ABSTRACT
Chronic obstructive pulmonary disease (COPD) is a growing health problem, and an underestimated and underdiagnosed disease in primary care. The aim of this survey was to study the feasibility of detecting undiagnosed COPD patients in the general practice population with the aid of a telephone questionnaire. The study was held in 2 general practices in the Netherlands. During 2 weeks, all patients registered with these 2 practices and aged between 40 and 75 years were contacted through a call center. Persons known with a previous history or diagnosis of COPD or asthma or comorbidity were excluded from the telephone list. The telephone interview used the Respiratory Health Screening Questionnaire (DB Price, 2006). Based on the score on this instrument, respondents were classified as having a low, moderate or high risk of having COPD. Smoking behaviour and BMI were also recorded. Patients with medium and high risk for COPD were invited for spirometry, performed by 2 experienced registered nurses. The results of the telephone interview and spirometric findings were assessed by the attending GP, who established the final diagnosis. The call center reached 1032 persons, 813 of whom answered the questions. The percentage of smokers was 49.2%, with an average number of pack-years of 17.9 (SD = 17); mean BMI was 26.1. Spirometry and analysis by the GP showed that 15.7% of the medium-risk group had previously undiagnosed COPD, versus 39.6% of the high-risk group. The number of undiagnosed COPD patients in the general practice population is considerable. Case finding can focus on moderate- and high-risk groups after telephone risk assessment.
INTRODUCTION
There is currently a worldwide increase in the prevalence of COPD (Citation1). The true prevalence of COPD in the Netherlands is higher than that found in the official registrations of COPD patients in general practices (Citation2,3). About 10% of the population above the age of 40 show symptoms compatible with COPD (Citation4). Only 26% of all COPD patients show clinical signs of the disease (Citation5). About 25–50% of all these COPD patients are known as such to their doctor (Citation6). There are various reasons why COPD patients do not visit their doctor. One reason is that many people with COPD are unaware of their condition. Doctors could play a pioneering role in changing this, through active case finding. Spirometry is not very frequently performed in general practices, and should be used more often to detect more COPD cases (Citation7,8). Early detection could be important to prevent further deterioration of lung function. This is particularly important for smokers, as the lung function in smokers decreases much faster (Citation9,10). There is also evidence that smoking cessation advice based on personal risk factors (pack-years, BMI, symptoms, lung function) is more successful than general advice (Citation11).
There have been several initiatives to develop case-finding programmes to detect COPD. There have been a number of surveys, but these have shown a wide variety in approach and outcome in different clinical settings (Citation12–19). Studies have concentrated on selected groups of persons who consulted their general practitioner (GP), for instance through surveys in the waiting room, or on a group of smokers. Unfortunately, the presence or absence of clinical findings was not helpful to detect airflow obstruction (Citation13). Van Schayck proposed that simple self-administered questionnaires can be used to identify persons for whom spirometric testing may be especially appropriate (Citation14,15). Vandervoorde found 30% undetected COPD among waiting-room patients (Citation16). Geijer found a prevalence of 34% of undetected COPD in male smokers (30% GOLD I and 4% GOLD II) (Citation17). Stratelis showed that 27% of smokers had COPD (Citation18). Piperno found moderate or severe COPD in 53% of smokers(Citation19).
Price and colleagues developed the Respiratory Health Screening Questionnaire as a tool to detect COPD in the general practice population (Citation20, 21). However, there is some controversy in the literature about the value of the RHS questionnaire in primary care populations. After external validation, Kotz et al. found that the RHSQ was not suitable to assess the risk of COPD in a population where only smokers are examined (Citation22). Development of a standardized questionnaire and scoring list will require further study, including prospective validation of items in different appropriate clinical settings.
Case finding in a general practice entails costs and extra workload. Previous research suggests that screening of the whole population with questionnaires and including spirometry is not feasible and not cost-effective (Citation23,24). Although COPD screening in routine general practice is not regarded as a feasible option, the cost of detecting a COPD case is relatively low (Citation15,Citation25,Citation26).
The aim of the present survey was to study the feasibility of COPD detection among all persons aged 40 to 75 years who were registered with 2 general practices, by applying the RHS questionnaire through a call center. The secondary aim was to assess the prevalence of previously unknown COPD in medium- and high-risk groups.
The research questions were:
How many persons are found to be at risk of having COPD in a general practice population, if every person aged 40 to 75 years who is not known to have COPD is assessed by telephone using the RHS questionnaire?
In how many persons who are at risk according to the RHSQ scores can the presence of COPD be confirmed? What extra workload does this approach involve, in terms of time?
METHODS
A cross-sectional observational study was undertaken in two general practices sharing the same building. The 2 practices are very similar and include a total of 4,200 patients. The surgery is located in a rural village in the center of the Netherlands.
All patients aged between 40 and 75 years were extracted from the electronic patient files. We excluded those persons who had already been diagnosed with asthma or COPD, those who had serious and complicated diseases such as a history of lung cancer, pneumoconiosis, tuberculosis, bronchiectasis or pneumonectomy, as well as those using oxygen supplementation and those who were unable to visit the practice due to lack of mobility. An experienced call center was selected, which operated in accordance with Dutch privacy regulations. The call center was asked to contact the eligible persons by telephone.
Unknown telephone numbers were, where possible, traced through the national internet telephone book. Known telephone numbers where there was no answer were called up to 3 times, including in the evenings up to 8 pm. No attempt was made to contact those for whom no telephone number was available. All persons were phoned personally. All respondents were asked to give informed consent. They were also told that the questions were asked on behalf of their own GP. It was explained to them that the purpose of the survey was to detect COPD, even if they had no complaints. They were also told that the outcome would be used to determine whether they would need to undergo spirometry at the surgery at a later date.
The average phone call was planned to last 6 minutes. During the telephone call, data were directly entered in Excel. The call center used 8 operators for the interviews. Within 2 weeks, all eligible persons had been contacted. After data processing, an RHS risk score was calculated for each respondent.
Only respondents at medium and high risk were then invited for spirometry by the practice receptionists, who tried to contact them by phone for a maximum of three times, on different days, between 9 am and 5 pm. Two well-trained, qualified asthma/COPD practice nurses performed spirometry over a period of 4 months. Each spirometry session took 30 minutes. Three evening spirometry sessions were offered to people who were too busy during the daytime.
The practice nurse and the doctor evaluated all spirometries with the accompanying questionnaires and clinical findings once a week. The GP also used his own Electronic Medical Record (EMD). Each new diagnosis was assessed by this GP with a special interest in COPD.
Instruments
The telephone calls used the ‘Respiratory Health Screening’ questionnaire (RHSQ) (see ). This is a short list with ten questions relating to important determinants of COPD. The criteria in this list are age, smoking, BMI, coughing, wheezing, sputum and allergy. The answers on the Respiratory Health Screening questionnaire were transformed into a score; therefore, the validated COPD case finding scorecard has been used (Citation21). First, 0–16.5 points was regarded as a low score, 16.5–19.5 points was a medium score and more than 19.5 points was a high score. Only respondents with medium or high scores were considered ‘at risk’ of having COPD, and only these 2 groups were asked to visit the surgery for spirometry. No spirometry was carried out in the low-risk group.
Each spirometry consultation took 30 minutes, and was based on the COPD guideline of the Dutch College of General Practitioners. Spirometry was performed before and 15 minutes after bronchodilation with four puffs of salbutamol 100 mcg by dosisaerosol. We used the Welch Allyn spirometer that was calibrated each morning. The FER index ( = Forced Expiratory Rate) was assessed using post-bronchodilatory data. Significant bronchodilation was considered present when FEV1post minus FEV1pre, divided by FEV1pre and multiplied by 100% was 12% or more. The diagnosis of COPD was accepted when the FER index was lower than 70%, after reading the flow volume curve and the time volume curve by a qualified nurse. These results in the presence of clinical signs suggestive of COPD were assessed by the attending doctor. The doctor also evaluated whether asthma could be diagnosed.
RESULTS
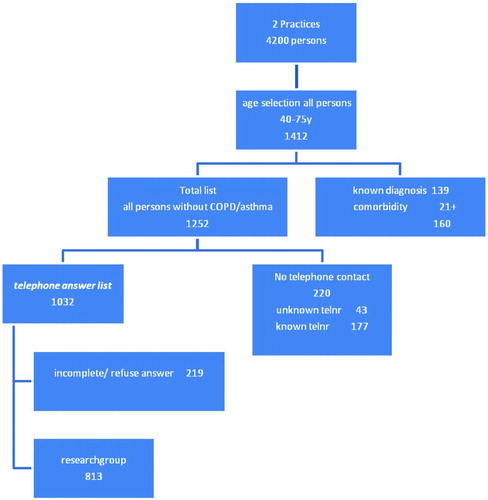
Of the 1,412 patients aged 40–75 years registered with the general practices, 1,252 were not known with a diagnosis of COPD or asthma, or serious invalidating co-morbidity leading to immobility. Two hundred-and-twenty persons could not be reached by telephone, so 1,032 persons were eventually contacted. Of these, 813 (= 78.8%) answered all the questions in the questionnaire as read to them by the call center operators (diagram ). Reasons for non-response were “refusing to answer questionnaires” or being unable to answer all items of the questionnaire. Some respondents refused to answer because they did not experience any respiratory symptoms or felt healthy and therefore felt that they could not answer the screening questions. Some of them thought that COPD had to do with smoking, and since they did not smoke, further questions did not make sense to them.
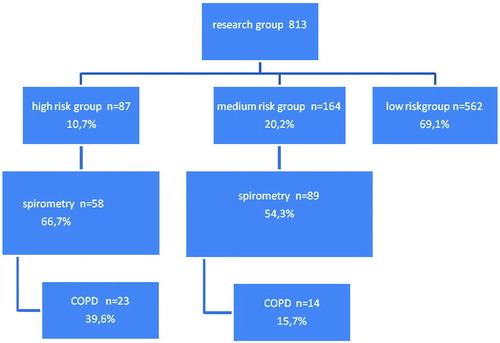
The questionnaire scores identified a high-risk group (11%), a medium-risk group (20%) and a low-risk group (69%) (diagram ). Men and those aged over 60 were overrepresented in the high- and medium-risk groups (). Forty-nine percent of the entire study population were former and/or current smokers, against 75% of the high-risk group. The smokers had an average of 31 pack-years. The medium-risk group included 59% smokers, with an average of 23 pack-years. Allergy was less frequent in the high- and medium-risk groups than in the study population as a whole, while coughing and wheezing were much more frequent than in the total study population.
Table 1. Distribution in the population of risk on COPD according to questionnaire data
Sixty-seven percent 67% of the high-risk group and 54% of the in the medium-risk group underwent spirometry (diagram 2). Most of those who did not show up for spirometry could not be reached via their home telephone number during the daytime. Of those who were contacted, hardly anyone refused spirometry in the end. Ten persons (2 in the high-risk group and 8 in the medium-risk group) were excluded from analysis because no valid spirometric findings could be obtained.
A diagnosis of COPD was established in 39.6% of the high-risk group and 15.7% of the medium-risk group (diagram 2). In both risk groups, about two thirds had GOLD-I COPD and about one third had GOLD-II COPD. GOLD III and IV were not detected. In the newly detected COPD group, 30,6% had COPD and asthma. The average FEV1 was 88,3% and FER 63,1%.
Even in the high-risk group, the score in terms of symptoms and allergy was rather low (). Very few of those in whom COPD was detected by this survey mentioned symptoms: 36% of them had a cough, 31% had productive sputum and 44% had symptoms of wheezing. Eighty-nine percent of them were current smokers. They had an average BMI of 24.6.
The results after questionnaires in this survey identified a risk of having COPD in 31% of the study population aged 40 to 75 years. Combining both risk groups after spirometry, 25% of the respondents were newly diagnosed with COPD. According to these data, 4 spirometries would be needed in the total risk group to find one COPD diagnosis.
The prevalence of COPD in the survey practices was 3.3% (139/4200) before the screening, rising to 4.2% (37/4200 = 0.9%) after the screening. The newly detected COPD patients comprised 21% of all COPD patients.
Impact on Resources
Time aspects and workload in this survey were assessed in a research setting. To translate the findings to routine general practice, we assumed that the screening and measurements would be spread out over one year (40 working weeks, or 200 days). We also assumed that the survey would not be done by a call center, but by the practice receptionist and practice nurse. An average practice in the Netherlands includes 700 persons in the target age group of 40 to 75 years. According to our findings (diagram 1), these would include (160/2) 80 persons with a previous diagnosis of asthma or COPD and/or known relevant comorbidity. Hence, 620 persons would have to be contacted, and 60% of these would provide completed questionnaires, while 60% would respond to a request for spirometry.
Extra time required by the receptionist for the telephone questionnaires
Since there would be 620 persons to be contacted by phone, and each takes about 6 minutes, the phone calls would require 62 extra receptionist hours. This corresponds to 90 minutes a week, which means 3 additional calls a day. In this scenario, the advantage is that when the receptionist makes the calls, she can identify medium or high risk using the questionnaire and make an appointment for spirometry at the surgery within the same call.
Time required for extra spirometries by the practice nurse
Sixty percent of the indicated population at risk were willing to complete a symptom questionnaire, corresponding to (60% of 620) 372 completed questionnaires in an average practice. This would identify (31%) 115 persons at risk, requiring (60% of 115) 69 spirometries. Since each spirometry consultation takes 30 minutes, the workload for 69 extra spirometries would be 35 extra practice nurse hours. Spread out over one year (40 wks) this would imply fewer than 2 extra spirometries a week.
Extra time investment for the GP
In 25% of the above-mentioned 69 spirometries, the GP would be involved to finalize the diagnosis of COPD; this means 17 additional new (20-minute) COPD consultations in one year, or 6 extra GP consultation hours. Furthermore, the GP would have to invest time to coach the practice nurse during the screening programme. In the first 8-week period this might take 30 minutes each week, and 1 hour every 4 weeks in the remaining 8 months. The total investment would thus be 12 GP coaching hours a year. After calculating this extra time workload in costs in the average Dutch general practice, it became clear that the process of detecting new COPD patients generates more income.
Follow-up on newly established diagnoses
A new GP appointment was made after each spirometry consultation when a new diagnosis was established. Two persons were referred to a pulmonologist directly after the GP consultation. These 2 later proved to have lung cancer. All persons with COPD were asked to come back for a new practice nurse consultation, where seven patients failed to show up within 3 months. All smokers were asked to come back for a stop smoking intervention. Six persons with newly found COPD were never smokers, 10 former smokers (27%) and 21 smokers (57%). Sixteen persons (43%) had complaints, while 7 of them did not receive medication. In the 37 persons with newly detected COPD, 11 persons (30%) also had asthma.
Three times during the present survey, doctors and nurses were asked to evaluate the consultations, spirometries and patient compliance. The nurses were very enthusiastic and motivated to do this extra work. No specific additional problems were identified in this survey, compared with the everyday work of the nurses.
DISCUSSION
This study showed that case finding results in the detection of new, undiagnosed COPD patients in normal general practice. The use of a telephone questionnaires call center was helpful in assessing the risk of COPD by means of a questionnaire and to select medium- and high-risk patients who could be invited for a consultation at the practice surgery (including spirometry). The strength of this study was that the personal approach, that is, asking questions by telephone to all people aged between 40 and 75 yrs registered with a GP practice, led to a very reasonable response rate.
The call center told every respondent that their own doctor had asked them to do this. The personal approach resulted in few incomplete questionnaires, although some people refused to answer. The most probable explanation for this is that people were being called by a person from an unknown call center rather than by their own doctor's practice receptionist. Another reason was that many people told the telephone operator that they had no symptoms. They argued that they were not the right person to answer the questions, because they did not feel ill and because they did not smoke. This is a difference with surveys in a different clinical setting, like interviews in waiting rooms, which focus on patients who visit their doctor with a “reason for encounter”. Persons with another known disease, or persons in the waiting room, including smokers, are probably more motivated to respond to questionnaire surveys.
The non-response to the receptionist's invitation for spirometry was in all cases due to the inability to reach these persons by telephone during the daytime. Many people in this age bracket work during the day, and of those who were reached by phone, very few refused spirometry.
If practices performed a screening programme all by themselves (without using a call center), we would expect a much higher response, as the receptionist could administer the telephone questionnaires herself, persons at risk could instantly make an appointment for spirometry, the receptionist could spread out her efforts to contact people at risk over one year and could also send a letter in case telephone contact fails, and the spirometries could also be spread out over one year.
We statistically compared the characteristics within the two risk groups between people who did undergo spirometry and those who did not. There were no significant differences, except for a higher average age and more pack-years in the medium-risk group without spirometry, which may have led to an underestimation of obstruction in this group.
What would have been the consequence of this screening in general practices for the prevalence of COPD? Based on the findings of the second Dutch National Survey (Citation27), the 40- to 75-year age group represents 33% (700 of 2,100 persons) of the total population registered with the general practices in our study. We assume that a maximum of 10% (700–70 = 630) of the persons of this group were already known with COPD. According to our data, 31% are at risk (195 out of 630) for COPD. One in 4 of these persons (49 persons) will have the diagnosis of COPD confirmed by spirometry. In an average GP practice, this would mean 119 (49+70) COPD patients after screening, with 41% (49/119) of all COPD patients not previously detected.
Table 2. Indicators after spirometry and diagnosis
The average prevalence of COPD in Dutch general practices is 3% (Citation28). According to our survey, a screening programme would result in the prevalence in average practices rising from 3.3% (70/2100) to 5.7% (119/2100). This implies a considerable effect of our programme in terms of increasing the percentage of COPD cases identified, especially in the earlier stages of the disease.
Our study was subject to some potential limitations. The burden of effort in this survey in general practice was in the telephone questionnaire, as many persons had to be called in a short time. The cost of engaging a call center for a general practice setting is a large expenditure. We did not offer spirometry to the low-risk group because of the large additional workload; the aim of this study was to detect only patients in the high-and medium-risk groups. The response rate for spirometry among those identified as high- or medium-risk by the questionnaire was rather low: this could be improved by sending those who could not be reached by phone a letter. Patients seem to respond more easily to phone calls by the practice receptionist than by a call center, which may have reduced the response rate. Time and cost restrictions did not seem to be insuperable in this single survey. The method of contacting persons by phone in this survey could be refined.
Patient satisfaction about the questionnaires was not assessed, but after spirometry, all respondents were asked by the practice nurse about their expectations and satisfaction.
Nearly all persons said they appreciated coming to the practice to undergo spirometry. Patients’ opinions about their spirometry consultation were nearly always positive. They found it important that doctors and nurses were interested in their health status. Patients appreciated the time that was available for support and advice in response to their questions during the consultation with the nurse. After the spirometry, most people said they wanted to return for further consultations. After spirometry, all smokers were offered assistance to give up smoking. Smokers with abnormal spirometry values, but also those with normal values, asked for more support to give up smoking. Since many of them had already tried a few times to quit, some did not want to make a new appointment immediately.
A recommendation to other GPs would be to invest greater efforts in detecting COPD patients in the 40–75-year-age group. Since our survey shows many more unknown COPD patients can be detected, the approach should be refeatured in other practices. [Dummy citation] [List 1]
Table 3. Respiratory Health Screening Questionnaire and Scorecard. [list 1]
CONCLUSION
The personal approach of using a telephone questionnaire to detect new COPD cases leads to a high response. The number of undiagnosed COPD patients in the general practice population is considerable. Time aspects do not appear to be an obstacle for starting a practice-led COPD case-finding project in the Netherlands. More research in GP practices should be done to detect regional differences, to assess cost-effectiveness and to focus on an approach involving only the GP's office itself, without external agencies like call centers.
ACKNOWLEDGMENTS
The authors thank the following persons: Mrs. Mascha Twellaar, Maastricht University for statistical procedures. Mrs. Astrid van Hamersveld, practice nurse in charge for the spirometries.
Declaration of interest
The authors report no conflicts of interest. There has been financial support by an unrestricted grant from Boehringer Ingelheim.The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, updated 2010. Available at: www.goldcopd.com. Accessed Aug 31, 2010.
- RIVM Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid 2003. Bilthoven: RIVM; 2003.
- Thiadens HA, Bock GH de, Dekker FW, Huysman JAN, Houwelingen JC, van, Springer MP, Identifying asthma and chronic obstructive pulmonary disease in patients with persistent cough presenting to general practitioners: descriptive study. BMJ 1998; 316:1286–1290.
- Tirimanna PR, Schayck CP van, Otter JJ den,Weel C van, Herwaarden CL van, Boom G van den Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract 1996; 46:277–281.
- Renwick DS, Conolly MJ. Prevalence and treatment of chronic airways obstruction in adults over the age of 45. Thorax 1996; 51:164–168.
- Siafakis NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, ERS consensus statement. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8:1398–1420.
- van Weel C. Underdiagnosis of asthma and COPD: is the general practitioner to blame? Monaldi Arch Chest Dis 2002; 57:65– 68.
- Schayck CP van, Heijden FMMA van Der, Boom G van den, Tirimanna PRS, van Herwaarden CLA. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax 2000; 55:562–565.
- Fletcher C, Peto R, Tinker C. The natural history of chronic airflow obstruction. BMJ 1977; i:1645–1648.
- Anthonissen NR, Conett JE, Kiley JP, Altose MD, Bailey WC, Buist AS. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the lung health study. JAMA 1995; 273:1497–1505.
- Bednarek M, Gorecka D, Wielgomas J. Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61:869– 873.
- Gingter C, Wilm S, Abholz HH. Is COPD a rare disease? Prevalence and identification rates in smokers aged 40 years and over within general practice in Germany. Fam Pract 2009; 26(1):3– 9.
- van Der Molen T, Willemse BWM, Schokker S, ten Hacken NHT, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Out 2003; 1:13.
- van Schayck CP, Halbert RJ, Nordyke RJ, Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology 2005; 10:323–333.
- van Schayck CP, Loozen JM, Wagena E, Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002; 324:1370.
- Vandevoorde J, Verbanck S, Gijssels L, Schuermans D, Devroey D, De Backer J, Kartounian J, Vincken W. Early detection of COPD: A case finding study in general practice. Respir Med 2007; 101:525–530.
- Geijer RMM. Detection of COPD in smokers. Thesis 2006. Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands.
- Stratelis G, Jacobsson P, Molstad S, Zetterstrom O. Early detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 years. Brit J Gen Pract, 2004; 54:201–206.
- Piperno D, Bart F, Serrier P, Zureik M, Finkielsztejn L. General practice patients at risk of chronic obstructive pulmonary disease. Presse Medical 2005; 34:1617–1622.
- Price DB, Tinkelman DG, Halbert RJ, Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006; 73:285–295.
- Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ; for the COPD Questionnaire Study Group. Scoring system and clinical application of COPD diagnostic questionnaires. Chest 2006; 129:1531–1539.
- Kotz D, Nelemans P, Schayck CP van, Wesseling GJ. External validation of a COPD diagnostic questionnaire. Eur Resp J 2008; 31:298–303.
- Calverley PMA, Nordyke RJ, Halbert RJ, Development of a population-based screening questionnaire for COPD. J COPD 2005; 2:225–232.
- Zielinski J, Bednarek M. Early detection of COPD in a high-risk population using spirometric screening. Chest 2001; 119:731– 736.
- Boom G van den, Schayck CP van, van Rutten-Mölken MPMH, Active detection of COPD and asthma in the general population: results and economic consequences of the DIMCA programme. Am J Resp Crit Care Med 1998; 158:1730–1738.
- Schayck van CP, Chavannes NH. Detection of asthma and chronic obstructive pulmonary disease in primary care. Eur Resp J 2003; 21(Suppl 39): 16S–22S.
- Linden MW van Der, Westert GP, Bakker DH de, Schellevis FG. Second National Study, 2004, NIVEL, Utrecht.
- Tabak C, Smit HA. De morbiditeit van astma en COPD in Nederland; leemtes in kennis opgevuld door aanvullende analyses en actualisering van beschikbare gegevensbronnen (RIVM-rapport 260855005/2002). Bilthoven: RIVM; 2002.