ABSTRACT
During the last decades progress has been made in the treatment of Chronic Obstructive Pulmonary Disease (COPD). We compared a random sample of patients admitted for an exacerbation in the period 2001–2005 (n = 101), with a random sample of patients hospitalized for the same reason in the period 1980–1984 (n = 51). Patients of the 2001–2005 cohort had a lower FEV1 (48 ± 3 vs. 41 ± 2% predicted, p = 0.01) for similar mean age, gender and body- mass index when compared to the historical sample. Co-morbidities, according to the Charlson's index, were more prevalent in the 2001–2005 cohort compared to the 1980–1984 cohort, with a reduction of hemoglobin (13.9 ± 0.2 gr/dl vs. 14.9 ± 0.2, p < 0.01) and higher prevalence of anemia in the most recent cohort. We found an increase in the use of cardiovascular drugs and respiratory medications over time with exception for the long-term use of oxygen. Despite lower FEV1 and more prevalent co-morbidities, no difference in length of hospitalization (13.6 ± 1.4 days vs. 12.7 ± 0.7 days, p = 0.52) and 30 months survival post-exacerbation was noted (66.6% vs. 69.3%, p = 0.85). Over the course of 20 years, the presentation of COPD patients admitted for an exacerbation seems to be changed towards a more severe phenotype with lower FEV1 and more co-morbidities. As the length of hospitalization and the overall survival were not different between the two samples, a currently improved management of COPD can be hypothesized.
Keywords: :
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease, a major cause of morbidity and mortality (Citation1–3) and is expected to become the third-leading cause of death by the year 2020 (Citation4). Over the last decades it became clear that COPD is not limited to the lungs, but has important systemic consequences and co-morbidities which affect disease severity and enhance progression (Citation5). Moreover, repetitive exacerbations have now been recognized as important contributors to disease deterioration (Citation6).
Many efforts have been made to diagnose COPD early and to treat patients better. Smoking cessation has been shown to reduce the rate of decline in forced expiratory volume in 1 second (FEV1) (Citation7), and smoking prevalence has decreased considerably in most of the developed Western countries. With the availability of long-acting anticholinergics, long-acting β2-agonists, inhaled steroids and fixed combinations, we now have treatments that improve FEV1, health status and reduce exacerbation rate. Even mortality is accepted to be reduced by fixed combinations of inhaled steroids and long-acting β2-agonists and by long-acting anticholinergics (Citation8,9). As exacerbations accelerate the rate of decline in FEV1, impair health status and hence, promote disease progression, a reduction in exacerbation rate would be expected to change the natural course of the disease. Data from a post-hoc analysis from TORCH appear to confirm this (Citation10).
Moreover, with the recognition of co-morbidities as important determinants of COPD severity, therapeutic improvements in other domains such as the cardiovascular field, might be reflected in better outcomes for COPD (Citation11–13). Overall, with the improved management of COPD, at present one would actually expect less severe COPD for a given age when compared to the past.
The present study was thus designed to examine a potential change in presentation of COPD patients admitted to our hospital for an exacerbation, over a 2-decade interval. We therefore compared the patients who followed the clinical pathway for COPD exacerbations in the period 2001–2005, with a retrospectively constituted sample of patients who were admitted for an exacerbation to our division in the period 1980–1984.
MATERIAL AND METHODS
Patient samples
Data from 427 COPD patients admitted to the clinical pathway from 2001 till 2005 were prospectively collected. The 51 first patients admitted in 2001 and the last 50 patients admitted in 2005 were enrolled as representative sample for the entire study period. They were compared with a group of 51 patients admitted during the period 1980–1984; those patients were retrospectively collected after analyzing 307 files received from our medical archives via ICD-9 coding (491: chronic bronchitis, 492.0: emphysematous bleb, 492.8: other emphysema, 496: chronic airway obstruction, not elsewhere classified and 518: other diseases of lung). All consecutive patients who had at least 1 pulmonary function performed and who fulfilled the definition of COPD and of an exacerbation were included. Fifty-one files were finally retained and the major reasons for non-retention were no acute exacerbation of COPD as primary diagnosis and absence of pulmonary function data in the file.
Diagnosis of COPD was based on the GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria (Citation14). In both groups, a smoking history (>10 pack-years) and a spirometry with forced expiratory volume in 1 second/ forced vital capacity (FVC) < 0.7 were mandatory for the diagnosis. All patients were admitted with an exacerbation of their disease, which was defined as an increase in symptoms of cough, dyspnea and sputum necessitating a change in treatment and hospitalization. Each patient was only included once in the study.
Patients who required intensive care admission for mechanical or non-invasive ventilation were excluded to avoid large variability in hospitalization length and survival (Citation15). Individual co-morbidities were also retrieved from medical files and subsequently Charlson's index and age-adjusted Charlson's index were calculated (Citation16). The index did not include COPD as previously suggested (Citation17). Overall, the following data could be collected in both cohorts: age, gender, anthropometric characteristics, FEV1, length of hospitalization, 30 months survival, co-morbidities, hemoglobin, hematocrit and creatinin at admission, and use of respiratory and cardiovascular medication.
Pulmonary function tests
Forced vital capacity (FVC) and FEV1 were determined by spirometry and the results were expressed in absolute values and as percentages of the adult reference values recalculated for all the patients using the European Respiratory Society 1993 equations (Citation18). All measurements were done under full therapy during the exacerbation. A FEV1/FVC ratio below 0.7 was mandatory for COPD diagnosis and inclusion. For the period 1980–1984, pulmonary function measurements were performed with a water-sealed spirometer (Lode Spirograph from Lode Instruments, Groningen, The Netherlands, Gould Spirometer from Gould Medical Products Inc., Dayton, Ohio or Godart Expirograph from Godart-Statham, Bilthoven, The Netherlands) according to guidelines of European Coal and Steel Community.
For the 2001–2005 period, pulmonary function measurements were performed either with the Jaeger spirometer (Jaeger Pneumoscreen Epich Jaeger, GmbH, Hoechberg, Germany) or with the Sensor Medics spirometer (Sensor Medics, Anaheim, CA, USA) according to the ATS/ERS guidelines. If multiple pulmonary function tests of the same patient were acquired during hospitalization, a mean FEV1 was calculated, which was then used in our data set.
Laboratory analysis
Hemoglobin and hematocrit were measured with the Coulter Counter Model S (Coulter Electronics, Luton, UK) from 1980 to 1982 and from 1982 until now with the Coulter Counter Model S Plus II (Coulter Electronics, Luton, UK) or with the Ortho ELT-8(Ortho Diagnostic Systems, Westwood, USA). Serum creatinin was determined by the kinetic Jaffé reaction from 1980 to 1984 with the Technicon SMAC (Technicon Instruments, NY, USA).
From 2001 to 2005, serum creatinin was determined by the kinetic Jaffé with the Hitachi Modular (Roche Diagnostics, Indianapolis, USA). The clearance of the creatinin was calculated according to the Cockroft-Gault equation. Anemia was defined as hemoglobin concentration <13 gr/ dl and <12 gr/ dl in males and females, respectively. Polycythemia was defined as hemoglobin concentration > 18 gr/ dl in males and > 16 gr/ dl in females.
Mortality data
Vital status was obtained from the date of admission until 30 months thereafter. This was done through the Belgian National Registry, after agreement from the National Commission of Privacy and formal approval of the Ethics Committee of our hospital. Causes of death were not obtained.
Statistical analysis
Variables between the 2 groups were compared by Student's t-tests. x2 was used for non-continuous variables. Correlations were calculated using Pearson regression coefficients. A stepwise multiple linear regression model was applied on hemoglobin levels looking at different covariates like age, sex, FEV1, body-mass index and the use of angiotensin converting enzyme (ACE)-inhibitors. Cox stepwise regression was performed to assess for potential explanatory variables for survival (FEV1, FEV1 percent predicted, age, body-mass index and hemoglobin). Survival analysis was performed by the method of Kaplan- Meier and Log-rank test was used to look for statistical significance. Two-sided p-values <0.05, were used as limits for statistical significance in all analyses.
RESULTS
Population characteristics
The characteristics of study patients are shown in . Age, weight, height, gender, GOLD classification and creatinin were not significantly different between the two groups. The more recent cohort had a statistically significant reduced airflow obstruction as compared to the former cohort (FEV1 1.07 ± 0.04 vs. 1.28 ± 0.1 L, p = 0.01; 48.4 ± 2.8 vs. 40.6 ± 1.5% predicted, p = 0.01), more severe co-morbidities according to the Charlon's index (2.26 ± 0.12 vs. 1.63 ± 0.16, p<0.05%, respectively) and significantly reduced hemoglobin levels (14.8. ± 0.2 vs. 13.8 ± 0.1, p < 0.05%, respectively)
Table 1. Baseline characteristics of study patients
Medical treatments
The medication used prior to admission is shown in . The use of respiratory medication changed profoundly between 1980–1984 and 2001–2005; short-acting inhaled β2-agonists (63 vs. 79%, p = 0.03), short-acting inhaled anti-cholinergics (20 vs. 71%, p < 0.01), inhaled steroids (18 vs. 78%, p < 0.01) and oral steroids (25 vs. 45%, p = 0.02) were more frequently used in the recent cohort. Notably, 30% and 75% of patients in the 2001–2005 cohort were on long-acting anti-cholinergics and long-acting ß2-agonists, medications that did not exist in the early 1980s. Similar observations were made for cardiovascular drugs; we found a greater use of aspirin, calcium-channel blockers and ACE-inhibitors in the 2001–2005 cohort, compared to the 1980–1984 cohort (19% vs 0%, 14% vs 4%, 16% vs 0%, p < 0.05, respectively). The use of vitamin K antagonists, statins, loop diuretics and beta blockers were not statistical significantly different. There were no significant differences in the use of home oxygen between the 2 cohorts and continuous positive pressure ventilation (CPAP) for sleep apnea was only used in 3 patients of the recent cohort.
Table 2. Use of medication prior to admission
As multidisciplinary respiratory rehabilitation for COPD patients in our hospital was only introduced after 1990, no direct comparison between the 2 cohorts was possible. However, only n = 6 or 6% of the admitted patients of the 2001–2005 cohort followed such a multidisciplinary program prior to admission.
Mean length of hospitalization and mortality
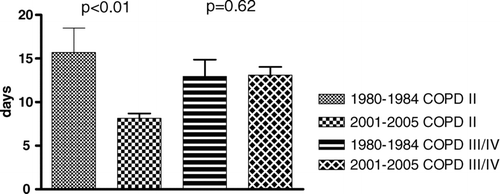
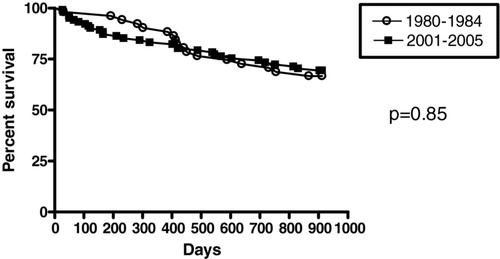
Mean length of stay was 13.6 ± 1.4 days in the 1980–1984 cohort, while it was 12.7 ± 0.7 days in the 2001–2005 cohort (p = 0.52). As the more severe patients had on the average a longer duration of stay, the latter was corrected for the enhanced severity. This yielded a significant reduction of the duration of stay for patients GOLD II (15.7 days ± 2.8 vs. 8.1 days ± 0.5, p < 0.01) but no modification for patients GOLD III/IV (13 ± 2 days vs. 14 ± 1 days, p = 0.62) (). The 30 months’ survival was not significantly different between the 2 cohorts: 66.6% for the 1980–1984 cohort and 69.3% for the more recent group (p = 0.85) (). Cox regression analysis could only reveal FEV1 and age as independent predictors for mortality, whereas body-mass index (BMI), Charlson's Index and hemoglobin levels did not contribute (r2 = 0.10, p = 0.02).
Hemoglobin levels
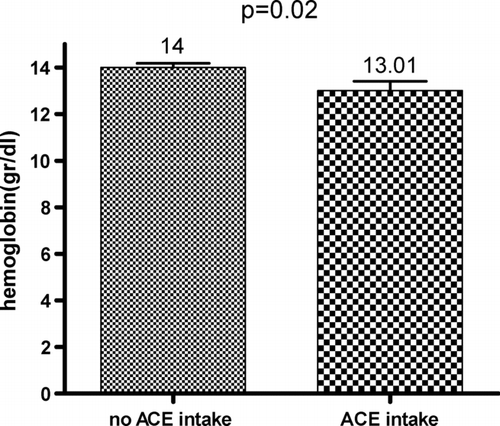
Hemoglobin and hematocrit were significantly lower in the 2001- 2005 cohort when compared to the 1980–1984 cohort (hemoglobin: 14.8 ± 0.2 vs. 13.8 ± 0.2 mg/dl, p < 0.01; hematocrit: 0.45 ± 0.01 vs. 0.43 ± 0.00, p = 0.02). The proportion of patients with polycythemia was significantly higher in the older cohort compared to the recent one (5/51 vs. 1/101, p = 0.02) whereas the proportion of patients with anemia was significantly lower (5/51 vs. 28/101, p = 0.01). In the cohort 2001–2005, the use of ACE-inhibitors was associated a lower hemoglobin (14 ± 0.2 vs. 13.01 ± 0.4 mg/dl, p = 0.02) () and with a higher prevalence of anemia (p = 0.04).
DISCUSSION
The present study suggests that the phenotypic presentation of COPD patients requiring hospital admission for an acute exacerbation has changed over the last decades. Compared to a historical cohort of the early 1980s (1980–1984), patients admitted for an exacerbation 20 years later (2001–2005) presented with a lower FEV1 and more co-morbidities for identical ages. Despite the more severe presentation, duration of hospital stay and 2.5-year survival post exacerbation was not different between both cohorts.
These changes are remarkable as they may indicate that over the last decades the severity of COPD has increased. Indeed, COPD patients of the most recent cohort had more severe disease, demonstrated by a 10% reduction of FEV1 for identical mean ages. They also presented with more prominent systemic consequences as suggested by a significant increase in Charlson index, a reduction in hemoglobin level and a non-significant reduction in BMI. If we assume that patients with a COPD exacerbation admitted to the hospital represent the most severe patients at a given point in time, our data suggest that we are currently dealing with more severe COPD. One explanation could be that patients who have COPD today, just started to smoke at younger ages, which would not be surprising given the maximal popularity of cigarettes in the 1960s and 1970s. An alternative explanation may be that COPD has become more aggressive over the last 20 years. Other potential risk factors for COPD and its presumed consequences that are not tobacco-related, such as inactivity, vitamin D deficiency, obesity, air pollution, etc. (Citation19–22) may well account for such difference, but to our knowledge, no direct evidence has supported this hypothesis so far.
It is tempting to speculate that when going back 25 years in time, patients generally died at earlier stages of their disease because of lack of treatment. Although our analysis is not the appropriate instrument to investigate such question, recent data from the National Vital Statistics for U.S. citizens seem to confirm that the average age of death for COPD has increased with 3 to 4 years over the last 20 years (Citation23). Although we would expect a concomitant increase in average age during hospitalization, the limited size and the retrospective nature of our analysis may explain the observed small and non-significant difference in our data set. For instance, most elderly COPD patients may have been referred to a geriatric division and will, therefore, be missed from inclusion in our study sample. Indirectly however, similar length of hospitalization and similar long-term survival for increased disease severity corroborate the idea of improved management of COPD patients at present. When correcting for enhanced severity, length of hospital stay in our hospital was even substantially reduced. To what extent these better outcomes for COPD patients are related to better diagnostic tools, better respiratory medication, more appropriate use of oxygen or respiratory rehabilitation remains unclear. Therapeutic improvements in the cardiovascular field, the cancer domain and other co-morbidities may definitely account for some, if not most of the observed improvements (Citation24,25). Certainly with regard to survival, better cardiovascular care may substantially impact on COPD outcomes (Citation11–13).
The present data suggest that primary care physicians of today less often refer exacerbations at moderate stages of COPD to the emergency department. A better awareness for COPD as well as a potentially improved management at home may be responsible for these observations (Citation26,27). A different policy with regard to admitting practices with more chronically ill patients managed in the outpatient setting is another plausible reason. It should be noted that we were not able to check the records of patients who were referred to the emergencies but not admitted, which would obviously be needed to substantiate one of the given explanations.
Another remarkable finding was the loss of hemoglobin over the last 25 years, which is consistent with our general impression that polycythemia has become more rare in COPD. Compared to the more recent cohort, a statistically significantly higher proportion of polycythemia was observed in the cohort of the 1980s, whereas anemia was more prevalent in the recent study group. A possible explanation may relate to higher levels of systemic inflammation with increasing disease severity. Alternatively, a more appropriate use of oxygen at home in COPD and a much better differential diagnosis with sleep apnea for “blue bloaters” may have reduced the prevalence of polycythemia in correctly diagnosed COPD. A final explanation might be found in the increasing use of ACE-inhibitors (Citation28). Our data confirm the observations of others that use of ACE-inhibitors associates with lower hemoglobin levels, independently of other covariates (Citation29).
The present study may be subject to bias due to the issues of selection. First, patients admitted to the intensive care were excluded from the analysis because of the lack of correct follow-up data in the early 1980s, but also to reduce the heterogeneity of clinical characteristics within each cohort. Second, sample sizes for both cohorts were rather limited. In particular for the earlier sample, the relatively small number of patients retained from original files was mainly due to the absence of spirometric data. Subjects in whom spirometry was not performed could have been the most severe cases, and many of them may have had COPD without spirometric confirmation, which may have introduced some selection bias. Nevertheless, we believe that our sample still represents a random sample of patients hospitalized at that time. Moreover, the differences between the two samples appear very clear and even with limited samples clear statistical significance was already obtained. It remains unclear, however, how patients hospitalized for COPD exacerbations were selected from the entire COPD population of our region at the 2 points in time. The present data do not give us insight in this matter.
In summary, our results suggest that in patients admitted with an acute COPD exacerbation, a modification occurred over the last 25 years towards more severe obstruction and more co-morbidities for similar ages. The enhanced severity with no apparent increase in length of hospital stay and mortality might be seen as an indirect proof of better disease management in general. A large multicenter review is now needed to support our hypotheses.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest 2003 May; 123(5):1684–1692.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004 Aug 14; 364(9434):613–620.
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006 Jan; 27(1):188–207.
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997 May 3; 349(9061):1269–1276.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007 Sep 1; 370(9589):797–799.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002 Oct; 57(10):847–852.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977 Jun 25; 1(6077):1645–1648.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007 Feb 22; 356(8):775–789.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct 9; 359(15):1543– 1554.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins C, Jones PW, Effect of Pharmacotherapy on Rate of Decline of Lung Function in COPD: Results from the TORCH Study. Am J Respir Crit Care Med 2008; 178:332–338.
- van Gestel YR, Hoeks SE, Sin DD, Simsek C, Welten GM, Schouten O, Effect of statin therapy on mortality in patients with peripheral arterial disease and comparison of those with versus without associated chronic obstructive pulmonary disease. Am J Cardiol 2008 Jul 15;102(2):192–196.
- Mortensen EM, Copeland LA, Pugh MJ, Restrepo MI, de Molina RM, Nakashima B, Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res 2009 Jun 3;10:45.
- Rutten FH, Zuithoff NP, Hak E, Grobbee DE, Hoes AW. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med 2010 May 24; 170(10):880–887.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001 Apr; 163(5):1256–1276.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003 Aug; 124(2):459–467.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined co-morbidity index. J Clin Epidemiol 1994 Nov; 47(11):1245–1251.
- Crisafulli E, Costi S, Luppi F, Cirelli G, Cilione C, Coletti O, Role of co-morbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008 Jun; 63(6):487–492.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993 Mar; 16:5–40.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med 2007 Mar 1; 175(5):458–463.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009 Apr 15; 179(8):630–636.
- Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax 2008 Dec; 63(12):1110–1117.
- Zanobetti A, Bind MA, Schwartz J. Particulate air pollution and survival in a COPD cohort. Environ Health 2008 Oct 10; 7:48.
- Brown DW, Croft JB, Greenlund KJ, Giles WH. Average age at death from COPD in the United States: 1980–85, 1990–95, 2000–05. COPD 2009 Jun; 6(3):152–154.
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009 Nov 15; 180(10):948–955.
- Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002 Aug 1; 166(3):333–339.
- Braman SS, Lee DW. Primary care management of chronic obstructive pulmonary disease: an integrated goal-directed approach. Curr Opin Pulm Med 2010 Mar; 16(2):83–88.
- Gross N, Levin D. Primary care of the patient with chronic obstructive pulmonary disease-part 2: pharmacologic treatment across all stages of disease. Am J Med 2008 Jul; 121(7 Suppl):S13–S24.
- Vlahakos DV, Kosmas EN, Dimopoulou I, Ikonomou E, Jullien G, Vassilakos P, Association between activation of the renin-angiotensin system and secondary erythrocytosis in patients with chronic obstructive pulmonary disease. Am J Med 1999 Feb; 106(2):158–164.
- Andreas S, Herrmann-Lingen C, Raupach T, Luthje L, Fabricius JA, Hruska N, Angiotensin II blockers in obstructive pulmonary disease: a randomised controlled trial. Eur Respir J 2006 May; 27(5):972–979.