ABSTRACT
Chronic Obstructive Pulmonary Disease (COPD) is defined by being “not fully reversible”, most guidelines recommend measurement of lung function after the administration of a bronchodilator. The objective of this study was to compare bronchodilator responsiveness (significant improvement in the FEV1 or FVC) to full-, partial- or “inverse’” reversibility in obstruction status in a population-based sample in Southeastern Kentucky. The study population was selected using random digit dialing of an adult population in Southeastern Kentucky as part of the Burden of Lung disease (BOLD) project. Lung function was assessed using spirometry pre- and post-bronchodilation. Subjects presence and severity of COPD was classified using modified Global Obstructive Lung Disease (GOLD) criteria. We examined the relation between changes in “obstruction” status (based on the FEV1/ FVC of 0.7) and the presence of “significant bronchodilator responsiveness” (based on ≥ 12% improvement in the FEV1 or the FVC). The final population with acceptable pre- and post-bronchodilator spirometry included 440 participants. 32/440 subjects (7.3%) changed from obstructed to unobstructed (full-reversibility), 19/440 (4.3%) changed from unobstructed to obstructed (“inverse”-reversibility), 389/440 (88.4%) had either no-change or partial-reversibility, and 65/440 (14.8%) had bronchodilator responsiveness. Among those with full-reversibility, only 9/32 (28.1%) had bronchodilator responsiveness, whereas among subjects with “inverse”-reversibility, 10/19 (52.6%) had bronchodilator responsiveness. Among all subjects with bronchodilator responsiveness, only 19/65 (29.2%) changed categories. Our findings suggest that significant bronchodilator responsiveness is not the same as “reversibility” of “obstruction”, even though these terms are often used interchangeably.
Keywords: :
BACKGROUND
COPD is a chronic disease of the lungs and is characterized by irreversible airflow limitation, and is currently the fourth-leading cause of death in the United States (Citation1, 2). GOLD defines COPD as a preventable and treatable disease with airflow limitation that is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases (Citation3). Both the American Thoracic Society (ATS) and the European Respiratory Society (ERS) have, in large part, adopted this definition (Citation4).
Response to a bronchodilator is thought to be important in COPD diagnosis and to distinguish COPD from asthma as the cause for airflow limitation, a disease that is clinically different from COPD in both pathogenesis and therapeutic response. Guidelines suggest that classification of COPD be made using spirometry performed after bronchodilator administration. One potential criterion of a significant response to a bronchodilator (i.e., the presence of “full”-reversibility) is the presence of a pre-bronchodilator ratio less than 0.7 but a post-bronchodilator ratio that is greater than or equal to 0.7, switching from “obstructed” to “unobstructed.”(Citation3). Of course, people also have the potential to switch from “unobstructed” to “obstructed” (which lacks a name but is listed as “inverse”-reversibility in this analysis).
Another potential criterion of a significant response is an improvement at or above a certain threshold in either of the components of the FEV1/ FVC ratio (bronchodilator responsiveness). Both the ATS and GOLD define this as a post-bronchodilator spirometric measurement increase ≥ 12% of the pre-bronchodilator measurement for the FEV1 or the FVC, along with an increase in the FEV1 or FVC of at least 200 mL (Citation5). Patients who demonstrate bronchodilator responsiveness but remain obstructed, based on their FEV1/ FVC are considered to have partial-reversibility.
According to the 2008 GOLD guidelines “Spirometry should be performed after the administration of an adequate dose of an inhaled bronchodilator (e.g., 400 μg salbutamol) (Citation5) to minimize variability. In a random population study to determine spirometry reference values, post-bronchodilator values differed markedly from pre-bronchodilator values (Citation6). Furthermore, post-bronchodilator lung function testing in a community setting has been demonstrated to be an effective method to identify individuals with COPD.” (Citation7, 8).
The purpose of this study is to better understand how bronchodilator administration changes FEV1 and FVC separately and how these changes affect the FEV1/ FVC. This study will determine the relation between bronchodilator responsiveness, as defined by a ≥ 12% increase in the FEV1 or the FVC after administration of a bronchodilator, and either full-, partial-, or “inverse”-reversibility. We used the Burden of Lung Disease (BOLD) protocol to complete the study (Citation9).
METHODS
BOLD is a community-based study conducted to provide a high-quality standardized tool to measure the country-specific prevalence of COPD. The study is a simple random sample of noninstitutionalized adults (age ≥ 18 years) from 26 counties in Southeastern Kentucky. Initially, participants were contacted by telephone through random-digit dialing. Answers to a minimal data questionnaire were obtained, and site or home visits were scheduled for adults who were at least 40 years in age to complete questionnaires and perform pre- and post-bronchodilator spirometry. Protocol for the BOLD study has been discussed elsewhere (Citation9). The present analysis was approved by the University of Kentucky Institutional Review Board.
Study Measures
Trained and certified technicians administered spirometry using the ndd EasyOne Spirometer. Measurements were made before and at least 15 minutes after two puffs of salbutamol (200 μg) administered via a metered dose inhaler with a spacer. Useable spirograms met ATS standards, with at least 2 acceptable and reproducible tests for both volume measurements. Adequate spirometry measurements were free from artifact, sudden stops, and back extrapolated volumes of less than 5% of FVC. Inclusion criteria for this study required subjects to have adequate pre- and post-bronchodilator spirometry. Predicted values from the Third National Health and Nutrition Examination Survey (NHANES III) were used in the analysis (Citation10).
Definitions
Demographic data included in this analysis were sex, age, body mass index (BMI), smoking status, and educational status (the studied population was 100% White). The study participants were classified, using the post bronchodilator lung function, into 6 lung function categories based on a modification of the GOLD criteria. Normal (no respiratory symptoms or airflow obstruction or restriction), Stage 0 (the presence of symptoms of cough, sputum, wheeze, or breathlessness without airflow obstruction or restriction), Restricted (FEV1/ FVC ≥ 70% and FVC < 80% of predicted), Stage 1 (FEV1/ FVC < 70% and FEV1 ≥ 80% predicted), Stage 2 (FEV1/ FVC < 70% and 50% ≤ FEV1 < 80% predicted), and Stage 3 or 4 (FEV1/ FVC < 70% and FEV1 < 50% predicted) (Citation3). GOLD stage 0 is not in the most recent guidelines, but we have included this stage because previous work (Citation11, 12) has demonstrated worse outcomes in this group of patients. We also included a “restricted” group, subjects with an FEV1/ FVC ≥70% and an FVC < 80% predicted.
Bronchodilator reversibility is defined according to how a person's obstruction classification changed between pre- and post-bronchodilator (based on the FEV1/ FVC of < 0.70) as either full-reversibility (change from obstructed to unobstructed), “inverse”-reversibility (change from unobstructed to obstructed), and no-change (no change in obstruction status). We classified subjects who were obstructed both pre- and post-bronchodilator, but also had bronchodilator responsiveness, as having “partial”-reversibility.
Significant bronchodilator responsiveness is defined as a ≥ 12% (from baseline) increase in either the FEV1 or the FVC. We did not use the 200 mL increase “floor” in our classification, although 64/65 subjects we classified as having bronchodilator responsiveness had at least a 200 mL increase in their FEV1 or FVC.
Statistical Analysis
Data analysis was completed using statistical software (Statistical Analysis Software, version 9.1; SAS Institute; Cary, NC). Descriptive statistics and frequency distributions were calculated for the eligible and the studied populations. To determine if there were any differences between the analyzed population and the eligible study cohort, χ2 analysis was used. Additional descriptive statistics and frequency distributions were calculated for those in the study population stratified by reversibility status and bronchodilator responsiveness. Least square means were used on the data to obtain p-values.
RESULTS
A total of 15,148 different telephone numbers were dialed. In 7,073 calls, individuals refused minimal participation in the study, could not be reached, or hung up. Another 6,011 telephone numbers dialed were ineligible due to an invalid telephone number with no forwarding information, no one in the household of eligible age to participate in the study, the individuals were institutionalized, or they did not speak English. A total of 1,046 individuals were eligible and provided at least part of the minimal data but refused full participation in the study; 971 individuals responded to the minimal data questionnaire and were willing to participate in the full protocol, but 575 actually participated. Acceptable pre- and post-bronchodilator spirometry and full data were collected from 440 participants, which comprised the final study cohort.
presents the frequency distributions for sex, age group, BMI, education, smoking status, respiratory symptoms, 12% increase in FEV1, 12% increase in FVC, 12% increase in either measurement, and GOLD COPD status of the final analyzed cohort (N = 440) and the eligible population (N = 575). Comparisons of all parameters were statistically insignificant, suggesting that the two groups were similar.
Table 1. Characteristics of analyzed population versus eligible study population*
presents the demographic information for the analyzed population stratified by those with full-reversibility (N = 32, 7.3%), “inverse”-reversibility (N = 19, 4.3%), and those who did not change in obstruction status (N = 389, 88.4%). Of the 100 subjects who were obstructed at baseline, 32 (32.0%) demonstrated full reversibility, and of the 340 subjects who were unobstructed at baseline, 19 (5.6%) demonstrated “inverse”-reversibility. Overall, 65/440 (14.8%) subjects had bronchodilator responsiveness. For subjects with full-reversibility 9/32 (28.1%) had bronchodilator responsiveness, while among those with “inverse”-reversibility 10/19 (52.6%) had bronchodilator responsiveness. Out of subjects who had no change to their obstruction status and were obstructed, 28/68 (41.2%) had bronchodilator responsiveness, comprising partial-reversibility. Among the other 321 subjects with no change, 18 (5.6%) had bronchodilator responsiveness. The mean FEV1, FVC, and FEV1/ FVC measurements before bronchodilator administration were 2.52 liters, 3.37 liters, and 0.743 respectively. The mean FEV1, FVC, and FEV1/ FVC measurements after bronchodilator administration were 2.61 liters, 3.42 liters, and 0.760, respectively.
Table 2. Characteristics of analyzed population by respiratory change status.
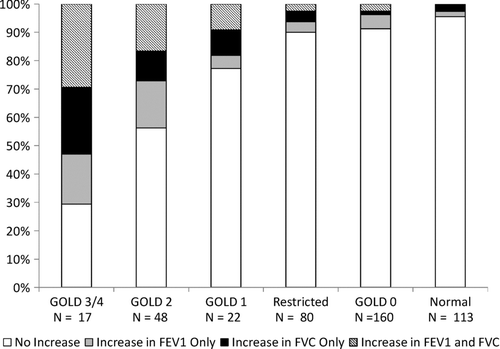
presents a bar chart of the percent change in FEV1 from baseline for participants, stacked by bronchodilator reversibility status. The demarcation distinguishes those who had significant bronchodilator responsiveness. The figure shows that it was modest increases in FEV1 that allowed people to change to unobstructed after bronchodilator administration. It also demonstrates that most people with bronchodilator responsiveness of the FEV1 did not also have bronchodilator reversibility. presents similar data for the FVC. Again, most subjects with bronchodilator responsiveness of the FVC did not also have bronchodilator reversibility. presents a bar chart of GOLD stage by bronchodilator responsiveness in either the FEV1, the FVC, or both of these measures. Among subjects with GOLD Stage 3 or 4, 12/17 (70.6%) had bronchodilator responsiveness, and 21/48 (43.8%) among those with Stage 2 disease.
Figure 1. The increase in the forced expiratory volume in one second (FEV1) following administration of 200 μg of salbutamol, and the presence of full-reversibility (change from obstructed to unobstructed based on the FEV1/ forced vital capacity (FVC) ratio of < 0.70), partial reversibility (> = 12% increase in the FEV1 or the FVC but no change in obstructive status), inverse reversibility (change from unobstructed to obstructed based on the FEV1/ FVC ratio of < 0.70), and no reversibility (not in any of the above categories).
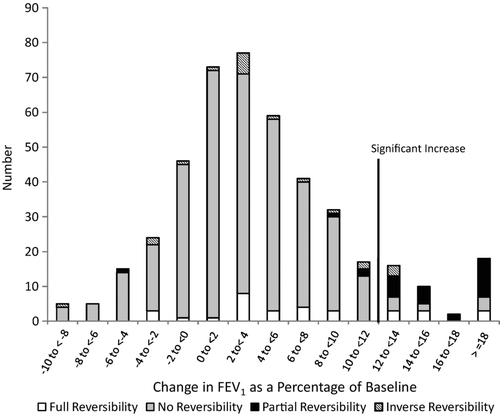
Figure 2. The increase in the forced vital capacity (FVC) following administration of 200 μg of salbutamol, and the presence of full-reversibility (change from obstructed to unobstructed based on the forced expiratory volume in 1 second (FEV1) / FVC ratio of < 0.70), partial reversibility (> = 12% increase in the FEV1 or the FVC but no change in obstructive status), inverse reversibility (change from unobstructed to obstructed based on the FEV1/ FVC ratio of < 0.70), and no reversibility (not in any of the above categories).
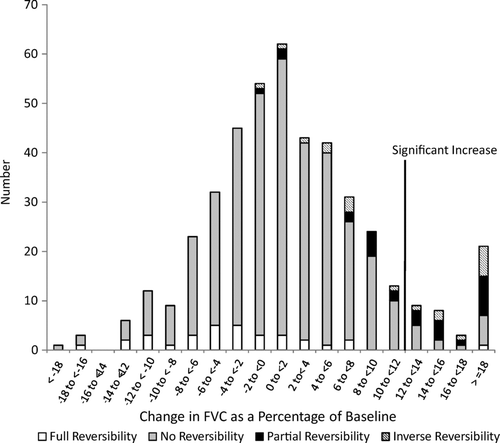
Figure 3. The post-bronchodilator lung function classification using the modified Global Initiative on Obstructive Lung Disease (GOLD) scheme as described in the methods, and the presence of bronchodilator responsiveness as indicated by a 12% or great increase in the forced expiratory volume in 1 second (FEV1), the forced vital capacity (FVC), or both. Those in GOLD stages 1, 2, 3 and 4 with bronchodilator responsiveness comprise “partial reversibility”.
DISCUSSION
In this study of 440 subjects with adequate pre- and post-bronchodilator lung function measurement, we found that 11.6% of participants experienced bronchodilator reversibility. Specifically, 7.3% changed from obstructed to unobstructed (full-reversibility) and 4.3% changed from unobstructed to obstructed (“inverse”-reversibility) after bronchodilator administration. An additional 28/440 (6.4%) had evidence of bronchodilator responsiveness but remained obstructed, comprising partial-reversibility. Ironically, only 9/32 (28.1%) of subjects with full-reversibility met criteria for bronchodilator responsiveness, whereas 10/19 (52.6%) of subjects with “inverse”-reversibility met these criteria. By definition, 100% of subjects with partial-reversibility met criteria for bronchodilator responsiveness, although this comprised 28/68 (41.2%) of subjects who remained obstructed following a bronchodilator. Overall, 46/343 (11.8%) of subjects who did not change “obstruction” categories met criteria for bronchodilator responsiveness.
These findings challenge some of the underlying assumptions about pre- and post-bronchodilator measurements and their usefulness in classifying COPD or in separating COPD (as not fully reversible) and asthma (as fully reversible). The 2008 GOLD guidelines acknowledge that “while post-bronchodilator FEV1/ FVC and FEV1 measurements are recommended for the diagnosis and assessment of severity of COPD, the degree of reversibility of airflow limitation (e.g., FEV1 after bronchodilator or glucocorticosteroids) is no longer recommended for diagnosis, differential diagnosis with asthma, or predicting the response to long-term treatment with bronchodilators or glucocorticosteroids”(Citation8). Our analysis shows that most people who were “fully reversible” met this definition on the basis of relatively small changes in their FEV1 and FVC, thus, they have “reversibility of obstruction” in the absence of having bronchodilator responsiveness.
Conversely, subjects with “inverse”-reversibility (a category which is not, to our knowledge, acknowledged in any guidance document) had the largest proportion of people demonstrating bronchodilator responsiveness because of a large FVC response. Prior to bronchodilator administration, 9 (47.4%) of the subjects with “inverse”-reversibility were classified as restricted, 8 of whom experienced bronchodilator responsiveness; while 2 of the 10 subjects classified as normal experienced bronchodilator responsiveness.
Others have shown that the presence of “reversibility” in patients with COPD is common and can vary in individuals over short periods of time. For example, Calverley et al. demonstrated that in baseline data from subjects enrolled in a clinical COPD trial, over a 2-month period, 52.1% of subjects changed their bronchodilator responsiveness status. They concluded that “classifying patients as ‘responders’ and ‘non-responders’ can be misleading and does not predict disease progression” (Citation13). Similarly, in the baseline data from another larger clinical trial, Tashkin et al. found that over 65% of subjects with documented COPD had at least a 15% increase in their FEV1 after bronchodilator administration (400 μg of salbutamol and 80 μg of ipratropium) (Citation14). This finding is similar to ours in Stage 3 or 4 subjects, where 70% had a 12% increase in either their FEV1 or their FVC following 200 μg of salbutamol.
Prior studies have noted individual changes in FEV1 and FVC after bronchodilator administration. Both Perez-Padilla et al. and Johannessen et al. found a greater increase in FEV1 compared to FVC for patients who had a significant increase in FEV1/ FVC after bronchodilator administration (Citation7,Citation15). In both studies for those with partial-reversibility, a consistent increase in FVC was seen, explaining why FEV1/ FVC levels remained decreased after bronchodilator administration.
Our group of subjects with “inverse”-reversibility had the highest proportion of those who experienced a 12% increase in either FEV1 and/or FVC (52.6%). This is contrary to what bronchodilator reversibility testing is hoping to capture and therefore has important implications for public health and clinically. For example, the NHANES 2007–2008 spirometry protocol states for bronchodilator administration “Only participants with abnormal baseline spirometry values showing airflow obstruction defined as FEV1/ FVC% equal to or less than the lower limit of normal (LLN), or an observed FEV1/ FVC% equal to or less than 70% will be selected for bronchodilator reversibility testing” (Citation16). This important group of people would be missed entirely since they are not obstructed at baseline.
This study also showed that the majority of subjects who did experience bronchodilator responsiveness, defined by a 12% increase in the FEV1 or the FVC were still classified as obstructed according to GOLD criteria. These findings support the conclusions from Cazzola et al. that bronchodilator therapy should not be considered only for those who become unobstructed after bronchodilator administration (Citation17). This analysis shows that patients who are classified as having COPD according to GOLD criteria can have significant bronchodilator responsiveness, potentially highlighting the overlap of COPD and asthma (Citation14,Citation18,Citation19).
There are some limitations to this study analysis. Of the 2,017 subjects potentially eligible for inclusion in this study, only 575 participated and 440 were ultimately included in this analysis. Studies with low participation rates are more subject to bias. Individuals who refused to participate or who did not meet the strict eligibility criteria for this study, having adequate lung function measurements for both pre- and post-bronchodilator spirometry, may be different from those who were included. Our study design, however, which compares the same individuals before and after a bronchodilator administration, should limit the bias that low participation introduces.
This study may not be generalizable to populations outside rural Kentucky. Further, the study sample sizes for both the full-reversibility and the “inverse”-reversibility cohorts were small. The small sample sizes for both the fully reversible and “inverse” reversible groups limited further analysis such as regression analysis of factors that may affect disease severity such as age and BMI. Finally, the dose of salbutamol used in this study (200 μg) was less than what was recommended for clinical testing of reversibility (400 μg). Even with these important limitations, the findings in this project present important new knowledge in understanding FEV1 and FVC in COPD reversibility after bronchodilator administration.
In conclusion, little overlap was found between the presence of bronchodilator responsiveness as indicated by a 12% increase in the FEV1 or FVC and the presence of full-, partial-, and “inverse”-reversibility as indicated by a status change in the FEV1/ FVC following administration of a bronchodilator. Only a minority of subjects with full-reversibility demonstrated bronchodilator responsiveness, and most subjects with significant bronchodilator responsiveness did not change obstruction categories. We believe these findings question the rationale for using post-bronchodilator lung function measurements alone to classify and categorize COPD. This also challenges the wisdom of not doing bronchodilator testing in people with “non-obstructed” lung function, in that a percentage of the population will have an improvement in their FVC that renders them “obstructed” following testing.
This may be particularly true among patients being evaluated for respiratory complaints. The finding that values following a bronchodilator are different does not mean they are better in classifying disease or predicting outcomes. Appropriate longitudinal studies using both pre- and post-bronchodilator lung function measurements are necessary to identify long-term outcomes for patients who experience bronchodilator reversibility and/or responsiveness and to determine which measurement is more useful prognostically.
Declaration of interest
David M. Mannino has served on advisory boards for Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Sepracor, Astra-Zeneca, Novartis and Ortho Biotech and has received research grants from Astra-Zeneca, GlaxoSmithKline, Novartis and Pfizer. Heather A. Prentice, Glyn G. Caldwell, and Heather M. Bush report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765-773.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ 2002; 51:1–16.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van WC, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med 2006; 173:1316–1325.
- Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax 2005; 60:842–847.
- Rodriguez-Roisin R, Rabe KF, Anzueto A, Bourbeau J, Calverley P, Casas A, DeGuia TS, Fukuchi Y, Hui DS, Jenkins C, Kocabas A, Martinez FJ, van Weel C, Vestbo J. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2008 Update). www.goldcopd.org. 2009.
- Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, Crapo RO, Jensen RL, Burney PG. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005; 2:277–283.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–187.
- Mannino DM, Doherty DE, Buist AS. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100:115–122.
- Mannino DM. GOLD stage 0 COPD: is it real? Does it matter? Chest 2006; 130:309–310.
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58:659–664.
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J 2008; 31:742–750.
- Perez-Padilla R, Hallal PC, Vazquez-Garcia JC, Muino A, Maquez M, Lopez MV, de Oca MM, Talamo C, Valdivia G, Pertuze J, Jardim J, Menezes AM. Impact of bronchodilator use on the prevalence of COPD in population-based samples. COPD 2007; 4:113–120.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Laboratory Protocol. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc .gov/ nchs/data/nhanes/nhanes_07_08/Bronchodilator.pdf. 2008.
- Cazzola M, Donner CF, Hanania NA. One hundred years of chronic obstructive pulmonary disease (COPD). Respir Med 2007.
- Mannino DM. Coexisting asthma and COPD: Confused clinicians or poor prognosticator? Chest 2008; 134: 1–2.
- Shaya FT, Dongyi D, Akazawa MO, Blanchette CM, Wang J, Mapel DW, Dalal A, Scharf SM. Burden of concomitant asthma and COPD in a Medicaid population. Chest 2008; 134:14–19.