ABSTRACT
Streptococcus pneumoniae (S. pneumoniae) is recovered from sputum of patients with chronic obstructive pulmonary disease (COPD) during stable disease and exacerbations. In patients with community acquired pneumonia, antibiotic exposure in the prior 3–6 months is associated with recovery of antibiotic resistant isolates of S. pneumoniae. Whether the same relationship is seen in COPD is not known. From April 1994 to June 2004, 127 adults with COPD were enrolled in a prospective longitudinal study. Sputum isolates of S. pneumoniae were characterized with susceptibility testing and pulsed-field gel electrophoresis (PFGE). The relationship between antibiotic use in the previous 3 and 6 months with either new acquisition of a resistant pneumococcal isolate or development of resistance (4-fold increase in MIC) in a pre-existing colonizing pneumococcal strain was determined. A total of 194 pneumococcal isolates were recovered from 38 patients. Among 71 newly acquired and 4 resistance-emergent strains analyzed further, rates of resistance to penicillin (MIC ≥2), erythromycin (MIC ≥1), tetracycline (MIC ≥8) and trimethoprim/sulfamethoxazole (MIC ≥4) were 8%, 24%, 17% and 16% respectively. Flouroquinolone resistance was not seen. Among strains isolated from patients exposed to a macrolide within 6 months, 53.6% displayed erythromycin resistance vs. 14% of strains without such exposure (p = 0.00085). Similar associations were not seen for other antibiotics. Macrolide use in the previous 6 months is associated with macrolide resistance in sputum isolates of S. pneumoniae. Recent antibiotic exposure may help in determining appropriate antibiotic treatment in these patients.
| Abbreviations | ||
| COPD: | = | Chronic Obstructive Pulmonary Disease |
| FEV1: | = | Forced Expiratory Volume in 1 Second |
| FVC: | = | Forced Vital Capacity |
| MIC: | = | Minimum Inhibitory Concentration |
| PFGE: | = | Pulsed-Field Gel Electrophoresis |
INTRODUCTION
Bacterial infection of the lower respiratory tract plays an important role in the course and pathogenesis of chronic obstructive pulmonary disease (COPD) (Citation1–3). Non-typeable Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae and Pseudomonas aeruginosa cause intermittent or chronic respiratory tract infection in the setting of COPD (Citation4–12). Exacerbations are characteristic of the course of COPD and approximately 50% of episodes are caused by bacterial infection with S. pneumoniae being responsible for 10–15% of all exacerbations (Citation1). S. pneumoniae colonizes the respiratory tract of 15–17% of adults with COPD at any one time (Citation13,14). Oral macrolides, β-lactams, fluoroquinolones, tetracyclines or trimethoprim-sulfamethoxazole are recommended antibiotic therapy for the treatment of exacerbations of COPD (Citation1). In the past 2 decades, resistant S. pneumoniae strains have become prevalent (Citation15–17). Antibiotic exposure in the previous three months or six months has been associated with invasive infections, mainly bacteremic community acquired pneumonia, with antibiotic-resistant strains of S. pneumoniae (Citation18–22).
Adults with COPD are frequently exposed to antibiotics for exacerbations and other comorbid conditions. Furthermore, these patients have a considerable bacterial load in the sputum. We hypothesized that this combination of frequent antibiotic exposure and large bacterial loads will result in selection of resistant pneumococcal strains in the sputum of patients with COPD with recent antibiotic exposure. We tested this hypothesis by determining if antibiotic exposure in the prior 3 and 6 months will lead to a significant increase in respiratory tract isolation of resistant strains of S. pneumoniae in adults with COPD.
PATIENTS AND METHODS
COPD Study Clinic
The study protocol was approved by the institutional review board of the Veteran Affairs Western New York Healthcare System, Buffalo, New York. This prospective longitudinal study of bacterial infection in COPD at the Buffalo Veterans Affairs Medical Center has been described previously (Citation7,8). From April 1994 to June 2004, 127 adults with COPD were enrolled to maintain a cohort of approximately 50 patients. Fifty subjects were enrolled at the beginning of the study in 1994 and additional subjects were enrolled as necessary to maintain the cohort at approximately 50 subjects. An average of 8 (range: 3–15) additional subjects were enrolled each year from 1995 to 2004. Inclusion criteria were at least 15 pack-years of smoking history, the presence of chronic bronchitis (Citation23); the absence of asthma and bronchiectasis on clinical assessment; an ability to comply with a schedule of monthly clinical visits; and the absence of immunosuppressive or other life-threatening disorders. The patients were seen monthly, as well as whenever they had symptoms suggestive of an exacerbation, at an outpatient clinic.
At each visit, clinical information, sputum and serum samples were obtained. The patients were questioned about the status of their chronic respiratory symptoms (dyspnea, cough, sputum production, viscosity, and purulence), and the responses were graded as 1 (at the usual level), 2 (somewhat worse than usual), or 3 (much worse than usual). A minor worsening of two or more symptoms or a major worsening of one or more symptoms prompted a clinical assessment of the cause. If the patient had fever (a temperature that exceeded 38.3°C), appeared ill, or had signs of consolidation on examination of the lungs, a chest X-ray was obtained to rule out pneumonia. If other causes of the worsening of symptoms, such as pneumonia, upper respiratory infection, and congestive heart failure, were ruled out, the patient was considered to be having an exacerbation of COPD. Patients were prescribed antibiotics for exacerbations of COPD for 5 to 10 days. The determination of whether the patient had stable disease or an exacerbation was made before the results of sputum cultures were available.
Table 1. Characteristics of patients with atleast one S. pneumoniae isolate compared with those without any S. pneumoniae isolate from sputum culture.
Sputum samples
Samples of sputum that were spontaneously expectorated were homogenized by incubation at 37°C for 15 minutes with an equal volume of 0.1 percent dithiothreitol. Serial dilutions of homogenized sputum in phosphate-buffered saline were placed on blood, chocolate, and MacConkey agar plates. Bacterial identification was performed with the use of standard techniques.
Streptococcus pneumoniae characterization
All isolates of S. pneumoniae were serotyped using previously described methods (Citation24) at Erie County Medical Center, Buffalo, NY (Dr. Daniel Amsterdam) or at the Houston Veterans Affairs Medical Center, Houston, TX (Dr. Daniel Musher). Non-typeable strains (25/199) were subjected to DNA analysis with AccuProbe (Gen-Probe, San Diego, CA) to reliably identify S. pneumoniae (Citation25–27). Five of the 25 non-typeable strains were negative by AccuProbe analysis and were excluded from further analysis.
Pulsed-field gel electrophoresis (PFGE)
All confirmed isolates of S. pneumoniae (194) were subjected to molecular typing by pulsed-field gel electrophoresis (PFGE) (Citation28). Sma I (250 U/ml) was used for DNA digestion, followed by PFGE with contour-clamped homogenous field electrophoresis (CHEF-DR II: Bio-Rad) at 200V (14°C for 20 hours; initial forward time –1 second; final forward time –20 seconds). After ethidium bromide staining, the gels were photographed with the Bio-Rad Gel Doc 1000 system (Bio-Rad). Isolates differing by ≤ 3 bands were considered closely related and assigned to the same PFGE type (Citation29).
Each S. pneumoniae strain was designated as new to the patient or pre-existing, based on molecular typing. A new strain was defined as a strain that had not been previously isolated from sputum samples obtained from previous visits since the patient's enrollment in the study. A pre-existing strain was defined as a strain that had been isolated previously from the sputum obtained from previous visit/s since the patient's enrollment in the study.
Antimicrobial susceptibility
Susceptibility testing was performed on all 194 isolates of S. pneumoniae using the Clinical Laboratory Standards Institute (CLSI) broth microdilution MIC method (Citation30). Resistance was defined according to 2005 CLSI interpretive standards (Citation31). There were no changes in definition of macrolide resistance between 2005 and 2008 CLSI standards (Citation31, 32). Similarly, there were no changes in breakpoints for penicillin resistance for patients without meningitis who can be treated with oral penicillin (Citation32, 33).
Statistical analysis
Numeric variables are presented as mean ± SD (or SEM) or median (range) when the data were not normally distributed. Antibiotic use during the prior 3 and 6 months was ascertained from study clinic and electronic medical records. Antibiotics were grouped as β-lactams, macrolides, tetracyclines and trimethoprim/sulfamethoxazole. In order to prevent multiple representation of the same strain, 2 kinds of strains were included in the analysis of antibiotic exposure and susceptibility: 1) new strains of S. pneumoniae or 2) pre-existing strains with ≥4 -fold increase in MIC of an antibiotic compared to previous isolates of the same strain from that patient. Fisher's exact test was used to determine relationship between isolation of these strains of S. pneumoniae and antibiotic use in previous 3 and 6 months.
To determine other factors that may relate to resistant S. pneumoniae isolation, patients with and without isolation of macrolide resistant S. pneumoniae from sputum cultures were compared by unpaired t-test for continuous data with normal distribution, by the Mann-Whitney test for continuous data without normal distribution, and by Fisher's exact test for nominal data. A univariate analysis was performed; proportions were compared with use of the odds ratio (OR) as a measure of association by means of a logistic regression model, in which resistance to erythromycin was considered a dependent variable and factors potentially related to antibiotic resistance were considered independent variables. Multivariate analyses were performed via stepwise logistic regression; macrolide resistance was the dependent variable. Statistical significance was considered to be present when p < 0.05. MedCalc® statistical software (version 9.3.8) was used to analyze the data.
RESULTS
Subject demographics and strain distribution
A total of 127 adults with COPD participated in clinic visits from April 1994 to June 2004. A total of 5092 sputum samples were obtained from these patients over this time period (mean: 40.1, range: 1–128). At least one pneumococcal isolate was recovered from 38 of the 127 patients (29.9%). Characteristics of patients with and without S. pneumoniae isolation from sputum culture were compared and no significant differences were found between these two patient groups (). The 38 patients with ≥1 culture yielding S. pneumoniae made a total of 2074 clinic visits. Overall rate of recovery of S. pneumoniae for these 38 patients was 9.4% (194 isolates/2074 visits). The rate of recovery of S. pneumoniae from individual patients ranged from 0.9% (1 of 111 visits) to 59.3% (16 of 27 visits). A single strain (one PFGE type) was isolated from 22 of the 38 patients; 16 patients carried multiple strains (up to 6 strains in one patient) over time. The rate of recovery and genetic relatedness of these isolates are summarized in . Serotypes of 194 S. pneumoniae isolates are described in .
Table 2. Patients with S. pneumoniae isolated from at least one sputum culture.
Table 3. Serotypes of S. pneumoniae isolates.
Antimicrobial susceptibility
Analysis of 194 S. pneumoniae isolates by PFGE determined that 71 independent strains were acquired by the 38 patients. Seventy-five strains were included in subsequent analyses, of which 71 were new strains and 4 were preexisting strains with a ≥4-fold increase in MIC to an antibiotic. Eighteen (24%) of these 75 strains had an erythromycin MIC ≥1, 6 (8%) strains had a penicillin MIC ≥2 and 6 (8%) strains showed intermediate susceptibility (MIC 0.12–1) for penicillin. Thirteen (17%) strains were resistant to tetracycline (MIC ≥8) and 12 (16%) strains were resistant to trimethoprim/sulfamethoxazole (MIC ≥4). Fluoroquinolone resistance was not seen. Resistance to at least 1 of these 4 antibiotics was seen in 26 strains (35%).
Antibiotic exposure and resistance
The association between antibiotic exposure and subsequent occurrence of resistant strains was examined at 2 time points, 3 and 6 months after exposure. Details of antibiotic exposure in the previous 3 and 6 months are described in . Of the pneumococcal strains isolated from patients exposed to a macrolide (predominantly azithromycin) within the past 3 months, 54.5% displayed erythromycin resistance vs. 18.7% of strains isolated without such antibiotic exposure (p = 0.0187, ). This difference was maintained at 6 months, when 53.6% of strains isolated after exposure displayed erythromycin resistance in comparison to 14% of pneumococcal strains isolated without such antibiotic exposure (p = 0.00085, ).
Figure 1. Effect of exposure to a macrolide antibiotic in previous 3 and 6 months and sputum isolation of macrolide resistant S. pneumoniae in COPD patients.
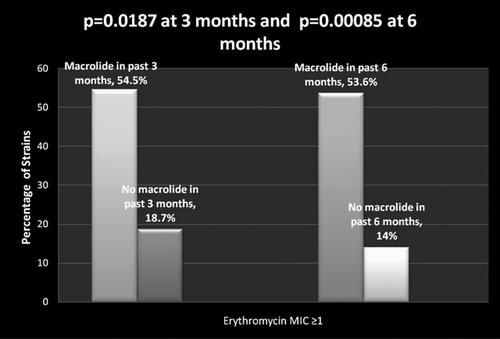
Table 4. Antibiotic exposure in the previous 3 and 6 months in patients with the 75 S. pneumoniae isolates included in this analysis.
There were no significant differences in penicillin non-susceptibility (MIC ≥0.12, p = 0.19 for 3 months and p = 0.27 for 6 months) or penicillin resistance (MIC≥2) between strains isolated from patients with or without β-lactam use in the previous 3 months or 6 months (). A trend was observed for increase in trimethoprim/sulfamethoxazole resistance in strains isolated from patients exposed to this antibiotic at 3 months (42.9% vs. 13.2%, p = 0.077) but this difference was not seen at 6 months (). Tetracycline exposure was too infrequent to provide useful data.
Table 5. Exposure to β-lactam, macrolide, or trimethoprim/sulfamethoxazole (TMP Sulfa) in past 3 and 6 months with isolation of S. pneumoniae resistant to those antibiotics from COPD patients
Cross-resistance development and antibiotic exposure were also examined at the 3- and 6- month time points. Though no significant cross-resistance was observed to any of the antibiotics assessed, a trend for resistance to tetracyclines was seen in patients with prior exposure to macrolides (33.3%) at 6 months when compared to those without such exposure (12.3%, p = 0.069).
Whether other demographic characteristics of patients influenced the prevalence of macrolide resistant S. pneumoniae was addressed by distinguishing all patients with at least one strain of macrolide resistant pneumococcus (n = 12) from those without any such isolation (n = 26). In a univariate logistic regression, age, race, sex, forced expiratory volume in 1 second percent predicted (FEV1%), forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio and pack years of smoking did not differ between these patients, while the use of macrolide antibiotics in prior 6 months continued to show an increased prevalence in those with at least one resistant strain in both univariate (odds ratio 8.6, 95% CI: 1.32 to 56.24; p = 0.02) and multivariate (odds ratio 8.4, 95% CI: 1.79 to 39.44; p = 0.007) analyses.
DISCUSSION
Antimicrobial resistance among S. pneumoniae is a serious global problem that complicates the management of infections caused by this pathogen, including exacerbations of COPD (Citation15–17). Several previous studies have identified prior treatment with antimicrobial agents as a major predisposing factor for infection with drug resistant S. pneumoniae; however these observations were limited to invasive pneumococcal infections including community acquired pneumonia (Citation18–22, Citation34–36). Our study extends this association between antibiotic use and prevalence of resistant pneumococci to mucosal carriage by this pathogen in adults with chronic lung disease. One can speculate that such individuals, besides suffering from exacerbations from these antibiotic resistant strains, could transmit these strains to other individuals in the community.
There are several differences in this study and prior studies showing effects of prior antibiotic use on infections with resistant S. pneumoniae. Our study population comprised adult patients with COPD while prior studies included all patients with pneumococcal infection and a large proportion were young children. S. pneumoniae were isolated from expectorated sputum during regular monthly visits and during exacerbations in our study while many previous studies have used S. pneumoniae isolates from different body sites. Most of the antibiotics were administered during hospitalization in the prior studies (Citation18–21) while the majority of our patients received outpatient antibiotics. Despite these differences, prior antibiotic use was found to be a risk factor for respiratory infection with resistant S. pneumoniae in patients with COPD.
Current guidelines of use of antibiotics to treat exacerbations of COPD have stressed that antibiotic use in the previous 3 months be considered and an agent of a different class be used in these patients (Citation1). Evidence to support this recommendation had been extrapolated from observations made in community acquired pneumonia and pneumococcal bacteremia. Our data provides direct support for this recommendation and suggests that for macrolide exposure, a period of 6 months would be appropriate, rather than only 3 months as currently suggested.
Our observations were most definitive for the association between azithromycin use in the previous 3 and 6 months and the isolation of macrolide resistant strains. This observation concurs with previous findings from several investigators. Moreno (Citation37) found that prior antibiotic therapy was associated with isolation of erythromycin resistant strains of S. pneumoniae in univariate analysis in a prospective study of all hospitalized patients with S. pneumoniae isolates. Reinert (Citation38) found an association between isolation of erythromycin resistant strains in invasive pneumococcal infections in Germany and consumption of azithromycin, clindamycin and roxithromycin; no correlation was found between consumption of erythromycin and erythromycin resistance.
Vanderkooi and colleagues (Citation22) found use of penicillin, trimethoprim/sulfamethoxazole, clarithromycin and azithromycin in previous 3 months as risk factors for invasive pneumococcal infections with macrolide-resistant isolates. Extensive use of macrolides in the past 2 decades and the persistence of long acting drugs such as azithromycin at low levels for prolonged periods after acute exposure likely account for the emergence of erythromycin resistant streptococci (Citation39). Increasing evidence supports the clinical relevance of macrolide resistance to the outcome of respiratory pneumococcal infections. Kelley (Citation40) found prior use of either azithromycin or clarithromycin for 3–5 days in 4 patients with treatment failure and low-level macrolide resistant pneumococci in a retrospective study of 41 patients. Another prospective study of patients with pneumococcal bacteremia showed that resistant isolates were more common after failure of macrolide therapy than after failure of nonmacrolide antibiotics or without prior antibiotic therapy (Citation41).
We did not find any association between β-lactam antibiotic exposure in previous 6 months and isolation of penicillin nonsusceptible/resistant S. pneumoniae. Though there was a trend at 3 months, we also did not find an association between trimethoprim-sulfamethoxazole exposure in previous 6 months and isolation of trimethoprim-sulfamethoxazole resistant S. pneumoniae. Many prior studies have found such associations in invasive pneumococcal infection. Nava (Citation20) and Clavo-Sanchez (Citation19) found previous use of β-lactam antibiotics as an independent risk factor for infection with penicillin resistant strains of S. pneumoniae in hospitalized patients with pneumococcal infections. Pallares and co-workers (Citation21) showed that 65% of patients infected with resistant pneumococci gave a history of antibiotic use in the previous 3 months, compared with only 17% of controls with susceptible pneumococcal infections. Vanderkooi and colleagues (Citation22) found that risk factors for infection with penicillin resistant pneumococci were previous use of penicillin, trimethoprim-sulfamethoxazole, and azithromycin within 3 months (Citation18, Citation42).
The frequency of isolation of S. pneumoniae was relatively low in our cohort, which is comprised of mostly patients with moderate to severe COPD. Several other investigators have reported a low rate of isolation of S. pneumoniae in patients with moderate to severe COPD, with a higher rate of isolation in milder disease (Citation8, Citation43, Citation44). This finding has not been explained, though it may be related to increased immunity to this pathogen with repeated exposures over time in this disease. A high degree of variability in carriage and turnover of pneumococcal strains among patients was also seen in this study and needs further investigation as to underlying mechanisms.
Several limitations of our study need to be considered. We did not have adequate numbers of resistant strains and/or antibiotic exposures to some of the antibiotics, such as fluoroquinolones, and tetracyclines. Observations regarding relationship of use of these classes of drugs with antibiotic resistance in COPD therefore could not be made. Observations from pneumococcal community acquired pneumonia (CAP) and invasive diseases suggest that such relationships are also likely to exist in COPD. Azithromycin was the predominant macrolide used in our study population; therefore, our observations should be extrapolated with caution to other macrolides such as clarithromycin and erythromycin. In fact the differences in pharmacokinetics of these agents suggest that they may have differential effects on subsequent pneumococcal resistance (Citation22). Isolation of pneumococci from our patients included colonization and only a minority of strains were isolated at exacerbation. Therefore, whether our observations are relevant to exacerbation treatment and clinical outcome is not directly assessable in this study.
In summary, S. pneumoniae is recovered from the sputum of a proportion of COPD patients with a high degree of variability in carriage and turnover of strains. Macrolide use in the previous 6 months is associated with macrolide resistance in sputum isolates of S. pneumoniae. Recent antibiotic exposure may help to determine appropriate antibiotic treatment in adult patients with COPD.
Declaration of interest
H. Desai has no conflicts of interest to disclose. S. Richter has received research funding from Abbott Laboratories, BD Diagnostics, Cerexa, and Schering –Plough. G. Doern received research funding (Cerexa, Schering-Plough, Bayer), speaking honoraria (Cubist, Schering-Plough) and has been a consultant (Abbott Diagnostic, Astellas, and Forest Laboratories). K. Heilmann has no conflicts of interest to disclose. C. Dohrn has no conflicts of interest to disclose. A. Johnson has no conflicts of interest to disclose. A. Brauer has no conflicts of interest to disclose. T. Murphy has received consulting fees from Glaxo Smith Kline, Merck and Mpex and has received grant support from Sanofi Aventis. S. Sethi has received research funding (Bayer), speaking honoraria (Bayer, Pfizer) and has been a consultant (Bayer, Pfizer, Mpex, GSK, Schering-Plough). All authors contributed to the research and writing of the article.
ACKNOWLEDGEMENTS
Work was performed at Western New York Veteran Affairs Healthcare System, Buffalo, New York; and University of Iowa, Iowa City, Iowa. The Study clinic was funded by VA Merit Review. Also, Bayer Corporation funded additional analysis of the S. pneumoniae isolates.
REFERENCES
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365.
- Wilson R. The role of infection in COPD. Chest 1998; 113(4 Suppl):242S–248S.
- White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax 2003; 58(1):73–80.
- Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177(8):853–860.
- Fagon JY, Chastre J, Trouillet JL, Domart Y, Dombret MC, Bornet M, Gibert C. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis 1990; 142(5):1004–1008.
- Monso E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, Ausina V. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995; 152(4 Pt 1):1316–1320.
- Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 2005; 172(2):195–199.
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347(7):465–471.
- Sethi S, Muscarella K, Evans N, Klingman KL, Grant BJ, Murphy TF. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 2000; 118(6):1557–1565.
- Sethi S, Wrona C, Grant BJ, Murphy TF. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169(4):448–453.
- Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1498–1505.
- White AJ, Gompertz S, Bayley DL, Hill SL, O'Brien C, Unsal I, Stockley RA. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 2003; 58(8):680–685.
- Zalacain R, Sobradillo V, Amilibia J, Barron J, Achotegui V, Pijoan JI, Llorente JL. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J 1999; 13(2):343–348.
- Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002; 57(9):759–764.
- Jones ME, Karlowsky JA, Blosser-Middleton R, Critchley IA, Karginova E, Thornsberry C, Sahm DF. Longitudinal assessment of antipneumococcal susceptibility in the United States. Antimicrob Agents Chemother 2002; 46(8):2651–2655.
- Mera RM, Miller LA, Daniels JJ, Weil JG, White AR. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander Project. Diagn Microbiol Infect Dis 2005; 51(3):195–200.
- Reinert RR. Resistance phenotypes and multi-drug resistance in Streptococcus pneumoniae (PROTEKT years 1–3 (1999–2002). J Chemother 2004; 16 Suppl 6:35–48.
- Bedos JP, Chevret S, Chastang C, Geslin P, Regnier B. Epidemiological features of and risk factors for infection by Streptococcus pneumoniae strains with diminished susceptibility to penicillin: findings of a French survey. Clin Infect Dis 1996; 22(1):63–72.
- Clavo-Sanchez AJ, Giron-Gonzalez JA, Lopez-Prieto D, Canueto-Quintero J, Sanchez-Porto A, Vergara-Campos A, Marin-Casanova P, Cordoba-Dona JA. Multivariate analysis of risk factors for infection due to penicillin-resistant and multidrug-resistant Streptococcus pneumoniae: a multicenter study. Clin Infect Dis 1997; 24(6):1052–1059.
- Nava JM, Bella F, Garau J, Lite J, Morera MA, Marti C, Fontanals D, Font B, Pineda V, Uriz S Predictive factors for invasive disease due to penicillin-resistant Streptococcus pneumoniae: a population-based study. Clin Infect Dis 1994; 19(5):884–890.
- Pallares R, Gudiol F, Linares J, Ariza J, Rufi G, Murgui L, Dorca J, Viladrich PF. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N Engl J Med 1987; 317(1):18–22.
- Vanderkooi OG, Low DE, Green K, Powis JE, McGeer A. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis 2005; 40(9):1288–1297.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(5 Pt 2):S77–121.
- Ertugrul N, Rodriguez-Barradas MC, Musher DM, Ryan MA, Agin CS, Murphy SJ, Shayegani M, Watson DA. BOX-polymerase chain reaction-based DNA analysis of nonserotypeable Streptococcus pneumoniae implicated in outbreaks of conjunctivitis. J Infect Dis 1997; 176(5):1401–1405.
- Denys GA, Carey RB. Identification of Streptococcus pneumoniae with a DNA probe. J Clin Microbiol 1992; 30(10):2725–2727.
- Mundy LS, Janoff EN, Schwebke KE, Shanholtzer CJ, Willard KE. Ambiguity in the identification of Streptococcus pneumoniae. Optochin, bile solubility, quellung, and the AccuProbe DNA probe tests. Am J Clin Pathol 1998; 109(1):55–61.
- Whatmore AM, Efstratiou A, Pickerill AP, Broughton K, Woodard G, Sturgeon D, George R, Dowson CG. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “Atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect Immun 2000; 68(3):1374–1382.
- McEllistrem MC, Stout JE, Harrison LH. Simplified protocol for pulsed-field gel electrophoresis analysis of Streptococcus pneumoniae. J Clin Microbiol 2000; 38(1):351–353.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33(9):2233–2239.
- Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In., 6th edn. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2003
- Performance standards for antimicrobial susceptibility testing: 15th informational supplement. In Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2005; M100–S115.
- Performance standards for antimicrobial susceptibility testing: 18th informational supplement. In Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008; M100–S118.
- MMWR. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae—United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2008; 57(50):1353–1355.
- Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev 1990; 3(2):171–196.
- Hofmann J, Cetron MS, Farley MM, Baughman WS, Facklam RR, Elliott JA, Deaver KA, Breiman RF. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med 1995; 333(8):481–486.
- Butler JC, Hofmann J, Cetron MS, Elliott JA, Facklam RR, Breiman RF. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis 1996; 174(5):986–993.
- Moreno S, Garcia-Leoni ME, Cercenado E, Diaz MD, Bernaldo de Quiros JC, Bouza E. Infections caused by erythromycin-resistant Streptococcus pneumoniae: incidence, risk factors, and response to therapy in a prospective study. Clin Infect Dis 1995; 20(5):1195–1200.
- Reinert RR, Al-Lahham A, Lemperle M, Tenholte C, Briefs C, Haupts S, Gerards HH, Lutticken R. Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany. J Antimicrob Chemother 2002; 49(1):61–68.
- Seppala H, Nissinen A, Jarvinen H, Huovinen S, Henriksson T, Herva E, Holm SE, Jahkola M, Katila ML, Klaukka T Resistance to erythromycin in group A streptococci. N Engl J Med 1992; 326(5):292–297.
- Kelley MA, Weber DJ, Gilligan P, Cohen MS. Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin Infect Dis 2000; 31(4):1008–1011.
- Daneman N, McGeer A, Green K, Low DE. Macrolide resistance in bacteremic pneumococcal disease: implications for patient management. Clin Infect Dis 2006; 43(4):432–438.
- Robins-Browne RM, Kharsany AB, Koornhof HJ. Antibiotic-resistant pneumococci in hospitalized children. J Hyg (Lond) 1984; 93(1):9–16.
- Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest 1998; 113(6):1542–1548.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD Study Group of Bacterial Infection in COPD. Chest 1999; 116(1):40–46.