ABSTRACT
Aclidinium bromide is a novel, long-acting, inhaled muscarinic antagonist in development for the treatment of chronic obstructive pulmonary disease (COPD). The aim of this study was to assess the rate of onset of bronchodilation with aclidinium compared with placebo and tiotropium. This was a double-blind, double-dummy, multicenter, crossover study in COPD patients with a post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥30% and <60% predicted. On study days, patients received single doses of aclidinium 200 μg, tiotropium 18 μg, or placebo. Serial spirometry was conducted from 10 minutes to 3 hours post-dose. The primary variable was the percentage of patients with an increase in FEV1 of ≥10% above baseline at 30 minutes post-dose. Other assessments included change from baseline in FEV1 and dyspnea over 3 hours post-dose. A total of 115 patients entered the study. Significantly more patients had an increase in FEV1 of ≥10% above baseline at 30 minutes with aclidinium and tiotropium versus placebo (49.5% and 51.8% versus 13.8%; p < 0.0001). At 30 minutes, the relative increase from baseline in FEV1 was significantly higher for aclidinium and tiotropium versus placebo (12% and 11% versus 3%; p < 0.0001). Aclidinium and tiotropium also significantly increased FEV1 (p < 0.01) and improved the perception of dyspnea compared with placebo at all measured time points from 10 minutes to 3 hours post-dose. In conclusion, aclidinium provided effective bronchodilation, similar to that seen with tiotropium, with significant improvements compared with placebo observed from 10 minutes post-dose.
INTRODUCTION
Treatment with bronchodilators is central in chronic obstructive pulmonary disease (COPD) and long-acting bronchodilators such as inhaled anticholinergics are recommended for patients with COPD ranging from moderate to very severe disease (Citation1). At present, tiotropium bromide is the only available long-acting anticholinergic agent. As individual patient responses to therapy are variable, the development of new long-acting anticholinergic agents is warranted.
Aclidinium bromide is a novel, long-acting muscarinic antagonist, currently being developed as a maintenance treatment for patients with COPD. In preclinical studies, aclidinium demonstrated potent antagonism of human muscarinic receptors with a long residence time at M3 receptors and a shorter residence time at M2 receptors (Citation2), in addition to rapid hydrolysis of the active compound in human plasma to two inactive metabolites (Citation3). These properties suggest that aclidinium may provide sustained bronchodilation and a low potential for systemic side effects in the clinical setting. Early clinical studies have confirmed that aclidinium displays long-lasting bronchodilatory activity, low and transient systemic exposure, and a good safety profile with a low incidence of cardiovascular side effects (Citation4–7). In Phase II studies, aclidinium, at doses of 100–900 μg, provided significant improvements in forced expiratory volume in 1 second (FEV1) from 30 minutes post-dose (Citation4,Citation6). The aim of this study was to assess the rate of onset of bronchodilation with aclidinium 200 μg compared with placebo and tiotropium in patients with COPD.
MATERIALS AND METHODS
Patients
Men and non-pregnant, non-lactating women aged ≥40 years with stable COPD (Citation1), post-salbutamol FEV1 ≥30% and <60% of the predicted value (Citation8), and post-salbutamol FEV1/forced vital capacity ratio (FVC) <70% were included if they were current or previous cigarette smokers with a smoking history of ≥10 pack-years. All patients provided written informed consent.
Exclusion criteria included: a history or current diagnosis of asthma, allergic rhinitis, or atopy; an eosinophil count ≥600 cells/mm3; a respiratory tract infection or COPD exacerbation within 6 weeks of the screening visit; or hospitalization for acute COPD exacerbation within 3 months of the screening visit. Clinically significant respiratory conditions other than those related to the inclusion criteria, clinically significant cardiovascular conditions, and clinically relevant medical findings or abnormalities unrelated to COPD at screening precluded patients from the trial.
The use of long-acting, oral, intra-nasal, or parenteral anticholinergic drugs and methyl-xanthines was prohibited within 72 hours of the screening visit. Cromolyn sodium, nedocromil, and H1-antihistamines were discontinued at least 6 weeks prior to the screening visit, and continuous oral or parenteral corticosteroids had to be discontinued or reduced to the equivalent of 10 mg prednisone per day at least 4 weeks before the screening visit. Patients were allowed to continue with short-acting anticholinergic drugs and short-acting inhaled or oral β2-agonists, provided treatment was interrupted at least 12, 6, and 24 hours, respectively, before each visit. Fixed inhaled combinations of a short-acting β2-agonist plus a corticosteroid or short-acting anticholinergic agent, or a long-acting β2-agonist plus a corticosteroid, were permitted if treatment was discontinued at least 12 and 24 hours, respectively, before each visit. Salbutamol was the only rescue medication permitted during the washout period and had to be discontinued at least 6 hours prior to any visit until after completion of the last spirometry assessment.
Study design
This was a double-blind, double-dummy, crossover study conducted at 22 centers in Germany, The Netherlands, and the United Kingdom. The study consisted of three, one-day treatment periods, separated by washout periods of 5–7 days. Patients were randomized to 1 of 6 treatment sequences and received single doses of: aclidinium 200 μg and placebo to tiotropium 18 μg; tiotropium 18 μg and placebo to aclidinium 200 μg; or placebo to aclidinium 200 μg and placebo to tiotropium 18 μg. Aclidinium 200 μg and placebo to aclidinium 200 μg were administered via the Genuair® inhaler. Tiotropium 18 μg and placebo to tiotropium 18 μg were administered via the HandiHaler. As aclidinium and tiotropium were administered using different inhaler devices, a double-dummy technique was employed to ensure blinding.
The primary objective of the study was to assess the rate of onset of bronchodilation with aclidinium 200 μg compared with placebo in patients with COPD. The rates of onset of bronchodilation for aclidinium 200 μg versus tiotropium 18 μg and tiotropium 18 μg versus placebo were secondary objectives.
Measurements
FEV1 and FVC assessments were carried out at 10, 20, 30, 45, 60, 120, and 180 minutes post-dose and inspiratory capacity (IC) was measured at 30, 60, and 180 minutes post-dose according to the ATS/ERS recommendations on spirometric assessments (Citation8). Dyspnea assessments were measured at the same time points as FEV1 and FVC, prior to the spirometry assessments. The change from baseline in the perception of dyspnea was assessed using a bipolar Visual Analog Scale ranging from -100 (very much worse) to +100 (very much better), with 0 (no change) in the middle).
The primary efficacy variable was the percentage of patients achieving an FEV1 increase of ≥10% from baseline at 30 minutes post-dose. The main secondary efficacy variables were the change from baseline in FEV1 at 30 minutes post-dose and the normalized area under the curve from 0 to 3 hours (AUC0–3h) for FEV1. Other secondary efficacy endpoints included the percentage of patients with an increase in FEV1 of ≥10%, ≥12%, and ≥15% from baseline, and the change from baseline in FEV1, FVC, IC, and perception of dyspnea, throughout the study period.
Adverse events (AEs) were recorded throughout each visit and at a follow-up visit within 24 days after Visit 3 (or after early discontinuation). At the follow-up visit, other safety investigations such as laboratory tests, vital signs, and 12-lead electrocardiograms (ECGs) were also performed.
Statistical analysis
The percentage of patients achieving a FEV1 increase from baseline ≥10% at 30 minutes after dosing was analyzed by logistic regression. All other efficacy endpoints were analyzed by means of Analysis of Covariance (ANCOVA) model for crossover designs including sequence, subject, period, treatment, and country as factors and baseline as a covariate. Safety endpoints were analyzed by means of descriptive statistics.
RESULTS
Patients
A total of 115 patients were randomized and 107 patients completed the study (). The safety and intent-to-treat population consisted of 115 patients. The baseline demographic and clinical characteristics are shown in . Of the 8 patients who did not complete the study, 5 were discontinued due to AEs, one due to protocol non-compliance, one due to personal request, and one due to “other reason”.
Table 1. Demographic and clinical characteristics at baseline (n = 115)
Efficacy
Primary variable
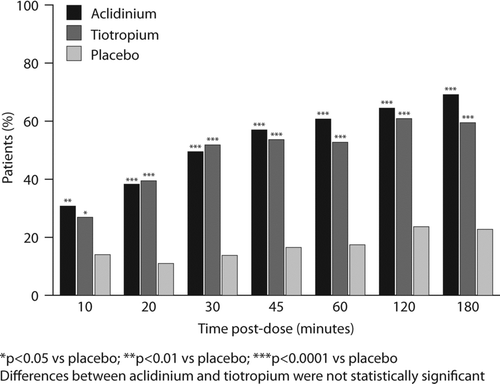
Significantly more patients had an increase in FEV1 of ≥10% above baseline at 30 minutes with aclidinium and tiotropium compared with the placebo (49.5% and 51.8% versus 13.8%; p < 0.0001 based on odds ratio; ). Moreover, the percentage of patients with an increase in FEV1 of ≥10% was significantly greater with aclidinium and tiotropium versus placebo from 10 minutes to 3 hours post-dose (). There were no significant differences between aclidinium and tiotropium at any time point.
Secondary variables
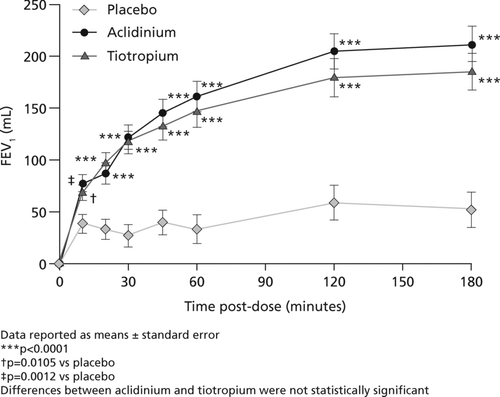
At 30 minutes post-dose, the mean increase in FEV1 from baseline was significantly greater for aclidinium (122 mL; 12% relative increase) and tiotropium (118 mL; 11% relative increase) compared with placebo (27 mL; 3% relative increase; p < 0.0001). Aclidinium and tiotropium also significantly increased FEV1 compared with placebo from 10 minutes to 3 hours post-dose (). No significant differences between aclidinium and tiotropium were observed at any time point.
The normalized AUC0–3h FEV1 was 127 mL higher following treatment with aclidinium compared with placebo (p < 0.0001) and 110 mL higher following treatment with tiotropium compared with placebo (p < 0.0001). The percentage of patients with an increase in FEV1 of ≥12% and ≥15% was significantly greater with aclidinium and tiotropium versus placebo from 20 minutes to 3 hours post-dose. No significant differences were observed between the active treatments at any time point.
Similar changes from baseline were observed for FVC as for FEV1, with statistically significant increases above placebo observed at 10 minutes post-dose with aclidinium (134 mL, p < 0.0001) and tiotropium (100 mL, p = 0.002). IC significantly improved following administration of either active treatment from 30 minutes post-dose when compared with placebo (p <0.0001). There were no significant differences between aclidinium and tiotropium at any time point for FVC or IC.
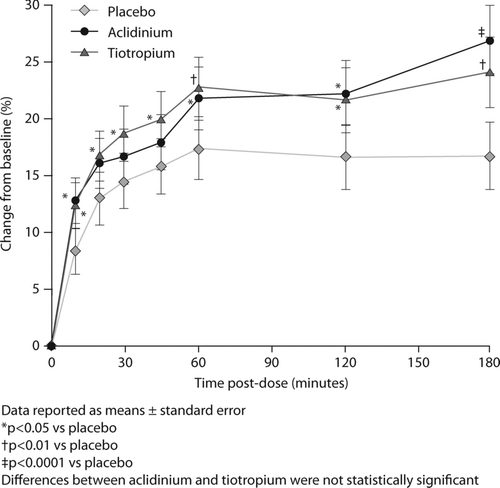
An improvement from baseline in the perception of dyspnea was observed at all time points after treatment with aclidinium compared with placebo; these differences reached statistical significance at 10, 60, 120, and 180 minutes (p < 0.05 at 10, 60, 120 minutes; p < 0.0001 at 180 minutes; ). Similar improvements were observed after treatment with tiotropium, with statistically significant differences versus placebo observed at 10, 20, 30, 45, 60, 120 and 180 minutes post-dose (p < 0.05 at 10, 20, 30, 45 and 120 minutes; p < 0.01 at 60 and 180 minutes; ). There were no significant differences between aclidinium and tiotropium at any time point.
Safety
The incidence of AEs was similar in each treatment arm (). Headache was the most frequently reported AE with 14 patients reporting 17 incidents (). Anticholinergic side effects were reported by 2 patients (1 of dry mouth and 1 of dizziness), following aclidinium treatment; both of them were mild in intensity and only dry mouth was considered to be related to the study drug. There was a total of 2 serious AEs reported following administration of tiotropium (left-side brain infarction and COPD exacerbation) that led to study withdrawal; neither event was considered to be related to the study drug. Three further patients were withdrawn due to COPD exacerbation (two in the placebo group and one in the aclidinium group). There were no deaths during the study. No clinically relevant changes in laboratory tests, vital signs, or 12-lead ECGs were observed at the follow-up visit.
Table 2. Adverse events reported by ≥2 patients in each treatment arm
DISCUSSION
This study fully characterizes the onset of action of aclidinium 200 μg, showing that aclidinium 200 μg significantly improves FEV1 compared with placebo at all time points from 10 minutes to 3 hours post-dose. The onset of bronchodilation and magnitude of effect observed with aclidinium 200 μg during the first 3 hours post-dose were similar to that seen with tiotropium 18 μg, although this comparison was primarily exploratory.
In this study, the criterion used for bronchodilator response was an increase in FEV1 of ≥10% from the baseline value. Any threshold of improvement can be debated as these are most often chosen arbitrarily and no ‘gold standard’ exists. According to ATS/ERS recommendations, a positive bronchodilator response can be defined as an increase in FEV1 and/or FVC ≥12% of control and ≥200 mL, although no distinction was made between COPD and asthma (Citation9). However, in patients with COPD, reversibility of airflow limitation is neither a stable (Citation10) nor predictive feature (Citation11, 12). Therefore, it may not be appropriate to intend to achieve the same degree of bronchodilation in COPD and asthma studies. For patients with COPD, regulators consider a change in FEV1 of 5–10% from baseline to be clinically meaningful (Citation13). The results using the ≥10% threshold in this study are corroborated by the results using different thresholds (≥12% and ≥15%) and the correlation between the improvements in lung function and dyspnea.
This is the first comparison of the onset of action of two long-acting anticholinergic drugs. Our findings regarding onset of action are generally in accordance with previous findings for tiotropium, although the responses for both products were slightly smaller in the present study than one would have expected based on prior research (Citation14,15). Given that changes in lung function alone may be less important to patients than improvement in symptoms, it is therefore reassuring to see that the improvements in lung function observed with aclidinium are mirrored by improvements in dyspnea. Even small improvements in bronchodilation observed at the earlier time points were related to dyspnea improvements. It is also possible that the clinically meaningful change in FEV1 has a different threshold in patients with severe or moderate COPD.
For those with severe COPD, a lower FEV1 threshold may constitute a change associated with improvement in dyspnea, whereas for the more general moderate to severe COPD population a higher FEV1 improvement (i.e., >100 mL) constitutes a clinically meaningful difference. It should also be noted that the improvements in dyspnea may have been underestimated in this study, as it is possible that those patients who did not perceive an improvement at rest may have done so during exercise due to an effect on dynamic hyperinflation (Citation16).
In this study, single inhaled doses of aclidinium were safe and well tolerated in patients with COPD, with a low incidence of AEs similar to that observed with placebo. This is consistent with the good safety profile demonstrated by aclidinium in previous Phase I/II clinical studies (Citation4–7). In conclusion, aclidinium provided effective bronchodilation in COPD patients, similar to that seen with tiotropium, with significant improvements compared with placebo observed from 10 minutes post-dose.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
We thank Dr. Catherine Hoare from Complete Medical Communications, who provided medical writing support. This study was funded by Almirall, S.A., Barcelona, Spain, and Forest Laboratories, Inc., NY, USA. Meritxell Falques, Anna Ribera, and Esther Garcia Gil are employees of Almirall. Genuair® is a registered trademark of Almirall, S.A.
REFERENCES
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Gavalda A, Miralpeix M, Ramos I, Otal R, Carreno C, Vinals M, Domenech T, Carcasona C, Reyes B, Vilella D, Gras J, Cortijo J, Morcillo E, Llenas J, Ryder H, Beleta J. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther 2009; 331:740–751.
- Gavaldà A, Sentellas S, Alberti J, Anton SF, Salva M, Beleta J. Aclidinium bromide, a novel long-acting anticholinergic, is rapidly inactivated in plasma. Am J Respir Crit Care Med 2008; 177 (Abstract Issue): A-654.
- Chanez P, Burge PS, Dahl R. Aclidinium bromide provides long-acting bronchodilation in patients with COPD. Pulm Pharmacol Ther 2009; Epub ahead of print 14 August.
- Jansat JM, Lamarca R, Garcia-Gil E, Ferrer P. Safety and pharmacokinetics of single doses of aclidinium bromide, a novel long-acting, inhaled antimuscarinic, in healthy subjects. Int J Clin Pharmacol Ther 2009; 47:460–468.
- Joos GF, Schelfhout VJ, Kanniess F, Ludwig-Sengpill A, Garcia Gil E, Montejo EM. Bronchodilator effects of aclidinium bromide, a novel long-acting anticholinergic, in COPD patients: a Phase II study. Eur Respir J 2007; 30 (Suppl 51):210S.
- Schelfhout VJ, Joos GF, Garcia Gil E, Montejo EM. Bronchodilator/bronchoprotective effects of aclidinium bromide, a novel long-acting anticholinergic: a Phase I study. Eur Respir J 2007; 30(Suppl 51):356S.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Calverley PMA, Burge PS, Spencer S, Anderson J, Jones P, for the ISOLDE Study Investigators. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58:659–664.
- Anthonisen NR, Lindgren PG, Tashkin DP, Kanner RE, Scanlon PD, Connett JE, for the Lung Health Study Research Group. Bronchodilator response in the lung healthy study over 11 yrs. Eur Respir J 2005; 26:45–51.
- Hansen EF, Phanareth K, Laursen LC, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:1267– 1271.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Molken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31:416–469.
- van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ, for the Dutch Tiotropium Study Group. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. Thorax 2000; 55:289–294.
- van Noord JA, Smeets JJ, Custers FLJ, Korducki L, Cornelissen PJG. Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary disease. Eur Respir J 2002; 19:639–644.
- Casaburi R, Porszasz J. Reduction of hyperinflation by pharmacologic and other interventions. Proc Am Thorac Soc 2006; 3:185–189.