ABSTRACT
Chronic obstructive pulmonary disease (COPD) is a leading and increasing cause of death, the extent of which is underestimated as a consequence of underdiagnosis and underreporting on death certificates. Data from large trials, such as the Lung Health Study, Towards a Revolution in COPD Health (TORCH), Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT), European Respiratory Society Study on Chronic Obstructive Pulmonary Disease (EUROSCOP), and Inhaled Steroids in Obstructive Lung Disease (ISOLDE), have shown that the causes of death in patients with mild COPD are predominantly cancer and cardiovascular disease, but as COPD severity increases, deaths due to non-malignant respiratory disease are increasingly common. In practice, mortality of patients with COPD can be predicted by a variety of measures including: forced expiratory volume in one second (FEV1), the ratio of inspiratory and total lung capacities, exercise capacity, dyspnea scores, and composite indices such as the body-mass index (B), degree of airflow obstruction (O), degree of functional dyspnea (D), and exercise capacity (E) (BODE) index. Smoking cessation improves survival in COPD patients, and in select patients with advanced disease, oxygen therapy, lung volume reduction surgery, or lung transplantation may also improve survival.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the fourth-leading cause of death in the USA (Citation1), and it is projected to be the third-leading cause of death worldwide by 2020 (Citation2). Moreover, while mortality due to other common causes of death in the USA appears to be decreasing, the death rates due to COPD are increasing (Citation3). COPD mortality rates are likely underestimated as a consequence of underdiagnosis and because co-morbidities are commonly recorded as causes of death (Citation4). This article reviews the evidence for the prevalence of death caused by COPD, examines how causes of death differ according to the stage of disease severity, and explores the impact of current COPD treatments on mortality.
PATIENT DEMOGRAPHICS AND COPD AS A CAUSE OF MORTALITY
The demographics of patients who die from COPD in the USA are changing, reflecting trends in smoking habits during the 20th century. Between 1980 and 2004, mortality among white males reached a plateau, whereas during the same period, mortality among women and African-Americans increased (Citation1). In the year 2000, for the first time there were more deaths from COPD in women than men (Citation5). Between 1980 and 2004, the age-adjusted mortality increased by 162% in Caucasian women (from 14.2 to 37.2 deaths/100,000 population) and 187% in African-American women (from 6.2 to 17.8 deaths/100,000 population) (Citation1). In contrast, age-adjusted mortality has been stable in Caucasian men (50.3 deaths/100,000 population) and increased by only 29% in African-American men (29.4 to 37.9 deaths/100,000 population) over the same time interval (Citation1).
Mortality from COPD is increasing particularly rapidly in elderly women. Between 1980 and 2004, mortality rates more than tripled in women ≥75 years old (Citation1). In part, this is because the elderly are particularly prone to secondary complications of COPD such as pneumonia, but it is also a likely cohort effect, reflecting the high smoking prevalence among young adults after World War II. This is compounded by the dramatic reduction in competing mortality risks from heart disease and stroke over the past 3 decades (Citation1).
Table 1. Causes of death in COPD; data from major clinical trials.
Even with this striking increase in mortality, it is likely that COPD mortality rates are underestimated (Citation4). First, airflow obstruction is often not diagnosed (Citation6, 7). Results from the National Health and Nutrition Examination Surveys (NHANES) I and III suggest that an estimated 24 million Americans have airflow limitation, while only 10 million report a physician diagnosis of COPD or a related disease (Citation5). In a Swedish survey of 1237 adults, 14% had evidence of COPD by spirometry, but less than a third of these individuals had been given a physician diagnosis of COPD, in spite of the fact that most were also symptomatic (Citation8). Underdiagnosis is particularly prevalent in the elderly, in whom half of smokers may eventually develop the disease (Citation8).
There is evidence that many patients who have a documented diagnosis of COPD do not have the condition indicated as an underlying or contributing cause of death on their death certificates (Citation9, 10). Patients with COPD are likely to have multiple co-morbid conditions, particularly coronary artery disease and lung cancer, which may interfere with the accurate determination of cause of death (Citation4). For example, in the study of COPD mortality trends in Tecumseh, Michigan, only 21% of men (62 of 293) and 6% of women (9 of 157) with a clinical diagnosis of COPD had it listed on their death certificate as a primary or contributing cause of death (Citation10). In the Towards a Revolution in COPD Health (TORCH) trial of patients with moderate to severe COPD, among 395 adjudicated deaths that also had informative death certificates, COPD was mentioned on only 58% of the certificates. Even among those patients whose adjudicated cause of death was a COPD exacerbation (n = 127), COPD was underreported: COPD was not listed as the main cause of death in 34% and was not mentioned at all in 21% of these death certificates (Citation9).
Although vital statistics are likely to underestimate the true burden of COPD, patients with more severe airflow limitation are more likely to have COPD reported as an underlying or contributing cause of death. In a follow-up study of NHANES I participants who had pulmonary function testing, mention of COPD on death certificates was increasingly common with worsening severity of obstruction. For example, COPD was listed as the underlying cause of death on death certificates of 1.3% of participants with mild COPD, 4.4% of those with moderate disease, and 23.1% of those with severe disease. COPD was mentioned on the death certificates of 3.8% of participants with mild disease, 17.8% of those with moderate disease, and 47.7% of those with severe COPD (Citation11).
AIRFLOW OBSTRUCTION IS ASSOCIATED WITH INCREASED MORTALITY
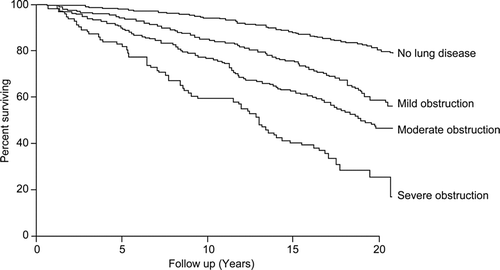
Reduction in forced expiratory volume during the first second (FEV1) is associated with increased mortality in the general population (Citation12). Even in people with mild COPD, the risk for all-cause mortality is increased compared to those with normal lung function () (Citation11). In the NHANES I study, the hazard ratio (HR) for death in participants with mild COPD was 1.2 (95% confidence interval [CI] 1.01–1.4) in an adjusted proportional hazards model (Citation11). For participants with moderate COPD, the HR for death was 1.6 (95% CI 1.4–2.0), and in those with severe COPD, the HR was 2.7 (95% CI 2.1–3.5) (Citation11).
Interestingly, when NHANES I participants were stratified by smoking status, there was no increased mortality risk in never-smokers with mild, moderate, or severe airflow limitation relative to those with no lung disease, whereas current and former smokers both had increased risk of death with worsening severity of airflow obstruction (Citation11). These data suggest that impaired lung function by itself is not necessarily the cause of death, but that FEV1 may be a surrogate measure of the biological effects of cigarette smoking, which leads to other fatal diseases, such as cardiovascular disease and cancer.
CAUSES OF DEATH IN COPD
The distribution of causes of death in patients with COPD varies with disease severity (). In mild and moderate COPD, lung cancer and cardiovascular disease are the most common causes of mortality. The Lung Health Study (LHS) III included 5887 smokers with asymptomatic mild to moderate airway obstruction that was identified by screening spirometry, and during 14 years of follow-up, there were 731 deaths in this cohort (Citation13). Cancer was the most common cause of death (54%), particularly lung cancer, followed by cardiovascular disease (22%). Only 8% of deaths were attributed to non-malignant respiratory disease (Citation13).
Similar results were found in the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease (EUROSCOP) of 1277 active smokers with mild to moderate COPD. Among the 18 patients who died during this 3-year study, 6 died from lung cancer, and 6 died from sudden cardiac death or myocardial infarction (Citation14). Only 1 death was related to a COPD exacerbation, and other causes of death included gastric carcinoma, ruptured aortic aneurysm, pulmonary embolism, and 2 deaths due to trauma (Citation14).
In patients with COPD of moderate severity, cardiovascular disease and malignancy continue to be the predominant causes of death, but deaths due to respiratory disease are more common than in patients with mild COPD. In the Inhaled Steroids in Obstructive Lung Disease (ISOLDE) trial of 751 patients with moderate COPD (mean post-bronchodilator FEV1 1.41 L), there were 68 deaths over 3 years, with cardiovascular disease (32%) and cancer (32%) comprising the most common causes of death. Non-malignant respiratory disease was the cause of death in 22% of deaths in this study (Citation15).
In patients with severe COPD, respiratory disease is an even more common cause of death. In the 3-year TORCH trial of 6184 patients with moderate to severe COPD (mean post-bronchodilator FEV1 1.22 L), there were 911 adjudicated deaths, with 35% due to respiratory disease, 26% due to cardiovascular disease, and 21% due to malignancy, most commonly lung cancer (Citation16, 17). Respiratory disease was also responsible for a high proportion of deaths in the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial. During this 4-year study of 5993 patients with moderate-to-very severe COPD (mean post-bronchodilator FEV1 1.32 L), there were 941 deaths, with 39% due to respiratory disease, 22% due to neoplasm, and 16% due to cardiovascular disease (Citation18, 19). Mortality data from a prospective cohort of 625 COPD patients (mean post-bronchodilator FEV1 1.25 L) used for validation of the BODE index (body-mass index (B), degree of airflow obstruction (O), degree of functional dyspnea (D), and exercise capacity (E)) further support that respiratory disease may be the predominant cause of death in severe COPD (Citation20). Of the 162 deaths that occurred in this cohort over a median follow-up of 28 months, 61% were attributed to respiratory insufficiency, with the remaining deaths due to myocardial infarction (14%), lung cancer (12%), and miscellaneous causes (13%) (Citation20).
Zielinski et al. (Citation21) examined the causes of death in 215 patients with COPD and chronic respiratory failure requiring long-term oxygen therapy. The majority of deaths occurred in a hospital setting, but 26% of the deaths (n = 56) were unexpected and 20% of patients (n = 43) died in their sleep. Acute or chronic respiratory failure was the most common cause of death (38%), followed by heart failure (13%), pulmonary infection (11%), pulmonary embolism (10%), arrhythmia (8%), and lung cancer (7%) (Citation21). Accordingly, 59% of deaths were due to nonmalignant respiratory causes in this group of hypoxemic patients with COPD.
In summary, the predominant causes of mortality in patients with mild COPD are cardiac disease and malignancy, especially lung cancer. As COPD severity increases, deaths due to respiratory disease are increasingly common.
Table 2. Measures that predict all-cause mortality risk in COPD patients.
PREDICTORS OF MORTALITY IN COPD
Reduction in FEV1 has been used as a measure for COPD staging, and it has long been known to predict mortality in COPD (Citation22, 23). However, additional factors have been identified that are independent predictors of increased mortality in COPD patients. COPD is now considered a systemic disease with manifestations other than just airflow limitation, and hence many of these predictors represent other pathophysiologic alterations that occur with the disease.
There is growing support in the literature that the increased work of breathing and accompanying sensation of dyspnea in COPD are manifestations of end-expiratory hyperinflation, rather than just slowing of expiratory airflow. The inspiratory capacity (IC), a measure of hyperinflation, is an independent predictor of mortality in COPD that is easy to assess with routine lung function testing. In a cohort study of 689 predominantly male patients with moderate-to-very severe COPD (mean post-bronchodilator FEV1 1.17 L), the ratio of inspiratory capacity to total lung capacity (IC/TLC) predicted both all-cause and respiratory mortality and was more sensitive and specific than FEV1 (Citation24). In this study, an IC/TLC ratio of ≤25% was associated with significantly shorter survival time compared with IC/TLC ratio >25%, and the adjusted HR for all-cause mortality was 1.97 (95% CI 1.36–2.85) for an IC/TLC ratio ≤25% (Citation24).
Exercise capacity measured by the 6-minute walk distance (6MWD) is also an independent predictor of mortality in COPD. In a 2-year study of 198 predominantly male patients with severe COPD (mean FEV1 1.04 L), the 6MWD decreased over time, and this decline was independent of changes in FEV1 (Citation25). Moreover, 6MWD was a better predictor of mortality than FEV1 (Citation25). Peak oxygen uptake (VO2max) during cycle ergometry is another measure of exercise capacity that also predicts mortality in patients with COPD. In a study of 150 male outpatients with varying COPD severity (mean pre-bronchodilator FEV1 1.01 L), there were no deaths among those individuals in the upper quartile with a VO2max >995 mL/min over the subsequent five years, whereas over half of participants died among those in the lower quartile with a VO2max <654 mL/min during that same time period (Citation26). As with the 6MWD, VO2max was an independent predictor of death and was superior to FEV1 in predicting mortality (Citation26).
Severity of dyspnea has also been shown to predict mortality in COPD, when measured using a standardized assessment method. In a 5-year prospective study of 227 patients with COPD of variable severity (mean FEV1 1.03 L), there were no statistically significant differences in 5-year survival by COPD stage based on FEV1 (p = 0.08) (Citation27). However, dyspnea grade based on the Medical Research Council (MRC) scale did correlate with mortality and was superior to FEV1 in predicting death (Citation27). Patients who had the most severe dyspnea (“too breathless to leave the house, or breathless after undressing”) were more likely to die than those with only mild dyspnea (“when hurrying on the level or walking up a slight hill”), with a hazard ratio of 61.3 (95% CI 13.2–285.4; p < 0.001) (Citation27).
Cachexia and decreased muscle mass are systemic manifestations of COPD associated with increased mortality (Citation28, 29). Studies have demonstrated that a low body mass index (BMI) predicts mortality in patients with COPD (Citation30–32), and although results from clinical trials of nutritional supplementation have been discouraging, there is some evidence that those individuals who do gain weight have improved survival (Citation33). In the National Emphysema Treatment Trial (NETT) cohort of 609 patients with severe emphysema (mean post-bronchodilator FEV1 27% predicted), low BMI was a weak predictor of mortality, but this relationship persisted even after adjustment for extent of emphysema using computed tomographic (CT) quantification (Citation32).
Several of the risk factors that independently predict COPD mortality were combined to generate a composite index called the BODE index, with scores ranging from 0 (lowest risk) to 10 (highest risk). The BODE index was validated in a prospective cohort of 625 patients with a wide range of COPD severity, and it was superior to FEV1 alone in its ability to predict all-cause mortality (Citation20). A modified BODE index also predicted mortality in the NETT cohort (Citation32), and a decrease in the modified BODE index over time correlated with improved survival and was a better predictor of survival than change in any of its individual components (Citation34).
Exacerbations of COPD characterized by increases in cough, phlegm, and dyspnea are common in many patients with COPD, yet occur rarely in others with the same magnitude of airflow limitation. Although most exacerbations are mild and many are not reported to medical caretakers, these events are strong predictors of subsequent death. In a prospective 5-year study of 304 patients with stable COPD, individuals who suffered three or more exacerbations requiring hospitalization or emergency room care during one year (n = 36) were more likely to die than those without exacerbations (adjusted HR 4.13, 95% CI 1.80–9.45) (Citation35). Diffuse mucus plugging of small airways may be a predisposing factor for frequent exacerbations, as examination of NETT surgical LVRS specimens (n = 101) demonstrated that COPD patients with the most diffuse plugging had the highest subsequent mortality (Citation36).
Among potential biomarkers, C-reactive protein (CRP) is a non-specific measure of systemic inflammation associated with an increased risk of mortality in patients with COPD (Citation37). However, it remains to be determined if CRP is a predictor of mortality in COPD specifically, or whether it is a more generic marker of cardiovascular and cancer risk (Citation37–39). In a small cohort of patients with moderate to very severe COPD (n = 218), CRP was not a significant predictor of death after adjusting for other known predictors including BMI, FEV1 % predicted, dyspnea grade, IC/TLC ratio, 6MWD, and BODE index (Citation40). One analysis including serum samples from 4787 participants in the Lung Health Study suggested that the ratio of fibronectin to CRP, a surrogate for lung inflammation and repair processes, is more predictive of all-cause mortality than either biomarker alone in individuals with COPD. However, with regard to cause-specific mortality, the ratio of fibronectin and CRP levels was predictive of cardiovascular but not respiratory mortality, so it may just be an indicator of cardiovascular risk (Citation41).
In spite of the identification of multiple factors that predict mortality in COPD, it is still challenging for physicians to determine prognosis for their individual patients with COPD compared with other end-stage diseases, such as cancer or severe heart failure. In a study of 6451 Medicare beneficiaries enrolled in hospice programs, 32% of 200 patients with COPD survived for more than 6 months after their enrollment, even though Medicare guidelines stipulate that patients are only eligible for hospice if their life expectancy is less than 6 months (Citation42). Moreover, the range of survival times after hospice admission was greater for COPD than any other diagnosis except Alzheimer's disease (Citation42). The results of this study suggest that it is difficult for physicians to predict survival in individual patients with COPD.
In summary, FEV1 remains a useful predictor of mortality, but other measures provide additional information to the FEV1 in their ability to predict survival in patients with COPD. These alternatives, including composite measures, may better reflect the systemic consequences of COPD than measures of pulmonary impairment alone.
COPD TREATMENT CAN ALTER MORTALITY
Smoking cessation is essential in the treatment of COPD, as it is one of few interventions that will slow the progressive decline in lung function as well as improve survival in COPD. In the Lung Health Study, 5887 smokers with mild-to-moderate asymptomatic obstruction were randomized to smoking cessation intervention or usual care, their lung function was measured annually for 5 years, and they were followed for a total of 14.5 years. In those who quit smoking, the rate of decline in FEV1 was half of the rate observed in continuing smokers (Citation43). Moreover, all-cause mortality was reduced among participants randomized to the smoking cessation intervention compared with those who received usual care (8.83 per 1000 person-years versus 10.38 per 1000 person-years; p = 0.03) (Citation13).
There has been substantial controversy regarding the impact of inhaled corticosteroids (ICS) on COPD mortality. In a meta-analysis of 12 randomized controlled trials of a minimum duration of 6 months including 4370 COPD patients, there was no significant difference in all-cause mortality risk between ICS and placebo groups (relative risk 0.81, 95% CI 0.60–1.08) (Citation44). In contrast, a pooled analysis of seven randomized studies of a minimum duration of 1 year including 5085 individuals with COPD demonstrated a 25% reduction in risk of all-cause mortality in patients who received ICS compared with placebo (HR 0.75, 95% CI 0.57–0.99) (Citation45). Retrospective studies of mortality in COPD patients have also suggested that ICS may improve survival (Citation46–48), but this finding has been criticized because of concern about immortal time bias (Citation49). The strongest evidence available regarding the effect of ICS on survival comes from the TORCH trial, which specified all-cause mortality as the primary endpoint. In this study, COPD patients receiving fluticasone propionate (500 mcg twice daily, n = 1534) had a similar risk of mortality compared with those in the placebo group (n = 1524) in an intention-to-treat analysis (HR 1.06, 95% CI 0.89–1.27; p = 0.53) (Citation16).
There have been several investigations of the effect of inhaled bronchodilators on mortality in COPD. In a randomized placebo-controlled trial of ipratropium in the LHS, there was no significant difference in survival between placebo (n = 1,962) and ipratropium (n = 1961) treatment groups over 5 years (Citation50). There was a statistically significant increase in cardiovascular mortality in the ipratropium group compared with placebo (18 deaths versus 7 deaths, p = 0.03); however, the investigators attributed this finding to possible chance effect of multiple comparisons, because many of the patients who died had discontinued treatment prior to death and there was no evidence of dose-related effect (Citation50). Similarly, a meta-analysis of 17 randomized controlled trials of both long-acting and short-acting inhaled anticholinergic drugs that included 14,783 patients found no significant difference in all-cause mortality with anticholinergic use compared with control, but it did identify an increased risk of cardiovascular death, myocardial infarction, or stroke (RR 1.58, 95% CI 1.21–2.06 p < 0.001) in those using anticholinergic drugs (Citation54).
In contrast, results of the recent UPLIFT study showed no evidence of increased mortality associated with anticholinergic therapy (Citation18, 19). In this study, which examined mortality as a secondary endpoint, 5,993 patients with moderate-to-very severe COPD (mean post-bronchodilator FEV1 1.32 L) were randomized to inhaled tiotropium or placebo. At the end of the 1,440-day protocol-defined treatment period, mortality was reduced in the tiotropium group in an intention-to-treat analysis (HR 0.87, 95% CI 0.76–0.99; p = 0.034). Another analysis was performed for follow-up that occurred 30 days after discontinuing therapy (1,470 days), and the trend toward reduced mortality in the tiotropium group persisted but was no longer statistically significant (HR 0.89, 95% CI 0.79–1.02; p = 0.086), due to excess deaths in the tiotropium group during the 30-day wash-out period and because of less complete follow-up at 1,470 days (Citation18, 19).
With regard to inhaled beta-agonists, the TORCH trial randomized 6112 patients with moderate to severe COPD (mean post-bronchodilator FEV1 1.22 L), to inhalational therapy with salmeterol alone, fluticasone alone, salmeterol/fluticasone combination, or placebo (Citation16). All-cause mortality was the primary endpoint, and there was no significant difference in 3-year mortality with salmeterol (HR 0.88, 95% CI 0.73–1.06; p = 0.18) or fluticasone (HR 1.06, 95% CI 0.89–1.27; p = 0.53) alone compared with placebo. However, the analysis of unadjusted results showed a significant mortality benefit for those who received the combination treatment compared with placebo (HR 0.82, 95% CI 0.68–0.99; p = 0.04). After statistical adjustment for an interim analysis by the data monitoring board, the results were no longer statistically significant, but there was still a trend towards decreased mortality in the group treated with the salmeterol/fluticasone combination compared with placebo (HR 0.83, 95% CI 0.68–1.002; p = 0.052) (Citation16).
TREATMENT IN ADVANCED DISEASE
For patients with severe COPD, additional treatment options may include supplemental oxygen, LVRS, and lung transplantation. In patients with COPD who have resting hypoxemia, supplemental oxygen therapy improves survival (Citation51, 52). In the Nocturnal Oxygen Therapy Trial (NOTT), 203 COPD patients with a resting arterial oxygen tension ≤ 55 mmHg (or ≤ 59 mmHg with evidence of chronic hypoxemia) were randomly assigned to receive continuous supplemental oxygen (24 hours/day) or nocturnal oxygen (12 hours/day).
After a mean follow-up of 19.3 months (minimum 12 months), the relative risk of death for patients receiving nocturnal oxygen compared with continuous oxygen was 1.94 (95% CI 1.17–3.24) in an intention-to-treat analysis (Citation51). The findings were similar in the Medical Research Council (MRC) study of 87 COPD patients with resting hypoxemia (arterial oxygen 40–60 mmHg) randomized to receive oxygen (≥ 15 hours per day) or no oxygen, where the 5-year mortality rate in the oxygen group was less than half that observed in the control group (Citation52). The effect of oxygen on mortality in patients with less severe resting hypoxemia or with hypoxemia associated with exercise or sleep remains to be determined.
LVRS is another treatment option that improves survival in selected patients with COPD. In the NETT, 1218 patients with severe emphysema were randomized to undergo LVRS or continue with medical management, and there was no difference in the primary endpoint of all-cause mortality between treatment groups after a median follow-up of 2.4 years (RR 1.01, p = 0.90). However, among a subgroup of 290 patients with predominantly upper lobe emphysema and low exercise capacity, the relative risk of death was 0.47 for those in the surgery group compared with the medical therapy group (p = 0.005) (Citation53).
After extending follow-up to a median of 4.3 years, there was an overall mortality benefit for the surgery group when comparing all 1218 randomized patients (RR 0.85, p = 0.02) (Citation54). Survival curves comparing the surgery group and medical management group demonstrated that early mortality risk was higher in the surgery group due to perioperative deaths, but these curves subsequently crossed with higher mortality risk in the medical management group after extended follow-up. However, in the subgroup of patients with predominantly upper lobe disease and low exercise capacity, the survival curves never crossed, and the surgery group maintained a survival advantage over the medical management group throughout the study (Citation54).
Early studies of lung transplantation in COPD patients suggested that transplantation improved exercise capacity and quality of life but not survival (Citation55, 56). However, more recent evidence indicates that transplantation may confer a survival benefit in select patients with advanced COPD. In a non-proportional hazards analysis from an observational study that included 647 COPD patients listed for transplantation in the United Kingdom, the estimated hazard ratio of death was 0.64 (95% CI 0.48–0.86) after transplantation relative to continued listing (Citation57). There has never been a randomized trial comparing single and bilateral lung transplantation in COPD, but results from observational studies suggest that long-term survival is superior with bilateral lung transplantation, especially in younger COPD patients (Citation58–60).
SUMMARY
Airflow obstruction is associated with increased mortality, even with mild impairment. In mild to moderate COPD, most deaths are due to cardiovascular disease and lung cancer, but as COPD severity increases, respiratory deaths are increasingly common. Smoking cessation has been shown to decrease mortality, but this is primarily due to the effects of smoking on cardiovascular disease and lung cancer risk. Pharmacotherapy with long-acting bronchodilators may favorably affect survival in moderate-to-severe COPD, but further investigations are necessary to confirm this. Oxygen therapy, LVRS, and bilateral lung transplantation improve survival in selected patients with advanced COPD.
ACKNOWLEDGMENTS
This article was developed on the basis of author presentation and discussions at the “Long-Term Considerations in the Course and Treatment of COPD” meeting in Miami, Florida, December 9–10, 2008. This meeting, last author's participation, and manuscript preparation were supported by Boehringer Ingelheim Pharmaceuticals, Inc. and Pfizer, Inc. Editorial assistance was provided by Rachael Profit, PhD, of Envision Scientific Solutions. The article reflects the concepts of the authors and is their sole responsibility. It was not reviewed by Boehringer Ingelheim Pharmaceuticals, Inc. and Pfizer, Inc., except to ensure medical and safety accuracy.
Declaration of interests
Dr. Cristine Berry has no conflicts of interest to declare. Dr Robert Wise has served as a paid consultant for AstraZeneca, Boehringer-Ingelheim Pharmaceuticals, Inc., Emphasys Medical (now part of Pulmonx Inc.), GlaxoSmithKline, Mpex Pharmaceuticals, Novartis Pharmaceuticals Corporation, Pfizer, Inc., Schering-Plough (now part of Merck), and Spiration, Inc. He has received research funding from Boehringer-Ingelheim Pharmaceuticals, Inc. and GlaxoSmithKline. The authors are responsible for the content and writing of this paper.
REFERENCES
- National Institutes of Health. National Heart, Lung, and Blood Institute (NHLBI). Morbidity and mortality: 2009 chartbook on cardiovascular, lung, and blood diseases. 2009 October 2009.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997 May 24; 349(9064):1498–504.
- Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA 2005 Sep 14; 294(10):1255–9.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of co-morbidities. Eur Respir J 2006 Dec;28(6):1245–57.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ 2002 Aug 2;51(6):1–16.
- Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med 2001 Aug 1; 164(3):372–377.
- Coultas DB, Mapel DW. Undiagnosed airflow obstruction: prevalence and implications. Curr Opin Pulm Med 2003 Mar; 9(2):96–103.
- Lundback B, Lindberg A, Lindstrom M, Ronmark E, Jonsson AC, Jonsson E, Not 15 but 50% of smokers develop COPD?—Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003 Feb; 97(2):115–122.
- Drummond MB, Wise RA, John M, Zvarich MT, McGarvey LP. Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD 2010 Jun; 7(3):179–185.
- Higgins MW, Keller JB. Trends in COPD morbidity and mortality in Tecumseh, Michigan. Am Rev Respir Dis 1989 Sep; 140(3 Pt 2):S42–48.
- Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003 May; 58(5):388–393.
- Bang KM, Gergen PJ, Kramer R, Cohen B. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest 1993 Feb; 103(2):536–540.
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005 Feb 15; 142(4):233–239.
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999 Jun 24; 340(25):1948–1953.
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000 May 13; 320(7245):1297–1303.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007 Feb 22; 356(8):775–789.
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007 May; 62(5):411–415.
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009 Nov 15; 180(10):948–955.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct 9; 359(15):1543–1554.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004 Mar 4; 350(10):1005–1012.
- Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997 Feb; 52(1):43–47.
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986 Jan; 133(1):14–20.
- Traver GA, Cline MG, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis 1979 Jun; 119(6):895–902.
- Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005 Mar 15; 171(6):591–597.
- Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004 Jan; 23(1):28–33.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003 Feb 15; 167(4):544–549.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002 May; 121(5):1434–1440.
- Barnes PJ, Celli BR. Systemic manifestations and co-morbidities of COPD. Eur Respir J 2009 May; 33(5):1165–1185.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005 Jul; 82(1):53–59.
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996 Mar; 153(3):961–966.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999 Dec; 160(6):1856–61.
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006 Jun 15; 173(12):1326–1334.
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 Jun; 157(6 Pt 1):1791–1797.
- Martinez FJ, Han MK, Andrei AC, Wise R, Murray S, Curtis JL, Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med 2008 Sep 1; 178(5):491–499.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005 Nov; 60(11):925–931.
- Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007 Sep 1; 176(5):454–459.
- Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007 Feb 1; 175(3):250–255.
- Donaldson GC. C-reactive protein: does it predict mortality? Am J Respir Crit Care Med 2007 Feb 1; 175(3):209–210.
- Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006 Oct; 61(10):849–853.
- de Torres JP, Pinto-Plata V, Casanova C, Mullerova H, Cordoba-Lanus E, Muros de Fuentes M, C-reactive protein levels and survival in patients with moderate to very severe COPD. Chest 2008 Jun; 133(6):1336–1343.
- Man SF, Xing L, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Circulating fibronectin to C-reactive protein ratio and mortality: a biomarker in COPD? Eur Respir J 2008 Dec; 32(6):1451–1457.
- Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med 1996 Jul 18; 335(3):172–178.
- Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000 Feb; 161(2 Pt 1):381–390.
- Gartlehner G, Hansen RA, Carson SS, Lohr KN. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med 2006 May-Jun; 4(3):253–262.
- Sin DD, Wu L, Anderson JA, Anthonisen NR, Buist AS, Burge PS, Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 2005 Dec; 60(12):992–997.
- Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest 2006 Sep; 130(3):640–646.
- Soriano JB, Vestbo J, Pride NB, Kiri V, Maden C, Maier WC. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J 2002 Oct; 20(4):819–825.
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001 Aug 15; 164(4):580–584.
- Suissa S. Inhaled steroids and mortality in COPD: bias from unaccounted immortal time. Eur Respir J 2004 Mar; 23(3):391–395.
- Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002 Aug 1; 166(3):333–339.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980 Sep; 93(3):391–398.
- Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981 Mar 28; 1(8222):681–686.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003 May 22; 348(21):2059–2073.
- Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006 Aug; 82(2):431–443.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998 Jan 3; 351(9095):24–27.
- Stavem K, Bjortuft O, Borgan O, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant 2006 Jan; 25(1):75–84.
- Titman A, Rogers CA, Bonser RS, Banner NR, Sharples LD. Disease-specific survival benefit of lung transplantation in adults: a national cohort study. Am J Transplant 2009 Jul; 9(7):1640–1649.
- Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Single vs bilateral, sequential lung transplantation for end-stage emphysema: influence of recipient age on survival and secondary end-points. J Heart Lung Transplant 2001 Sep; 20(9):935–941.
- Thabut G, Christie JD, Ravaud P, Castier Y, Brugiere O, Fournier M, Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet 2008 Mar 1; 371(9614):744–751.
- Thabut G, Ravaud P, Christie JD, Castier Y, Fournier M, Mal H, Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008 May 15; 177(10):1156–1163.