ml, both results being significant at p < 0.0001. Other spirometric outcomes were similarly improved. In both studies, there were trends toward improvement in the number, severity, and time to first acute exacerbation in the roflumilast-treated subjects and these were sometimes statistically significant, although neither study had been powered for the analysis of acute exacerbation outcomes. Similar trends toward improvement were seen in other efficacy outcomes.
These two studies provide useful and practical information. Many patients with GOLD stage II or worse COPD will already be treated with a long-acting bronchodilator in accordance with the GOLD guidelines. If these do not provide sufficient clinical benefit, the patient and practitioner should consider what additional treatments to use. It is therefore important to know that roflumilast can provide further improvements in lung function and possibly reduce the frequency of acute exacerbations in patients already using a long-acting bronchodilator.
SAFETY OUTCOMES
The therapeutic precursor of synthetic PDE4 inhibitors, theophylline—a nonselective inhibitor of PDEs, has some major safety issues. It suffers from a narrow therapeutic margin, such that nausea and emesis occur at blood levels that are only marginally higher than those that are therapeutic. At only slightly higher levels than these therapeutic levels, serious and sometimes fatal adverse events such as tachyarrhythmias and convulsions are not rare. Of further concern, there is uncertainty about dosing as the metabolism and inactivation of theophylline varies substantially among patients and can also be altered by cigarette smoking, viral infections, and drug–drug interactions with many other agents that are commonly used in COPD patients. It is therefore necessary to measure blood levels of the drug from time to time. The seizures and arrhythmias with theophylline treatment are thought to be due to effects distinct from its PDE inhibition, and these side effects have not been seen in studies with selective PDE4 inhibitors to date. Nevertheless, because of this history, the safety profile of roflumilast has been carefully scrutinized in each of the long-term clinical studies.
The safety data reviewed here are taken from the reports of clinical trials, which include only the events that are relatively common. Less-common events that may be ascertained from pooled analysis of all studies were not possible from the review of published data. The total number of subjects that reported adverse events was nearly always greater in the roflumilast group than the placebo group and, where different doses were employed, was dose-related. Diarrhea was always among the most significant and frequently reported adverse effects, typically occurring in 8–9% of patients receiving 500 μg of roflumilast, or two to four times more commonly than in patients receiving placebo (). Nausea also tended to be reported more often, in 3–5% of the roflumilast-treated patients versus about 2% in the placebo arm. Studies in mice have provided indirect evidence that some of the gastrointestinal (GI) events of concern are due to the inhibition of PDE4D in the brain (Citation77, 78). In this respect, it is interesting that cilomilast, whose development was discontinued due to a lack of consistent efficacy (adverse-effects were dose limiting), is 10-fold more selective for PDE4D than the other isoforms (Citation34, Citation79). In contrast, roflumilast (and the N-oxide) does not selectively target PDE4D (Citation33) and this could explain, at least in part, why it is better tolerated.
Table 3. Adverse events occurring in at least 2·5% of patients in one of the treatment groups of the M2–124 and M2–125 studies
Weight loss was also a relatively frequent adverse event in the 1-year paired studies, occurring in 6–12% of roflumilast subjects versus 1–3% in the placebo arm. The mean weight changes in that report were −2.09 kg and +0.08 kg in the roflumilast and placebo groups, respectively, with most of the change occurring in the first 6 months. The weight loss may be related to decreased appetite because this adverse event was also reported more commonly by patients in the active treatment arm, as well as nausea and diarrhea. Other adverse events that were reported by more than 2.5% of patients, but not occurring more frequently (≥2.5%) in the roflumilast arm were nasopharyngitis, influenza, upper respiratory infection, acute bronchitis, back pain, pneumonia, hypertension, insomnia, and headache. (Acute exacerbations were recorded as adverse events, but have been addressed as an efficacy outcome in this review). Most adverse events that could be attributed to roflumilast were said to have resolved during the course of the trial. However, it is likely that they contributed to withdrawals from the trials because withdrawals were frequently greater in the active treatment arms, the overall differences in withdrawal rates being almost entirely explained by withdrawals due to adverse events. One notes that withdrawal rates also tended to diverge in the first month of a trial, suggesting that adverse events became evident shortly after subjects entered the treatment phase so that those subjects who were unable or unwilling to tolerate the adverse event withdrew. In the paired 1-year pivotal studies (Citation73), withdrawals due to adverse events were more common in the active treatment arm than placebo group (101 for roflumilast vs. 83 for placebo). By the end of the trial, the number of withdrawals due to GI adverse events was almost entirely compensated for by a similar reduction in withdrawals due to acute exacerbations in the active treatment group (49 for roflumilast vs. 66 for placebo), thus, overall withdrawals were almost identical in the two arms.
Among the safety concerns of theophylline are the dangerous neurologic and cardiovascular side effects, so it was important to include records of any such events in clinical trials of a PDE4 inhibitor. Mortality rates in the 1-year roflumilast trials (Citation73) were virtually identical in the treatment and placebo groups, both about 2%. Cardiovascular events were not different between active and placebo groups, being 7% and 8%, respectively. Similarly, arrhythmias were uncommon being reported in 1% and 0.4% of subjects, respectively. Headache and insomnia tended to be more common in the active treatment than placebo arms. Pneumonia has not been a frequent adverse event in any of the long-term studies and has not been more frequently reported with roflumilast than with placebo administration.
In preclinical animal toxicity studies, mesenteric vasculitis was seen in some rodents and cynomolgus monkeys following administration of relatively high doses of a PDE4 inhibitor (Citation80). A similar pathology has been observed with inhibitors of other PDEs and with a variety of non-PDE vasodilators (Citation80). There is uncertainty, therefore, whether these instances of arterial disease are species-specific or drug-specific. In humans, Merck withdrew a PDE4 inhibitor in development because of adverse GI effects and there were a few cases of fecal occult blood with GlaxoSmithKline's cilomilast. These events have resulted in requirements for extra vigilance including routine occult blood studies and colonoscopies in the development programs of some PDE4 inhibitors. Instances of GI disturbance are increased following the use of roflumilast, as mentioned above, but none have reached the severity of serious bowel disease or malfunction. Nevertheless, clinical experience with roflumilast exposure in patients with COPD has been relatively brief, 1 year or less, and confined to a relatively small number of patients in comparison with the number of patients who might receive it after approval, so caution is warranted.
A final safety question concerns the potential for unfavorable interaction of roflumilast with other drugs. Patients with COPD are known typically to have multiple co-morbidities for which they may receive other medications. Roflumilast is metabolized by the CYP3A4 and CYP1A2, and its primary metabolite is also pharmacologically active. Thus, inducers of these systems, for example, rifampicin, have the potential to increase roflumilast CL lowering its efficacy. Correspondingly, agents that are metabolized by the same system could compete with roflumilast and delay its inactivation, in effect raising its level. A number of studies have been performed to evaluate such drug–drug interactions and their potential for adverse reactions (Citation59–67). The results published so far indicate that there is not a substantial effect of a range of potential agents and conditions on roflumilast levels. Thus, no dose adjustments of roflumilast are likely to be needed when co-administered with other drugs commonly used in COPD or in patients with liver failure. Equally, roflumilast blood levels are unaffected by meals or smoking (Citation40).
DISCUSSION
The clinical studies reviewed here strongly suggest that roflumilast has clinical efficacy in COPD by a mechanism unrelated to bronchodilation. It is likely, therefore, that the mechanism of action of roflumilast is due to the suppression of lung inflammation which, if so, would suggest it may be disease modifying in COPD. The clearest evidence of this would be a reduction in mortality or a decrease in the rate of decline of lung function. Conclusive demonstration of either of these outcomes generally requires a large prospective study carried out over several years. The Lung Health Study, for example, enrolled 5,887 subjects and followed them for 5 years. Before roflumilast will be subjected to such a study, highly suggestive indications of its anti-inflammatory potential and disease modification will be needed.
In support of the anti-inflammatory properties of roflumilast, we have cited evidence that roflumilast, like some other PDE4 inhibitors, suppresses the release of a broad range of inflammatory mediators from cells in vitro and reduces the recovery of inflammatory mediators and cells from the airways after challenges with pro-inflammatory agents. Roflumilast does not cause significant short-term relaxation of airways smooth muscle in vitro or rapid bronchodilation in humans with COPD. Yet airway function does improve with roflumilast following regular administration over a period of days or weeks and declines over a similar period when withdrawn. These findings can be most readily explained by an anti-inflammatory effect.
Of the clinical studies reviewed here, those outcomes that are most supportive of an anti-inflammatory action are the improvement in pre-bronchodilator FEV1 and the reduction in the incidence of acute exacerbations, both of which were primary outcomes in the most recent roflumilast studies (Citation73, Citation76) and both outcomes met predetermined goals. Pre-treatment FEV1 has been developed as an anti-inflammatory outcome in other studies, particularly those of LABA-ICS combinations in which the FDA's “combination rule” requires demonstration that each component contributes to the overall efficacy. As stated earlier, the magnitude of the increase in FEV1 due to roflumilast, between 40 and 100 ml, is not as great as would be required for a bronchodilator to be considered effective. But in the context of a study population that was sometimes selected for their non-responsiveness to albuterol and that was allowed to use long-acting bronchodilators, the result can be considered to indicate a meaningful improvement in lung function.
The reduction in the incidence of acute exacerbations, while not seen in every study, was substantial and statistically significant in both long-term studies that were powered for AECOPD as a co-primary outcome. Notably, this effect appeared to be greatest in the subgroup of patients with more severe disease and with more severe exacerbations. A reduction in AECOPDs has been reported for LABAs, LAMAs, ICSs, and their combinations, as well as theophylline (Citation3, Citation11–16, Citation81). We do not have a clear understanding of the mechanism by which any of these agents affects the frequency of AECOPDs. However, as inflammation is intensified during AECOPD events, a reduction in their frequency and severity would seem to be a requirement for an anti-inflammatory agent.
Concerning the safety of roflumilast, early study dropouts have nearly always been more frequent in the active treatment group compared to the placebo arm and this can be largely attributed to adverse GI events. In contrast, dropouts after the first few weeks were more common in the placebo groups and may be related to the lack of efficacy with control treatments. Adverse effects associated with roflumilast more commonly than control and their approximate frequencies include diarrhea (9%), nausea (5%), and weight loss (12%). The latter two events are a feature of PDE4 inhibition, possibly of the PDE4D isoform, and have been problematic with theophylline and all subsequent non-selective PDE4 inhibitors that have entered clinical trials. While the clinical developments of several PDE4 inhibitors have been discontinued because of GI adverse events, roflumilast appears to be among the least problematic in this respect. We have recently reviewed strategies that are being employed by pharmaceutical companies to increase the therapeutic ratio of PDE4 inhibitors (Citation82). With the recent elucidation of the co-crystal structure of the PDE4 active site (Citation83), it may become possible to design agents that avoid the GI problems altogether.
Roflumilast has two distinct advantages over theophylline. First, it lacks the dangerous induction of seizures and pro-arrhythmic side effects of theophylline (Citation84). Second, its elimination and pharmacokinetics are not significantly altered by food, tobacco smoking, and by the many drugs that alter the pharmacokinetics of theophylline (Citation84). Rifampicin is the only drug known to accelerate the inactivation of roflumilast. The phenomenon of fast- and slow-inactivators of the drug does not occur, nor are dose adjustments required when fever occurs. Measurements of blood levels are not required. Indeed, the mechanisms of action of roflumilast and theophylline differ to such an extent that the former should not be considered a novel formulation of the latter.
If roflumilast is approved for routine use in COPD, we expect it to find its role initially as a substitute or alternative for ICSs in the maintenance treatment of severe and very severe disease, particularly in patients who have frequent acute exacerbations. It may be used as a supplementary drug when symptoms are not adequately controlled by a long-acting bronchodilator alone. Whether it will have a role as a supplement to an ICS-LABA combination therapy, or indeed to supplant it, remains to be determined.
Declaration of interest
The authors were responsible for the content and writing of the paper. Each of the authors contributed equally to the writing of the manuscript. Editorial assistance was provided by Young Yoo, Autumn Kelly, Jennifer Jaworski, and Biplob Dass of Prescott Medical Communications Group. The manuscript was submitted to Nycomed and Forest, the manufacturers of roflumilast, for review of its factual content only.
ACKNOWLEDGEMENTS
Dr. Nicholas Gross has received honoraria from Nycomed and Forest, the manufacturers of roflumilast. He also received honoraria from Dey LP, AstraZeneca, GlaxoSmithKline, Boehringer-Ingelheim, Pfizer, Novartis, and Almirall. Dr. Mark Giembycz has received honoraria from Nycomed and Forest, the manufacturers of roflumilast. He also received honoraria and/or unrestricted educational grants from AstraZeneca, Gilead Sciences, GlaxoSmithKline, Otsuka, Proctor and Gamble, Sanofi-Aventis, Schering-Plough and the GSK (Canada)/Collaborative Innovation Research Fund. Dr. Stephen Rennard has received honoraria from Nycomed and Forest, the manufacturers of roflumilast. He has also received honoraria and grants from American Board of Internal Medicine, American College of Chest Physicians, Almirall, APT Pharma/Britnall, AstraZeneca, American Thoracic Society, Boehringer Ingelheim, California Allergy Society, Chiesi, COPDForum, Creative Educational Concept, France Foundation, Gerson, GlaxoSmithKline, Hoffmann LaRoche, Information TV, Novartis (Horsham), Oriel Therapeutics, Pearl Therapeutics, Pulmatrix, Schering Plough and UBC.
REFERENCES
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163:1256–1276.
- American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168:818–900.
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper 10.1183/09031936.04.00014304. Eur Respir J 2004; 23:932–946.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Tashkin DP. Long-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safety. Curr Opin Pulm Med 2010; 16:97–105.
- Rennard SI. Treatment of stable chronic obstructive pulmonary disease. Lancet 2004; 364:791–802.
- Connors AF, Jr., Dawson NV, Thomas C, Harrell FE, Jr., Desbiens N, Fulkerson WJ, Kussin P, Bellamy P, Goldman L, Knaus WA. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154:959–967.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Snow V, Lascher S, Mottur-Pilson C. The evidence base for management of acute exacerbations of COPD: clinical practice guideline, part 1. Chest 2001; 119:1185–1189.
- Ramirez-Venegas A, Ward J, Lentine T, Mahler DA. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest 1997; 112:336–340.
- Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, Wisniewski M, Rickard K. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1087–1092.
- Calverley PM, Rennard SI. What have we learned from large drug treatment trials in COPD? Lancet 2007; 370:774–785.
- Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343:1902–1909.
- Burge PS, Calverley PMA, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial 10.1136/bmj.320.7245.1297. BMJ 2000; 320:1297–1303.
- Vestbo J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J 2004; 24:206–210.
- Kojima S, Sakakibara H, Motani S, Hirose K, Mizuno F, Ochiai M, Hashimoto S. Incidence of chronic obstructive pulmonary disease, and the relationship between age and smoking in a Japanese population. J Epidemiol 2007; 17:54–60.
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006; 27:397–412.
- Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J 2005; 25:1084–1106.
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:1635– 1639.
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med 1997; 155:542–548.
- Barnes PJ. Mechanisms in COPD: differences from asthma. Chest 2000; 117:10S–14S.
- Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167:418–424.
- O’Byrne P M, Postma DS. The many faces of airway inflammation. Asthma and chronic obstructive pulmonary disease. Asthma Research Group. Am J Respir Crit Care Med 1999; 159:S41–S63.
- Yawn BP. Differential assessment and management of asthma vs chronic obstructive pulmonary disease. Medscape J Med 2009; 11:20.
- Torphy TJ. Action of mediators on airway smooth muscle: functional antagonism as a mechanism for bronchodilator drugs. Agents Actions Suppl 1988; 23:37–53.
- Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998; 157:351–370.
- Torphy TJ, Cieslinski LB. Characterization and selective inhibition of cyclic nucleotide phosphodiesterase isozymes in canine tracheal smooth muscle. Mol Pharmacol 1990; 37:206–214.
- Torphy TJ, Undem BJ. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax 1991; 46:512–523.
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 2006; 58:488–520.
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today 2005; 10:1503–1519.
- Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking? Br J Pharmacol 2008; 155:288–290.
- Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther 2001; 297:267–279.
- Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2001; 10:1361–1379.
- Giembycz MA. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma? Biochem Pharmacol 1992; 43:2041–2051.
- Press NJ, Banner KH. PDE4 inhibitors—a review of the current field. Prog Med Chem 2009; 47:37–74.
- Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here? Drugs 2000; 59:193–212.
- Currie GP, Butler CA, Anderson WJ, Skinner C. Phosphodiesterase 4 inhibitors in chronic obstructive pulmonary disease: a new approach to oral treatment. Br J Clin Pharmacol 2008; 65:803–810.
- Dastidar SG, Rajagopal D, Ray A. Therapeutic benefit of PDE4 inhibitors in inflammatory diseases. Curr Opin Investig Drugs 2007; 8:364–372.
- Field SK. Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs 2008; 17:811–818.
- Jeffrey AM, Luo FQ, Amin S, Krzeminski J, Zech K, Williams GM. Lack of DNA binding in the rat nasal mucosa and other tissues of the nasal toxicants roflumilast, a phosphodiesterase 4 inhibitor, and a metabolite, 4-amino-3,5-dichloropyridine, in contrast to the nasal carcinogen 2,6-dimethylaniline. Drug Chem Toxicol 2002; 25:93–107.
- Reid P. Roflumilast Altana Pharma. Curr Opin Investig Drugs 2002; 3:1165–1170.
- Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 2001; 297:280–290.
- Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med 2005; 172:848–853.
- Martorana PA, Lunghi B, Lucattelli M, De Cunto G, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm Med 2008; 8:17.
- Sanz MJ, Cortijo J, Taha MA, Cerda-Nicolas M, Schatton E, Burgbacher B, Klar J, Tenor H, Schudt C, Issekutz AC, Hatzelmann A, Morcillo EJ. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br J Pharmacol 2007; 152:481–492.
- Wollin L, Bundschuh DS, Wohlsen A, Marx D, Beume R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther 2006; 19:343–352.
- Wollin L, Marx D, Wohlsen A, Beume R. Roflumilast inhibition of pulmonary leukotriene production and bronchoconstriction in ovalbumin-sensitized and -challenged Guinea pigs. J Asthma 2005; 42:873–878.
- Grootendorst DC, Gauw SA, Baan R, Kelly J, Murdoch RD, Sterk PJ, Rabe KF. Does a single dose of the phosphodiesterase 4 inhibitor, cilomilast (15 mg), induce bronchodilation in patients with chronic obstructive pulmonary disease? Pulm Pharmacol Ther 2003; 16:115–120.
- Grootendorst DC, Gauw SA, Verhoosel RM, Sterk PJ, Hospers JJ, Bredenbroker D, Bethke TD, Hiemstra PS, Rabe KF. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007; 62:1081–1087.
- Hohlfeld JM, Schoenfeld K, Lavae-Mokhtari M, Schaumann F, Mueller M, Bredenbroeker D, Krug N, Hermann R. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial. Pulm Pharmacol Ther 2008; 21:616–623.
- Timmer W, Leclerc V, Birraux G, Neuhauser M, Hatzelmann A, Bethke T, Wurst W. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. J Clin Pharmacol 2002; 42:297–303.
- Gamble E, Grootendorst DC, Brightling CE, Troy S, Qiu Y, Zhu J, Parker D, Matin D, Majumdar S, Vignola AM, Kroegel C, Morell F, Hansel TT, Rennard SI, Compton C, Amit O, Tat T, Edelson J, Pavord ID, Rabe KF, Barnes NC, Jeffery PK. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168:976–982.
- Bethke TD, Bohmer GM, Hermann R, Hauns B, Fux R, Morike K, David M, Knoerzer D, Wurst W, Gleiter CH. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol 2007; 47:26–36.
- Hauns B, Hermann R, Hunnemeyer A, Herzog R, Hauschke D, Zech K, Bethke TD. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol 2006; 46:1146–1153.
- Neville KA, Szefler SJ, Abdel-Rahman SM, Lahu G, Zech K, Herzog R, Bethke TD, Gleason MC, Kearns GL. Single-dose pharmacokinetics of roflumilast in children and adolescents. J Clin Pharmacol 2008; 48:978–985.
- Nassr N, Huennemeyer A, Herzog R, von Richter O, Hermann R, Koch M, Duffy K, Zech K, Lahu G. Effects of rifampicin on the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy subjects. Br J Clin Pharmacol 2009; 68:580–587.
- David M, Zech K, Seiberling M, Weimar C, Bethke TD. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavailability. J Allergy Clin Immunol 2004; 113:S220–S221.
- Lahu G, Huennemeyer A, von Richter O, Hermann R, Herzog R, McCracken N, Zech K. Effect of single and repeated doses of ketoconazole on the pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol 2008; 48:1339–1349.
- Nassr N, Lahu G, Hunnemeyer A, von Richter O, Knoerzer D, Reutter F, Zech K, Hermann R. Magnesium hydroxide/aluminium hydroxide-containing antacid does not affect the pharmacokinetics of the targeted phosphodiesterase 4 inhibitor roflumilast. J Clin Pharmacol 2007; 47:660–666.
- Nassr N, Lahu G, von Richter O, Reutter F, Knoerzer D, Zech K, Erb KA, Schug B, Blume H, Hermann R. Lack of a pharmacokinetic interaction between steady-state roflumilast and single-dose midazolam in healthy subjects. Br J Clin Pharmacol 2007; 63:365–370.
- Hermann R, Nassr N, Lahu G, Peterfai E, Knoerzer D, Herzog R, Zech K, de Mey C. Steady-state pharmacokinetics of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet 2007; 46:403–416.
- Bethke TD, Giessmann T, Westphal K, Weinbrenner A, Hauns B, Hauschke D, David M, Lahu G, Zech K, Hermann R, Siegmund W. Roflumilast, a once-daily oral phosphodiesterase 4 inhibitor, lacks relevant pharmacokinetic interactions with inhaled salbutamol when co-administered in healthy subjects. Int J Clin Pharmacol Ther 2006; 44:572–579.
- Bohmer GM, Nassr N, Wenger M, Hunnemeyer A, Lahu G, Templin S, Gleiter CH, Hermann R. The targeted oral, once-daily phosphodiesterase 4 inhibitor roflumilast and the leukotriene receptor antagonist montelukast do not exhibit significant pharmacokinetic interactions. J Clin Pharmacol 2009; 49:389–397.
- Hermann R, Siegmund W, Giessmann T, Westphal K, Weinbrenner A, Hauns B, Reutter F, Lahu G, Zech K, Bethke TD. The oral, once-daily phosphodiesterase 4 inhibitor roflumilast lacks relevant pharmacokinetic interactions with inhaled budesonide. J Clin Pharmacol 2007; 47:1005–1013.
- Lahu G, Huennemeyer A, Herzog R, McCracken N, Hermann R, Elmlinger M, Zech K. Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int J Clin Pharmacol Ther 2009; 47:236–245.
- von Richter O, Lahu G, Huennemeyer A, Herzog R, Zech K, Hermann R. Effect of fluvoxamine on the pharmacokinetics of roflumilast and roflumilast N-oxide. Clin Pharmacokinet 2007; 46:613–622.
- Hunt SN, Jusko WJ, Yurchak AM. Effect of smoking on theophylline disposition. Clin Pharmacol Ther 1976; 19:546–551.
- Jusko WJ. Influence of cigarette smoking on drug metabolism in man. Drug Metab Rev 1979; 9:221–236.
- Powell JR, Vozeh S, Hopewell P, Costello J, Sheiner LB, Riegelman S. Theophylline disposition in acutely ill hospitalized patients. The effect of smoking, heart failure, severe airway obstruction, and pneumonia. Am Rev Respir Dis 1978; 118:229–238.
- Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005; 366:563–571.
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:154–161.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2:111–124.
- Hanania NA. The impact of inhaled corticosteroid and long-acting beta-agonist combination therapy on outcomes in COPD. Pulm Pharmacol Ther 2008; 21:540–550.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Rabe KF. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Giembycz MA. 4D or not 4D—the emetogenic basis of PDE4 inhibitors uncovered? Trends Pharmacol Sci 2002; 23:548.
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest 2002; 110:1045–1052.
- Torphy TJ, Barnette MS, Underwood DC, Griswold DE, Christensen SB, Murdoch RD, Nieman RB, Compton CH. Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther 1999; 12:131–135.
- Boswell-Smith V, Page CP. Roflumilast: a phosphodiesterase-4 inhibitor for the treatment of respiratory disease. Expert Opin Investig Drugs 2006; 15:1105–1113.
- Cyr MC, Beauchesne MF, Lemiere C, Blais L. Effect of theophylline on the rate of moderate to severe exacerbations among patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol 2008; 65:40–50.
- Gross NJ. The COPD Pipeline. COPD 2009; 6:488–489.
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol 2009; 28: 63–70.
- Gupta P, O’Mahony MS. Potential adverse effects of bronchodilators in the treatment of airways obstruction in older people: recommendations for prescribing. Drugs Aging 2008; 25:415– 443.