ABSTRACT
Reduced heart rate variability (HRV) is a predictor of poor outcome in several pathologies and in general population. Whether HRV is altered during normal daily activities or influenced by anticholinergic and β-adrenergic medications in chronic obstructive pulmonary disease (COPD) remains unknown. Forty-one clinically stable COPD patients and 19 healthy controls matched for age, sex and smoking history underwent a 24-hour ambulatory ECG recording during normal daily activities. HRV was assessed by standardized temporal and spectral analysis. COPD patients showed a reduced HRV (LF/HF ratio) compared with healthy controls (median [interquartile range]) during daytime (2.6 [1.5–3.8] vs. 3.5 [2.9–5.6]), nighttime (1.8 [1.1–4.3] vs. 4.2 [2.7–6.9]) as well as during the entire 24-hour (1.9 [1.5–3.4] vs. 3.9 [3.2–5.6]) recordings (all P < 0.005). There was no significant difference between the two groups in the time domain and in the low frequency or high frequency domain for the 24-hour period analysis. In COPD patients, the 24-hour LF/HF ratio positively correlated with forced expiratory volume in 1 second (FEV1) (r = 0.342, P = 0.028) and negatively correlated with age (r = −0.317, P = 0.044). In multiple regression analysis, LF/HF ratio was associated with FEV1 (P = 0.05) but not with age (P = 0.08). There was no difference of HRV between patients using or not anticholinergic or β-agonist medications. These results demonstrate that COPD patients have a reduced sympatho-vagal balance compared with healthy subjects. HRV correlates with disease severity and does not seem to be influenced by anticholinergic or adrenergic medications.
INTRODUCTION
Heart rate variability (HRV) is defined as the variation of time differences in millisecondes between consecutive heart beats over a given period of time. It reflects the balance of sympathetic and parasympathetic nervous systems inputs on cardiac activity (1). The clinical relevance of HRV was first documented in 1963 by Hon and Lee (Citation2) who noted that fetal distress was preceded by alterations in interbeat intervals before any appreciable change in heart rate itself. Wolf and al. (Citation3) subsequently observed that reduced sinus arrhythmia in response to breathing cycle was a morbidity and mortality predictor following acute myocardial infarction. Lower HRV is now recognized as a risk factor of cardiovascular mortality following myocardial infarct (Citation4). Reduced HRV has also been associated with a worsen prognosis in several cardiovascular and non-cardiovascular conditions (Citation1, 5) such as systemic hypertension, congestive heart failure, valvular heart disease, diabetes, obesity, renal failure, neurological diseases as well as in the healthy population (Citation6).
In chronic obstructive pulmonary disease (COPD), altered HRV has been observed under controlled laboratory conditions and generally in response to sympathetic and parasympathetic stimuli such as changes in body position or voluntary ventilation (Citation7–Citation11). Whether HRV is also altered during daily life activities in COPD and whether HRV is influenced by bronchodilators remains unknown. The objectives of this study were to compare HRV in COPD patients and healthy controls during normal daily life and to evaluate the influence of anticholinergic and β-adrenergic medications on HRV in COPD patients.
MATERIALS AND METHODS
Study population
Forty-one COPD patients aged between 45 and 80 years old were recruited at the Institut universitaire de cardiologie et de pneumologie de Québec. The diagnosis of COPD was based on smoking history and pulmonary function tests documenting chronic bronchial obstruction (forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio <0.70). Only moderate to severe COPD patients (FEV1 <80% of predicted) were eligible (Citation12). COPD caused by a α1-antitrypsin deficiency, patients with COPD exacerbation and/or use of systemic corticosteroid in the 3 preceding months and patients on long-term oxygen therapy were excluded.
The control group was composed of 19 healthy subjects matched for age, sex and smoking history recruited by community advertisement. Conditions with potential influence on HRV were excluded: any cardiovascular disease, significant arrhythmias and pacemaker, use of anti-hypertensive drugs including β-blockers and calcium channel blockers, hormonotherapy, oral corticosteroid, drug acting on the central nervous system or chronic use of non-steroidal anti-inflammatory drug, active cancer, thyroid disease and participation to a regular exercise program. This was evaluated by history taken from the patient, medical record assessment and pharmacotherapy revision with the patient's pharmacist. The research protocol was approved by the institutional ethics committee and participants gave their written consent.
Pulmonary function tests
COPD patients completed standardized pulmonary function tests. Lung volumes were measured by phlethysmography using a constant-volume chamber and carbon monoxide diffusing capacity was measured by single-breath method (Vmax Spectra 22D, SensorMedics, Yorba Linda, CA). All measurements were performed according to the European Respiratory Society recommendations (Citation13–15). Individual results were compared to the predicted values (Citation16). All controls performed a spirometry to exclude an unknown obstructive pulmonary disease.
Heart rate variability
A 24-hour ambulatory ECG recording was performed during daily life activities as previously published (Citation17). Data were separated into three periods: 1) 24-hour period, 2) daytime (8:00 to 20:00) and, 3) nighttime (0:00 to 6:00). HRV analysis was performed according to standards of measurement and interpretation of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Citation1). The parameters calculated in the time domain were: NN (mean of RR intervals), SDNN (standard deviation of all the RR intervals), SDANN (standard deviation of 5-minute average RR intervals), rMSSD (square root of the mean of the squares of consecutive RR interval differences) and pNN50 (proportion of RR intervals >50 ms). Parameters in the frequency domain analysis were: LF (low-frequency power; the area under the spectral curve from 0.04 to 0.15 Hz), HF (high-frequency power; the area under the spectral curve >0.15 Hz) and the LF/HF ratio. The parasympathetic modulation of the autonomous nervous system on cardiac activity is reflected by rMSSD, pNN50 and HF. The LF is considered as representing both sympathetic and parasympathetic components of autonomic function (Citation5). More importantly, SDNN, SDANN and the LF/HF ratio are markers of global HRV or sympathovagal balance.
Statistical analysis
HRV parameters are reported as mean ± standard deviation or median and interquartile range (Q1–Q3) when appropriated. Comparisons between groups were performed using unpaired Student's t-test or the Mann-Whitney Rank Sum test when variables were not normally distributed. Categorical variables were compared using the chi-square test. Pearson's correlation coefficients were used to evaluate relationships between HRV and patients characteristics.
Because SDNN, SDANN and the LF/HF ratio are markers of global HRV, correlations between these parameters and COPD severity (FEV1) as well as patients’ demographics were evaluated. Multivariate regression analysis was used to examine the independent effect of COPD severity, demographics and medication use on HRV, controlling for confounding variables and using variables with a P < 0.10 on univariate analysis. The program used was SigmaStat 3.0 statistical software (SPSS Inc., Chicago, IL, USA). A P < 0.05 value was considered statistically significant.
Table 1. Characteristics of the study population
RESULTS
Characteristics of study participants are presented in . Ambulatory ECG recording results are presented in . There was no difference between the two groups for minimal, mean or maximal recorded heart rates as well as percentage of time spent in tachycardia or bradycardia. For the 24-hour recording period, time domain analyses of HRV (NN, SDNN, SDANN, rMSSD and pNN50) did not show statistical significant difference between groups. However, the 24-hour SDNN and SDANN, both markers of sympathovagal balance, tended to be lower in COPD patients than in controls. For nighttime and daytime periods, only daytime rMSSD was significantly lower while pNN50 tended to be reduced in COPD patients compared to controls.
Regarding the frequency domain, a significant reduction of the LF/HF ratio was observed in COPD patients compared to healthy subjects during the 3 recording periods (). There was no significant difference in time and frequency domain analysis of HRV for COPD patients using or not β-agonists or anticholinergic medications ().
Table 2. Time and frequency domain analysis of heart rate variability in chronic obstructive pulmonary disease patients and controls
Table 3. Time-domain and frequency-domain analysis of heart rate variability in chronic obstructive pulmonary disease patients using or not ß-agonist and anticholinergic medications
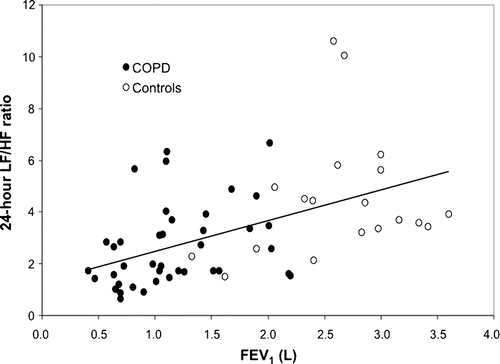
Among COPD patients, the 24-hour LF/HF ratio correlated with absolute FEV1 (r = 0.342, P = 0.028) and age (r = −0.317, P = 0.044). FEV1 tended to correlate with SDNN (r = 0.283, P = 0.073) and SDANN (r = 0.252, P = 0.112). In multivariate analysis, the 24-hour LF/HF ratio was related to FEV1 (P = 0.05) but not with age (P = 0.08). No relationship with other demographic characteristics or medication use was observed. Healthy controls did not show correlation between FEV1 and the LF/HF ratio (r = 0.206, P = 0.397). However, looking at all the participants, there was a strong correlation between HRV and FEV1 (r = 0.499, P = <0.001) even after a multivariate analysis (P < 0.001) ().
DISCUSSION
The present study documented that COPD patients showed reduced HRV compared to matched healthy subjects when studied in normal daily life. LF/HF ratio was significantly lower while SDNN and SDANN, also reflecting overall sympathovagal balance, tended to be decreased in COPD patients. HRV correlated with COPD severity. Conversely, HRV was similar for COPD patients using or not ß-adrenergic and anticholinergic medications.
HRV study consists in analyzing the variation of time between consecutive heart beats for a subject during a given period of time. Using time-domain analysis, the intervals between successive normal QRS complexes, so-called the R-R intervals, are determined and descriptive statistics are applied. Using frequency-domain analysis, variations of RR intervals are grouped under range of frequency powers to delineate parasympathetic and sympathetic influences.
Indeed, HRV reflects balance of the sympathetic and the parasympathetic nervous systems on cardiac activity; the former enhancing cardiac contractility and accelerating heart rate, the latter slowing it down. Some parameters reflect the parasympathetic activity (rMSSD, pNN50 and HF), while LF represents both sympathetic and parasympathetic systems. SDNN, SDANN and the LF/HF ratio are considered as markers of overall HRV and thus, sympatho-vagal balance (Citation1).
Previous studies have demonstrated reduced HRV in COPD patients assessed under controlled conditions with short-term measurements: before and after tilt position, after voluntary changes in ventilation and/or other stimuli selected to challenge the cardiac autonomic nervous system (Citation7–11). However, it as been reported that both breathing control and posture per se influence the autonomic balance, making these observations difficult to interpret (Citation18,19). Some of these studies also used breathing pacing to isolate the impact of respiratory rate on HRV (Citation7–10). These studies showed that even when this factor was controlled for, HRV was lower in patients suffering of COPD than in controls. This suggests that altered HRV is not solely influenced by altered breathing pattern.
The present study confirms an inherent alteration of HRV in COPD patients during normal daily life. The main marker of global HRV, the LF/HF ratio, was lower than controls for the 3 recording periods. Interestingly, there was no difference in the range and the mean heart rates of both groups. This reinforces the notion that HRV evaluation may unmask subtle preclinical changes in the modulation of the cardiac autonomic nervous system (Citation20).
Reduced HRV correlated with FEV1. In accordance with the literature (Citation21), HRV also decreased with aging. It is known that FEV1 is related to age as well (Citation22). After multivariate regression, we observed a significant correlation between the 24-hour LF/HF ratio and the FEV1. Healthy controls did not show this correlation between FEV1 and the LF/HF ratio, but the relationship was quite strong when all the participants were pooled.
In accordance with our results, several studies have reported reduced HRV in COPD patients (Citation7–11). Stein et al. (Citation23) also reported that indexes of HRV reflected severity of disease in COPD patients with α1-antitrypsin deficiency. This suggests that alteration in the autonomic modulation might be related to pulmonary dysfunction and pathologic course of COPD. One possible mechanism is a reactional increased vagal tone of heart rate modulation to counterbalance effect of chronic sympathetic stimulation. Elevated parasympathetic activity could be comprehended as the price to pay for adaptation to stress induced by COPD disease because of chronic airway obstruction, hyperinflation, hypoxemia, deconditioning as well as anxiety.
Figure 1. Low/high frequency ratio of chronic obstructive pulmonary disease patients and healthy controls for the 24-hour, daytime and nighttime recording periods. The group mean is represented by the horizontal bar. LF/HF = Low/high frequency ratio; COPD = chronic obstructive pulmonary disease. *P < 0.005.
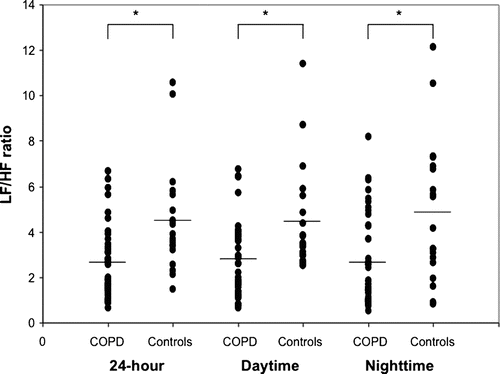
Figure 2. Correlation between the 24-hour low/high frequency ratio and lung function in chronic pulmonary obstructive disease patients and controls. Significant correlations between the 24-hour low/high frequency ratio (LF/HF) and the forced expiratory volume in 1 second (FEV1) were observed for chronic obstructive pulmonary disease (COPD) patients (r = 0.342; P = 0.028) as well as the overall study population (r = 0.499; P < 0.001), whereas no correlation was documented for the controls (r = 0.206; P = 0.397). L = liters.
We also studied inhaled medication as a potential contributing factor to altered HRV in COPD, as suggested by some authors (Citation7). We did not observe any significant difference of HRV parameters between patients using or not inhaled anticholinergic nor β-agonist regimen and medication had no independent influence on the LF/HF ratio in the multivariate analysis. A recent study of Cekici et al. (Citation24) reported that inhalation of therapeutic doses of salbutamol in healthy subjects resulted in significant haemodynamic changes and a shift of the sympatho-vagal balance towards increased sympathetic tone. Few studies reported that acute β-agonist inhalation increased sympathetic modulation of the cardiovascular autonomic balance in asthmatic patients (Citation25, 26), but there is no such study in COPD patients. HRV studies in COPD demonstrate that abnormal autonomic activity is characterized by lack of responsiveness to sympathetic stimulation (Citation7, Citation11). This could be related to chronically high intrinsic and extrinsic sympathetic stimulation maybe associated with a desensitivation and downregulation of β-receptors (Citation27). Thus, the absence of HRV difference between patients using or not inhaled β-agonists could underline COPD abnormal autonomic reactivity.
Lower HRV has been associated with a worsen prognosis and/or increased mortality in several cardiovascular and non-cardiovascular conditions (Citation1, Citation5, Citation6). COPD has been associated with an increased risk of cardiovascular diseases and arrhythmias (Citation28,29). This could be potentially related to subclinical cardiac autonomic nervous system disturbances (Citation30, 31). Whether HRV could be a clinical measure of overall physiologic status and prognosis in COPD patients remains unknown (Citation32). Similarly, improvements in HRV in response to drugs, supplemental oxygen therapy or pulmonary rehabilitation program (Citation33) requires further studies.
Limitations of our study should be discussed. Previous studies in COPD patients documented that hypoxaemia was associated with abnormalities in the autonomic nervous system (Citation9), which may be partially reversed by oxygen administration (Citation34,35). Although none of the COPD patients were hypoxemic at rest, some of the patients may have experienced exercise-induced or sleep-related hypoxemia. Thus, the influence of short-term hypoxemia on HRV in COPD remains unknown.
Similarly, as the level of daily life activity was not monitored, a more sedentary life style frequently observed in COPD (Citation36) could also have influenced the HRV analysis. Yet, similar heart rates during the Holter recordings support the fact that both groups were similarly active. Also, we could speculate that the presence of some degree of right heart failure secondary to potential increased pulmonary artery pressures might lead to altered HRV as encountered in heart failure (Citation1, 5). We unfortunately did not evaluate specifically this element.
Finally, HRV did not differ between patients using or not inhaled medications. However, differences in patient characteristics and absence of a placebo arm precluded any firm conclusions about the effect of anticholinergic and ß-agonist medication on HRV. On the other hand, the strict exclusion criteria to ensure that no other potential disease or medication could influence the autonomic nervous activity and response of patients and controls is a strength of our study.
In conclusion, our findings demonstrate that COPD patients have a reduced global HRV compared with healthy subjects during normal daily life. This decreased HRV correlates with disease severity and does not seem to be influenced by anticholinergic or adrenergic medications. The impact of reduced HRV on morbidity and mortality as well as its potential role in risk stratification of COPD patients needs to be studied.
Declaration of interest
This research was supported partly by the Quebec Heart Institute Research Foundation Corporation. Drs. Steeve Provencher and Paul Poirier are research-scholars from the Fonds de la Recherche en Santé du Québec (FRSQ). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996; 93(5):1043–1065.
- Hon EH, Lee ST. Electronic evaluations of the fetal heart rate, patterns preceding fetal death: further observations. Am J Obstet Gynecol 1963; 87:814–826.
- Wolf MM VG, Hunt D, Sloman JG. Sinus arrhythmia in acute myocardial infarction. Med J Aust 1978; 2:52–53.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59(4):256–262.
- Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med 1999; 50:249–261.
- Tsuji H, Larson MG, Venditti FJ, Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation 1996; 94(11):2850–2855.
- Volterrani M, Scalvini S, Mazzuero G, Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest 1994; 106(5):1432–1437.
- Bartels MN, Jelic S, Ngai P, Basner RC, DeMeersman RE. High-frequency modulation of heart rate variability during exercise in patients with COPD. Chest 2003; 124(3):863–869.
- Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur Respir J 1991; 4(10):1207–14.
- Camillo CA, Pitta F, Possani HV, Barbosa MV, Marques DS, Cavalheri V, Probst VS, Brunetto AF. Heart rate variability and disease characteristics in patients with COPD. Lung 2008;186(6):393–401.
- Pagani M, Lucini D, Pizzinelli P, Sergi M, Mela GS, Malliani A. Effects of aging and of chronic obstructive pulmonary disease on RR interval variability. J Auton Nerv Syst 1996; 59:125–132.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, Van Weel C, Zielinski J. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2007; 176:532–555.
- Macintyre N, Crapo RO, Viegi G, Johnson DC, Van Der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26(4):720–35.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26(3):511–522.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5):948–968.
- Poirier P, Bogaty P, Philippon F, Garneau C, Fortin C, Dumesnil JG. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in uncomplicated well-controlled men with type 2 diabetes. Metabolism 2003; 52:1056–1061.
- Novak V, Novak P, De Champlain J, Le Blanc AR, Martin R, Nadeau R. Influence of respiration on heart rate and blood pressure fluctuations. J Appl Physiol 1993; 74(2):617–626.
- Sanderson JE, Yeung LY, Yeung DT, Kay RL, Tomlinson B, Critchley JA, Woo KS, Bernardi L. Impact of changes in respiratory frequency and posture on power spectral analysis of heart rate and systolic blood pressure variability in normal subjects and patients with heart failure. Clin Sci 1996; 91(1):35–43.
- Poirier P, Bogaty P, Philippon F, Garneau C, Fortin C, Dumesnil JG. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in uncomplicated well-controlled men with type 2 diabetes. Metabolism 2003; 52:1056–1061.
- Utemani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 1998; 31(3):593–601.
- Guenard H. Respiration and aging. Rev Mal Respir 1998; 15(6):713–721.
- Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, Senior RM. Heart Rate Variability Reflects Severity of COPD in PiZ agr1-antitrypsin deficiency. Chest 1998; 113(2): 327–333.
- Cekici L, Valipour A, Kohansal R, Burghuber OC. Short-term effects of inhaled salbutamol on autonomic cardiovascular control in healthy subjects: a placebo-controlled study. Br J Clin Pharmacol 2009; 67(4):394–402.
- Jartti T, Kaila T, Tahvanainen K, Kuusela T, Vanto T, Välimäki I. The acute effects of inhaled salbutamol on the beat-to-beat variability of heart rate and blood pressure assessed by spectral analysis. Br J Clin Pharmacol 1997; 43(4):421–428.
- Eryonucu B, Uzun K, Güler N, Bilge M. Comparison of the acute effects of salbutamol and terbutaline on heart rate variability in adult asthmatic patients. Eur Respir J 2001; 17:863–867.
- Wallukat G. The β-adrenergic receptors. Herz 2002; 27(7): 683–690.
- Falk JA, Kadiev S, Criner GJ, Scharf SM, Minai OA, Diaz P. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5(4):543–548.
- Marquis K, Maltais F, Poirier P. Cardiovascular manifestations in patients with COPD. Rev Mal Respir 2008; 25(6):663–673.
- Yildiz P, Tükek T, Akkaya V, Sözen AB, Yildiz A, Korkut F, Yilmaz V. Ventricular arrhythmias in patients with COPD are associated with QT dispersion. Chest 2002; 122(6):2055–2061.
- Stewart AG, Waterhouse JC, Howard P. The QTc interval, autonomic neuropathy and mortality in hypoxaemic COPD. Respir Med 1995; 89:79–84.
- Lacasse M, Maltais F, Poirier P, Lacasse Y, Marquis K, Jobin J, LeBlanc P. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir Med 2005; 99(7):877–886.
- Marquis K, Maltais F, Lacasse Y, Lacourcière Y, Fortin C, Poirier P. Effects of aerobic exercise training and irbesartan on blood pressure and heart rate variability in patients with chronic obstructive pulmonary disease. Can Respir J 2008; 15:355–360.
- Scalvini S, Porta R, Zanelli E, Volterrani M, Vitacca M, Pagani M, Giordano A, Ambrosino N. Effects of oxygen on autonomic nervous system dysfunction in patients with chronic obstructive pulmonary disease. Eur Respir J 1999; 13:119–124.
- Bartels MN, Gonzalez JM, Kim W, De Meersman RE. Oxygen supplementation and cardiac-autonomic modulation in COPD. Chest 2000; 118:691–696.
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, Poirier P. The metabolic syndrome in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005; 25:226–232.