ABSTRACT
Recent randomised controlled trials suggest non-invasive ventilation may offer benefit in the long-term management of ventilatory failure in stable COPD. The best mode of ventilation is unknown and newer volume assured modes may offer advantages by optimising ventilation overnight when treatment is delivered. This study compares volume assured with pressure preset non-invasive ventilation. Randomised crossover trial including twenty five subjects previously established on long-term non-invasive ventilation to manage COPD with chronic ventilatory failure. Two 8-week treatment periods of volume assured and pressure preset non-invasive ventilation. The primary outcomes were daytime arterial blood gas tensions and mean nocturnal oxygen saturation. Secondary outcomes included lung function, exercise capacity, mean nocturnal transcutaneous carbon dioxide, health status and compliance. No significant differences were seen in primary or secondary outcomes following 8 weeks of treatment when comparing volume assured and pressure preset ventilation. Primary outcomes assessed: mean (standard deviation) PaO2 7.8 (1.2) vs 8.1(1) kPa, PaCO2 6.7 (1.1) vs 6.3 (1.2) kPa and mean nocturnal oxygenation 90 (4) vs 91 (3)% volume assured versus pressure preset, respectively. Volume assured and pressure preset non-invasive ventilation appear equally effective in the long-term management of ventilatory failure associated with stable COPD.
INTRODUCTION
Non-invasive positive pressure ventilation (NIPPV) is being used with increasing frequency to manage chronic ventilatory failure associated with COPD (Citation1). A third of all domiciliary NIPPV is prescribed for this reason (Citation2). Systematic reviews (Citation3,4) and meta-analysis (Citation5) do not support this practice, but more recent randomised controlled trials (RCTs) have shown significant improvements in health-related quality of life, exercise capacity, gas exchange (Citation6) and adjusted survival (Citation7). The importance of titrating NIPPV to augment ventilation overnight has become increasingly recognised (Citation8,9) and is now addressed in trial methodology (Citation6,7). Previous RCTs did not adequately assess whether nocturnal ventilation was increased (Citation10–13) or confirmed a lack of improvement (Citation14).
NIPPV is most commonly used during sleep and delivered by positive pressure techniques in either a volume-preset (vp-NIPPV) or pressure preset (pp-NIPPV) mode (Citation2). Each mode has limitations. Vp-NIPPV aims to deliver a preset volume of ventilation but studies demonstrate up to 50% reductions in delivered ventilation with mask leak (Citation15,16) and a marked drop in ventilation during sleep (Citation17). On the other hand, pp-NIPPV compensates well for mask leaks (Citation18) but constant inspiratory pressure leads to changes in ventilation according to patient effort, lung compliance and airway resistance (Citation16), and a reduction in ventilation during sleep (Citation17).
Volume assured (va-NIPPV) modes adjust inspiratory pressure automatically in an attempt to maintain a target minute ventilation. Theoretically they may be superior to both vp-NIPPV and pp-NIPPV by combining preset minute ventilation with leak compensation ability. One short term study in nine patients with stable COPD has shown equivalence in terms of daytime ABG, comfort and subjective sleep efficiency when comparing va-NIPPV and pp-NIPPV (Citation19). In subjects with obesity hypoventilation improved nocturnal ventilation with a reduction in mean transcutaneous CO2 (mPtcCO2) in comparison to pp-NIPPV (Citation20,21) was seen.
The purpose of this study was to compare pp-NIPPV with va-NIPPV, in stable COPD patients receiving domiciliary NIPPV to manage hypercapnic respiratory failure. The outcomes included daytime arterial blood gases (ABGs) self-ventilating, nocturnal ventilation during NIPPV, lung function, exercise capacity and health status. Our aim was to establish the safety and efficacy of va-NIPPV.
METHODS
The local research ethics committee granted approval for the protocol. Subjects were recruited between the 1st March 2006 and 30th October 2007. Data were collected prospectively. Written consent was obtained from all participants. Randomisation was performed by the hospital's research and development department, independently of the research team, using sealed opaque envelopes. The trial was assigned ISRCTN 84977419.
Population
Subjects were recruited from a cohort of patients under the care of the Respiratory Support and Sleep Centre (RSSC), Papworth Hospital NHS Foundation Trust, Cambridge, UK. All had received domiciliary NIPPV for at least 3 months with compliance of over 2 hours per day and were clinically stable. Based on a change in daytime PaCO2 of 0.7 kPa, a value shown to affect long-term survival in COPD patients on LTOT (Citation22), 23 patients in a crossover design would give an 80% power to detect a difference at a two sided 5% significance level.
The following entry and exclusion criteria were applied. Inclusion criteria: 1) A diagnosis of COPD supported by a smoking history of over 20 pack-years and lung function tests showing a Forced Expiratory Volume in 1 second (FEV1) <50% predicted, FEV1/Forced Vital Capacity (FVC) ratio <70% and Total Lung Capacity (TLC) >80% predicted. 3) Chronic ventilatory failure defined as a daytime PaCO2 over 7.5 kPa with a pH over 7.35 or a nocturnal PtcCO2 over 9 kPa prior to commencing pp-NIPPV. Exclusion criteria: 1) Over 80 years of age. 2) Other significant respiratory disease including interstitial lung disease, asthma, bronchiectasis, neuromuscular or restrictive chest wall disorders. 3) Clinical evidence of left ventricular dysfunction with a documented ejection fraction of <40%.
Clinical stability was confirmed during overnight assessment as part of routine follow-up using the following criteria: 1) No increase in breathlessness, wheeze, cough or sputum volume in the preceding 4 weeks. 2) Arterial PaCO2 within ±1 kPa of the patients’ last elective assessment. 3) Mean overnight oxygen saturation (mSpO2) within 5% of the patients’ last recorded value.
Intervention
Va-NIPPV was provided using an iVAPs (ResMed, Bella Vista, Australia) and Pp-NIPPV using a VPAP III STA (ResMed, Bella Vista, Australia). Both are similar in appearance, were not in use at the recruitment centre and trial subjects were blinded to the ventilators’ mode. Pp-NIPPV was set at similar pressure settings and back-up respiratory rates to that each subject had previously used. The inspiratory trigger was left in the default setting and cycling to expiration was at 25% of maximal inspiratory flow.
Va-NIPPV required a target minute ventilation (TgV). This was recorded using the va-NIPPV by applying pressure support ventilation whilst the subject was awake and at rest for 1 hour at similar pressure settings to that used previously by the subject. This gave an average figure of minute ventilation that was adopted as the TgV. The va-NIPPV was set to enable adjustment of inspiratory pressure up to 25 cm H2O, the maximum possible with this ventilator. Expiratory pressure was set at that previously used by the subject. To allow for subjects’ physiological deadspace, a value was calculated from the patient's height according to the manufacturer's recommendations. To correct for intentional mask leak, masks were quantified for leak characteristics and the appropriate mask setting selected on the va-NIPPV. The va-NIPPV back-up respiratory rate was set to that previously used by the subject. No other changes were made to the patient's mask, circuit configuration, humidification system, oxygen flow rate or routine COPD treatment during the trial.
Protocol
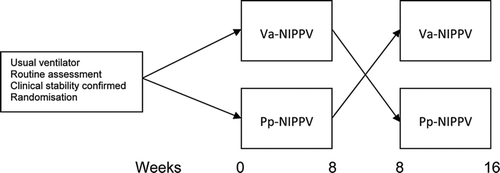
Subjects were contacted with written information prior to their routine clinical in-patient follow-up. Those who gave consent underwent a standard clinical appraisal including history, physical examination, ABG analysis and an overnight study using their established pp-NIPPV consisting of continuous pulse oximetry and transcutaneous PaCO2. Pre-treatment data were collected retrospectively from the patients’ case notes. Those who met clinical stability and entry/exclusion criteria were randomized in a 1:1 ratio to either pp-NIPPV or va-NIPPV in a crossover trial for 2 8-week treatment periods (). At the end of each treatment period subjects underwent outcome measurements as described next with nocturnal assessments performed using the trial ventilator.
Outcome measurements
Arterial blood gases were taken between 0900 and 1700 from the radial artery on the subject's usual oxygen flow rate following a minimum of 20 minutes rest and analysed at the point of care (GEM Premier 3000, Instrumentation Laboratory, Lexington, USA). The overnight SpO2 (3900 Datex-Ohmeda, Louisville, CO, USA) and PtcCO2 (TCM3 Radiometer, Copenhagen, Denmark) were recorded using finger probes and a heated electrode attached to the forearm, respectively. PtcCO2 was calibrated to a known CO2 concentration. Both signals were sampled once a second and analysed using an automated system (Download 2000, Stowood Scientific Instruments, Oxford, UK) that calculates mean values for the period monitored, typically 23.00 Hrs to 07.00 Hrs.
Spirometry, assessment of lung volumes using whole body plethysmography, gas diffusion using carbon monoxide uptake (breath hold method) and an incremental shuttle walking test assessing exercise capacity (Citation23) were performed by trained technicians blinded to the treatment group. Health status was assessed using the SF-36 (UK V1) (Citation24) and St George's Respiratory Questionnaire (SGRQ) (Citation25). Compliance with NIPPV was assessed by downloading hours of use from the ventilators. Ease of use, tolerability, comfort and the quality of sleep associated with the ventilators were assessed using a visual analogue scale (VAS).
Statistical analysis
The primary outcome measures were the daytime PaO2, PaCO2 and nocturnal mSpO2. Kolmogorov-Smirnov testing was utilised to establish a normal distribution. Data were compared using paired Student's t tests. Figures are presented as mean (SD) unless otherwise stated. Due to multiple endpoints Hochberg's principle (Citation26) was used to control type 1 error such that the null hypothesis was to be rejected if all three primary endpoints were significant at <0.05, any two significant at <0.025, or any one significant at <0.0167.
Table 1. Mean (SD) daytime arterial blood gases, nocturnal ventilatory indices, lung function, exercise capacity and demographic data prior to treatment with pp-NIPPV and at trial enrollment
RESULTS
Twenty-eight patients agreed to be screened for trial entry and 25 (13 male) met criteria and were randomised. One subject suffered a severe exacerbation whilst receiving va-NIPPV (randomised to va-NIPPV first) requiring prolonged hospital admission and withdrew consent. Twenty-four patients completed the trial protocol. Pre-treatment historical data and baseline data at trial enrolment are given in .
Table 2. Mean (SD) daytime arterial blood gases, nocturnal ventilatory indices, lung function and exercise capacity following 8 weeks each of va-NIPPV and pp-NIPPV.
Prior to trial entry 22 subjects used a NIPPY 2, one a NIPPY 3 and one a NIPPY 1(B and D Electromedical, Stratford upon Avon, UK) with a mean inspiratory positive airway pressure (IPAP) of 30(5) cm H20 and expiratory pressure (patient on NIPPY 1 excluded) of 4(2) cm H20 and mean compliance of 7.8(2.2) hours per day. Sixteen required additional oxygen supplementation using flow rates between 0.5–2.5 litres/minute. Most were experienced NIPPV users with only four having received NIPPV for less than 1 year.
Thirteen subjects were randomised to receive va-NIPPV first. Patients received va-NIPPV for a mean of 56 (Citation5) days and pp-NIPPV for 56 (Citation7) days. Compliance was 8.2 (3.6) vs. 7.7 (2.4) hours (p = 0.25), respectively. The va-NIPPV was used with a mean minute TgV of 11 (3.9) litres and the pp-NIPPV with a mean IPAP of 27 (Citation3) cm H2O and EPAP of 4 (Citation2) cm H2O.
Nocturnal ventilation and daytime ABGs
No significant differences were found following 8 weeks’ treatment between va-NIPPV and pp-NIPPV in terms of daytime ABGs self ventilating or mean oxygen saturations and transcutaneous PaCO2 during ventilation overnight (). Both ventilators provided adequate ventilation during use and compliance was similar.
Lung function and exercise capacity
The patients recruited to the trial had severe COPD with a mean FEV1 0.6 l (24% predicted) and marked gas trapping with a mean residual volume of 227% predicted. Due to the severity of their disease not all were able to perform repeated breathing manoeuvres to allow assessment of lung volumes via body plethysmography or gas diffusion. Similarly not all were able to perform a shuttle walk test. In those who performed two measurements to allow a comparison, no significant differences in FEV1, FVC, RV, TLC, gas diffusion or exercise capacity were seen following treatment with either ventilator ().
Health status and assessments using VAS
Following 8 weeks of treatment with each of the trial ventilators there were no significant differences between any domains of the SF-36 or SGRQ (). The VAS did not show any differences in ease of use, comfort, tolerability or subjective sleep quality.
Table 3. Mean (SD) of health status and visual analogue scores following 8 weeks each of va-NIPPV and pp-NIPPV
DISCUSSION
This study compared va-NIPPV with pp-NIPPV in a population with chronic ventilatory failure associated with severe COPD. No significant differences in outcomes were found when comparing ventilatory modes following eight weeks of treatment in terms of daytime ABGs, nocturnal ventilation, lung function and exercise capacity. Assessments of health status and the comfort and tolerability of the ventilator modes did not differ. Compliance with both modes of ventilation was good. Va-NIPPV and pp-NIPPV appeared equally effective in the long-term ventilation of patients with ventilatory failure as a consequence of COPD, in patients previously compliant with pp-NIPPV.
The major theoretical advantage of va-NIPPV is automatic adjustment of inspiratory pressures to maintain minute ventilation. Bench testing of va-NIPPV has confirmed that TgV is reached when lung compliance and resistance alter and that circuit leak has little effect on delivered minute ventilation (Citation27). However, maintained minute ventilation is no guarantee of constant alveolar ventilation. A rise in patient effort and respiratory frequency may lead to a situation where tidal volume falls, increasing dead space ventilation and reducing alveolar ventilation. This may lead to treatment failure (Citation28). Such a detrimental effect was not apparent in the current study. Small and probably clinically insignificant differences were seen between pp-NIPPV and va-NIPPV in terms of daytime PaCO2 and mean nocturnal oxygenation. It is unclear if this is a result of the ventilators used or ventilatory mode.
Considerable differences in the performance of positive pressure ventilators in terms of leak compensation, delivery of tidal volume and preset pressures are seen in bench tests of NIPPV and the results are not always predictable from the ventilators’ operating principles (Citation15,16). Previous investigation has shown little effect on nocturnal ventilatory parameters or sleep quality when comparing different pp-NIPPV ventilators (Citation29). Given the experimental nature of the va-NIPPV ventilator and the potential for negative effects, it was important to compare it with a clinically established pp-NIPPV ventilator, although this comparison does make the results more difficult to interpret.
When comparing sleep and wake, ventilation alters by up to 35% in COPD patients, and significant changes in ventilation are also apparent between sleep stages (Citation30). It is likely that different levels of pressure support will be required during these periods to adequately maintain ventilation. Pp-NIPPV with inspiratory pressures set to that tolerated by the patient during wakefulness may not control nocturnal hypoventilation (Citation7, Citation14). On the other hand, high intensity pp-NIPPV aimed at maximal improvements in ventilation may be poorly tolerated by some patients and cause difficulties initiating sleep.
In the largest RCT examining long-term NIPPV in COPD patients with ventilatory failure, 40% of subjects’ compliance was under 4 hours per night; a per protocol analysis suggested the duration of NIV use was an important determinant of survival (Citation7). Va-NIPPV may offer a way to achieve both improved comfort and compliance whilst augmenting nocturnal ventilation. In the current study experienced and compliant users of high intensity NIPPV were assessed and therefore little difference in compliance between ventilator modes would be expected. Subjects who are ventilator naive, intolerant of pressure effects or whom experience sleep disruption may benefit from ventilator modes that attempt to improve compliance by optimizing the pressure support applied. Further investigation in ventilator naive patients will be required to establish if this theory is correct.
Few data are available to assess which mode of ventilation is most effective in the long-term management of ventilatory failure associated with stable COPD. RCTs with positive results have used pp-NIPPV (Citation6–8). Survey data show that pp-NIPPV is the most commonly used modality (Citation2). A short-term crossover trial with 9 stable COPD patients comparing va-NIPPV and pp-NIPPV found no differences in outcomes (Citation19). Longer-term crossover studies in patients with chest wall deformity and in groups with mixed diagnoses including COPD have shown equivalence between pp-NIPPV and vp-NIPPV (Citation17,Citation31). Individual patients’ characteristics and their response to alternative modes of ventilation differ (Citation17) and this must be born in mind in clinical practice. The addition of new modes of ventilation provides alternatives in those who fail standard therapy.
This is the largest study to date comparing different modes of NIPPV in COPD patients with chronic ventilatory failure. No differences were found when va-NIPPV and pp-NIPPV were compared. Further clinical trials analysing the effects of the modulation of inspiratory pressure to maintain minute ventilation are now warranted in ventilator naive patients to assess if optimisation of inspiratory pressure to augment nocturnal ventilation and reductions in IPAP during wakefulness to aid sleep onset and improve comfort offer advantages over current modes of ventilation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors would like to thank ResMed for the loan of ventilator equipment and an unrestricted educational grant to support this research. ResMed had no involvement in the study design, in the data collection, analysis and interpretation or in the writing of the manuscript and the decision to submit the manuscript for publication.
REFERENCES
- Janssens JP, Derivaz S, Breitenstein E, Muralt B, Fitting JW, Chevrolet JC, Rochat T. Changing patterns in long-term non-invasive ventilation. Chest 2003; 123:67–79.
- Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Fauroux FB, Robert D, Schoenhofer B, Simonds AK, Wedzicha JA. Patterns of home mechanical ventilation use in Europe. Results from the Eurovent survey. Eur Respir J 2005; 25:1025–1031.
- Wijkstra PPJ, Lacasse Y, Guyatt GH, Goldstein R, Struik F. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No. CD002878.
- Kolodziej MA, Jensen L, Rowe B, Sin D. Systematic review of non-invasive positive pressure ventilation in severe stable COPD. Eur Respir J 2007; 30:293–306.
- Wijkstra PJ, Lacasse Y, Guyatt GH, Casanova C, Gay PC, Meecham Jones J, Goldstein RS. A meta-analysis of nocturnal non-invasive positive pressure ventilation in patients with stable COPD. Chest 2003; 124:337–343.
- Duiverman ML, Wempe JB, Bladder G, Jansen DF, Kerstjens HAM, Zijlstra JG, Wijkstra PJ. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 2008; 63:1052–1057.
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, O’Donoghue FJ, Barnes DJ, Grunstein RR. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomized controlled trial. Thorax 2009; 64:561–566.
- Meecham-Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538–544.
- Windisch W, Kostic S, Dreher M, Virchow JC, Sorichter S. Outcome of patients with stable COPD receiving controlled non-invasive positive pressure ventilation aimed at a maximal reduction of PaCO2. Chest 2005; 128:657–662.
- Strumpf DA, Millman RP, Carlisle CC, Grattan LM, Ryan SM, Erickson AD, Hill NS. Nocturnal positive-pressure ventilation via nasal mask in patients with severe COPD. Am Rev Respir Dis 1991; 144:1234–1239.
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe COPD during a 3 month controlled trial. Mayo Clin Proc 1996; 71:533–542.
- Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, Santolaria F. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, Ambrosino N. The Italian multicentre study on non-invasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:529–538.
- Lin CC. Comparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPD. Am J Respir Crit Care Med 1996; 154:353–358.
- Smith IE, Shneerson JM. A laboratory comparison of four positive pressure ventilators used in the home. Eur Respir J 1996; 9: 2410–2415.
- Mehta S, McCool FD, Hill NS. Leak compensation in positive pressure ventilators: a lung model study. Eur Respir J 2001; 17:259–267.
- Tuggey JM, Elliott MW. Randomised crossover study of pressure and volume non-invasive ventilation in chest wall deformity. Thorax 2005; 60:859–864.
- Highcock MP, Shneerson JM, Smith IE. Functional differences in bi-level pressure preset ventilators. Eur Respir J 2001; 17:268–273.
- Crisafulli E, Manni G, Kidonias M, Trianni L, Clini EM. Subjective sleep quality during average volume assured pressure support (AVAPS) ventilation in patients with hypercapnic COPD: A physiological pilot study. Lung 2009; 187:299–305.
- Storre JH, Seuthe B, Fiechter R, Milioglou S, Dreher M, Sorichter S, Windisch W. Average volume-assured pressure support in obesity hypoventilation. A randomised crossover trial. Chest 2006; 130:815–821.
- Janssens JP, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity-hypoventilation. Respir Med 2009; 103:165–172.
- Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med 1998; 158:188–193.
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47:1019–1024.
- Jenkinson C, Layte R, Wright L, Coulter A. The UK SF-36: An analysis and interpretation manual. 1996 Health Services Research Unit, University of Oxford.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. Georges Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
- Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802.
- Oscroft NS, Smith IE. A bench test to confirm the core features of volume-assured non-invasive ventilation. Respirology 2010; 15:361–364.
- Smith IE, Shneerson JM. Secondary failure of nasal intermittent positive pressure ventilation using the Monnal D: effects of changing ventilator. Thorax 1997; 52:89–91.
- Highcock MP, Morrish E, Jamieson S, Shneerson JM, Smith IE. An overnight comparison of two ventilators used in the treatment of chronic respiratory failure. Eur Respir J 2002; 20:942–945.
- Ballard RD, Clover CW, Suh BW. Influence of sleep on respiratory function in emphysema. Am J Respir Crit Care Med 1995; 151:945–951.
- Windisch W, Storre JH, Sorichter S, Virchow JC. Comparison of volume and pressure limited NPPV at night: a prospective randomised cross-over trial. Respir Med 2005; 99:52–59.